Abstract
• Aims The purpose of this Botanical Briefing is to stimulate reappraisal of root growth, root/shoot partitioning, and analysis of other aspects of plant growth under heterogeneous conditions.
• Scope Until recently, most knowledge of plant growth was based upon experimental studies carried out under homogeneous conditions. Natural environments are heterogeneous at scales relevant to plants and in forms to which they can respond. Responses to environmental heterogeneity are often localized rather than plant‐wide, and not always predictable from traditional optimization arguments or from knowledge of the ontogenetic trends of plants growing under homogeneous conditions. These responses can have substantial impacts, both locally and plant‐wide, on patterns of resource allocation, and significant effects on whole‐plant growth. Results from recent studies are presented to illustrate responses of plants, plant populations and plant communities to nutritionally heterogeneous conditions.
• Conclusions Environmental heterogeneity is a constant presence in the natural world that significantly influences plant behaviour at a variety of levels of complexity. Failure to understand its effects on plants prevents us from fully exploiting aspects of plant behaviour that are only revealed under patchy conditions. More effort should be invested into analysis of the behaviour of plants under heterogeneous conditions.
Key words: Biomass partitioning, environmental heterogeneity, foraging, plant communities, plant populations, precision, resource acquisition, resource allocation, root growth, root/shoot ratio, soil nutrients
INTRODUCTION
Until recently, most knowledge about plant growth and behaviour was based on experiments conducted under spatially homogeneous conditions. Although the results from such studies are not in question, it is becoming clear that they provide limited explanations of plant behaviour under natural conditions. Spatially homogeneous growing conditions in experiments are problematic because resource availability in natural environments is patchy rather than uniform, and most environments are patchy at scales relevant to (i.e. similar to, or smaller in size than) individual plants (Lechowicz and Bell, 1991; Jackson and Caldwell, 1993; Gross et al., 1995). Evidence is now accumulating showing that plants are strongly affected by heterogeneous rather than homogeneous resource distribution, even if the total resource supply remains the same. Responses to heterogeneity, and their strength, can differ between species. They also depend on developmental stage, and on the type of heterogeneity experienced.
Recent research has shown that spatial heterogeneity in essential resource availability can affect placement and growth of leaves and roots, the growth of whole plants, the intensity of inter‐plant competition, and the yield and structure of plant populations (Fransen et al., 2001; Facelli and Facelli, 2002; Day et al., 2003a, b, c) and communities (Wijesinghe et al., 2004). In this Botanical Briefing, we limit ourselves to reviewing the effects of spatial heterogeneity in soil nutrient supply on root growth and root/shoot (R/S) partitioning. Our purpose is to stimulate a reappraisal of root growth and R/S partitioning that considers the effects of environmental heterogeneity, and to encourage further study of these and other aspects of plant growth, under heterogeneous conditions.
RESPONSIVENESS OF ROOT PLACEMENT TO SOIL NUTRIENT HETEROGENEITY
The most important early studies on plant responses to spatial heterogeneity are those of Drew (1975), Drew and Saker (1975, 1978) and Drew et al. (1973). Main root axes of barley plants were grown vertically through either homogeneous substrates with high nutrient supply, or nutrient‐poor substrates containing a narrow, horizontally‐orientated layer of substrate with the same high nutrient concentration as in the homogeneous treatment. In the homogeneous treatment, development of first‐ and second‐order lateral roots was similar at all depths. In the heterogeneous treatments, lateral root development was poor where nutrients were scarce, but their proliferation was very strong if the narrow nutrient‐rich layer contained nitrate, ammonium or phosphate at high concentration. Importantly, relative growth rates were very similar in these heterogeneous treatments and in the homogeneous treatment. This is striking because the whole of the main root axis was exposed to nutrient‐rich substrate in the homogeneous treatment, whereas only a few per cent of it was within the nutrient‐rich layer in the heterogeneous treatments (Drew and Saker, 1975). Therefore, plants in heterogeneous conditions achieved disproportionate growth for the amount of nutrient‐rich substrate to which they were exposed.
Preferential utilization of habitat patches that are rich in essential resources (a manifestation of foraging: Hutchings and de Kroon, 1994) is a common (although not universal) plant response to heterogeneity. Robinson (1994) reviewed its frequency and the types of responses exhibited when soil nutrient supply is heterogeneous, and Hodge et al. (2000a, b, c) have compared responses in patches with different physical and chemical properties. Alterations in morphology caused by foraging are expected to promote resource uptake, at least of less mobile ions (Robinson and van Vuuren, 1998) and growth (Hutchings and de Kroon, 1994; Fransen et al., 1999). In cases where root proliferation in response to resource‐rich conditions is localized (e.g. Jackson et al., 1990), the cost in terms of new root production may be low compared with the potential resource acquisition benefits.
Some studies have shown that plants can respond with great subtlety to local differences in nutrient supply, closely matching the mass of roots produced in different patches to the relative quality of each patch. Gersani and Sachs (1992) reported that when pea root systems were divided equally between vessels containing solutions with different nutrient concentrations, the mass of new roots grown in each container correlated closely with the relative nutrient concentrations in each vessel. As the nutrient concentrations diverged, so did the root weights. Other studies seeking trade‐offs between root production in patches of different quality have produced inconsistent results (Robinson, 1994; Robinson and van Vuuren, 1998), although similar effects to those observed by Gersani and Sachs (1992) were reported by Gersani et al. (1998). They showed that the presence of competitors in part of the plant’s rooting zone resulted in preferential root production in the competitor‐free habitat. In contrast, Gersani et al. (2001) and Maina et al. (2002) reported that plants provided only with the possibility of competing for the same pool of resources increased their root production compared with plants provided with the same amount of resources per planta. This led to a trade‐off in terms of lower fitness (the ‘tragedy of the commons’, Hardin, 1968). Intriguingly, Falik et al. (2003) recently demonstrated that roots can distinguish potentially competing roots from the same and different plants. Significantly more roots were produced when competing roots belonged to a different plant than when they belonged to the same root system.
Preferential root placement in resource‐rich patches has also been reported in the clonal species Glechoma hederacea (Wijesinghe and Hutchings, 1999). Glechoma hederacea produces numerous ramets, each with its own root system, at intervals along branching stolons. Connections between ramets can persist for long periods, and ramets can be widely distributed. Thus, connected ramets often occupy substrate patches of different quality. Wijesinghe and Hutchings (1999) conducted an experiment on G. hederacea in which different treatments provided the same overall nutrient supply in patches arranged in a chequerboard pattern. There were two patch scales (12·5 × 12·5 cm and 25 × 25 cm) and different contrasts between good‐ and poor‐quality patches. In the treatment with maximum contrast, the substrate in good patches was 100 % compost and that in poor patches was 100 % sand. At the opposite extreme, both ‘good’ and ‘poor’ patches contained a mixture of 50 % compost and 50 % sand (i.e. this treatment was homogeneous). There were four further treatments with intermediate levels of contrast. At the whole clone level, the proportion of roots developed in rich and poor patches closely matched the relative quality of each patch type. Thus, when G. hederacea was subjected to heterogeneous conditions it produced more roots where there were more nutrients. It should be noted that if the plant’s capacity to acquire nutrients from different patches is correlated with the quantity of roots produced in different patches, nutrient concentration will eventually equalize throughout the substrate (Charnov, 1976) unless patches are replenished. We would then expect any further root growth to be uniformly distributed. Under natural conditions, soil nutrient status is very variable in time and space (Frankland et al., 1963; Davy and Taylor, 1974; Farley and Fitter, 1999), and therefore equalization of nutrient concentration and root growth throughout the substrate would rarely occur.
PRECISION OF ROOT PLACEMENT IN NUTRIENT‐RICH PATCHES
Variation in the ability of plants to display selective root growth in nutrient‐rich patches may explain differences in growth and competitiveness in heterogeneous conditions. The extent to which higher‐quality substrate patches are selected is referred to as precision. Several recent studies have sought correlations between precision and other aspects of performance. Campbell et al. (1991) found a significant negative relationship between scale of a species, measured as its proportional contribution to an artificially assembled community, and precision, measured as the proportion of its root growth over a given period that is within nutrient‐rich patches in a heterogeneous habitat. This measure of precision could be subject to high variability, especially for species with little root growth during the measurement interval (Hutchings et al., 2000). Wijesinghe et al. (2001) also recorded a significant negative relationship between root system mass (a more direct measure of scale than that used by Campbell et al., 1991) of six herbaceous species, and precision in root placement in nutrient‐rich substrate patches. In contrast, Einsmann et al. (1999) found no relationship between precision and root mass for a group of herbaceous and woody species; in fact, when the herbaceous species alone were considered there was a (non‐significant) positive relationship between scale and precision.
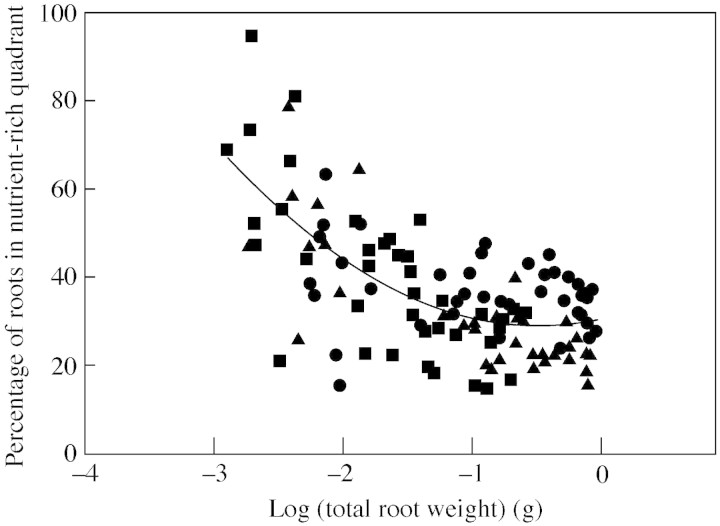
Although precision has been treated as if it were a fixed property of a given species, several lines of evidence show that it is not. For example, in spatially heterogeneous substrates, a plant can germinate with either none or all of its root system in nutrient‐rich conditions. As growth proceeds, precision may either increase or decrease, depending on whether new roots are projected into nutrient‐rich patches or not. If species rapidly increase the volume of substrate explored by their root systems they may locate nutrient‐rich patches faster than species with slow root expansion. Indeed, species that expand slowly, and plants suffering growth limitations due to nutrient shortage, may never access nutrient‐rich patches, even if the distance to them is very small (Hutchings et al., 2003). Precision will also depend on pattern of distribution of nutrient‐rich patches around the plant (Wijesinghe et al., 2001), and perhaps also on the nutrient status of the patches and the plant (Hodge et al., 1999). Finally, as suggested above, after displaying some degree of precision at an earlier stage of growth, roots may eventually occupy all available substrate equally. This was observed in an experiment in which single plants of two small (Poa annua and Briza media) and one large grass species (Arrhenatherum elatius) were grown at the centre of pots of which one quadrant contained nutrient‐rich substrate while the other three contained nutrient‐poor sand. Harvests were taken at different times and total root mass was measured. Precision of root placement in the nutrient‐rich quadrant was also measured as the proportion of roots within the nutrient‐rich quadrant. Whereas plants of all species tended to have high precision when small, precision fell as plant size increased. A trend line through the data suggests that, despite variation, the largest plants of all species had close to 25 % on average of their roots in the enriched quadrant (Fig. 1). It is notable, while considering the relationship between root system scale and precision, that there were no obvious differences in precision between the three species at any size.
Fig. 1. The relationship between percentage of root systems of plants of Arrhenatherum elatius (closed circles), Briza media (closed squares) and Poa annua (closed triangles) located in a nutrient‐enriched quadrant of a pot in which the plant was grown, and total root weight. Harvests were taken 22, 36, 45 and 57 days after the start of growth. The solid line has the form y = 30·49 + 6·29x + 6·61x2 (r2 = 0·44, P < 0·001). See text for further details of the experimental design.
BIOMASS PARTITIONING AND OPTIMIZATION OF RESOURCE ACQUISITION UNDER HETEROGENEOUS CONDITIONS
Many studies of single plants under homogeneous conditions have shown that root growth and biomass partitioning between roots and shoots is strongly influenced by ontogeny (Evans, 1972; Coleman and McConnaughay, 1995; Gedroc et al., 1996; Müller et al., 2000). Others (Bloom et al., 1985; Chapin et al., 1987; Shipley and Meziane, 2002) emphasize that biomass partitioning is adjusted to equalize the limitation of growth by different essential resources. Usually, both ontogeny and conditions impact upon R/S partitioning (Gedroc et al., 1996; Cahill, 2003). When conditions are heterogeneous rather than homogeneous, new possibilities for resource allocation arise. In addition to trading‐off allocation of biomass between different structures, plants can make trade‐offs in root and leaf placement in patches of different quality, as described above for pea (Gersani et al., 1998) and G. hederacea (Wijesinghe and Hutchings, 1999). In homogeneous conditions, resource deficiency is often ameliorated by increasing allocation to the part of the plant responsible for acquiring the most limiting resource (although some studies show increased allocation to roots following fertilization, associated with luxury nutrient consumption without immediate growth, e.g. van Wijk et al., 2003). In contrast, plants in heterogeneous conditions could invest more heavily in roots located where soil‐based resources are most abundant, and more in leaves where light is most abundant. Thus, contrasting strategies, adopted on a local basis, could increase resource acquisition under homogeneous and heterogeneous conditions. Several studies (Drew and Saker, 1975; Birch and Hutchings, 1994; Stuefer et al., 1994, 1996; Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997; Wijesinghe and Hutchings, 1997, 1999; Einsmann et al., 1999) indicate that plants in heterogeneous conditions maximize resource acquisition from sites of abundance rather than scarcity. As a consequence, acquisition efficiency (i.e. amount of resource acquired per unit of mass or effort invested in acquisition) increases (Kovar and Barber, 1988, 1989; Jackson et al., 1990; Jackson and Caldwell, 1996; Stuefer et al., 1996). In clonal species especially there can be considerable variation in resource allocation by different plant parts. Consequently, performance can often be greater in heterogeneous conditions than in homogeneous habitats with the same resources.
Not all heterogeneous environments permit heightened plant performance, however. For example, yield can be strongly influenced by patch scale, as follows. Although responses to patches of differing quality may be initiated quickly, morphological changes take time to complete (Oborny, 1994). If environmental quality is very variable, for example because patches are very small in scale, this could result in appropriate morphological responses to local conditions never being completed before plants grow beyond patches of a given quality. Consequently, resource acquisition might not be maximized, and the potential benefits of patchy resource supply would not be realised. When patch scale is very small, growth can even be poorer than in homogeneous conditions providing the same quantity of nutrients (Slade and Hutchings, 1987; Oborny, 1994; Wijesinghe and Hutchings, 1997). Ackerly (1997) describes analogous difficulties when conditions fluctuate rapidly through time.
ROOT GROWTH AND R/S RATIO OF PLANTS IN HETEROGENEOUS CONDITIONS
The contrasting effects of heterogeneous and homogeneous conditions on plant behaviour are most clearly seen in clonal species. For example, Birch and Hutchings (1994) grew G. hederacea in large boxes containing the same quantity of nutrients distributed either homogeneously or heterogeneously. In the heterogeneous treatment half of the nutrients were confined within a circular patch occupying just 10 % of the total area and volume of substrate, in the centre of the boxes. One ramet of G. hederacea was placed at the edge of each box, with a single short stolon directed towards its centre. Thus, plants in the heterogeneous treatment began growth in poorer conditions than plants in the homogeneous treatment, and the quality of 90 % of the substrate was poorer in the heterogeneous treatment.
Despite their initial handicap and the relatively poor quality of most of the substrate, plants in heterogeneous conditions had produced 2·7 times more leaf mass, 4·7 times more below‐ground mass and 2·5 times more total mass after 11 weeks than plants in homogeneous conditions. Whole clone R/S ratio was nearly twice as high in the heterogeneous treatment, suggesting, from optimization arguments used to predict R/S partitioning, that heterogeneous conditions were perceived by the plants as less favourable than homogeneous conditions. The yields contradict this interpretation. Whereas R/S ratio was similar for all parts of clones in homogeneous conditions, it varied significantly within clones in heterogeneous conditions, being significantly greater within the nutrient‐rich patch than in the surrounding nutrient‐poor area (Table 1). Development of the clones in heterogeneous conditions depended on local substrate quality. Local nutrient supply induced changes in ramet ontogeny. Compared with ramets in similar positions in the homogeneous treatment, roots developed earlier in nutrient‐rich patches and later in poor patches (see table 3 in Birch and Hutchings, 1994). Thus, biomass allocation patterns altered in ways expected to improve acquisition of locally abundant resources. The abundances of light and soil‐based resources are often negatively spatially correlated in natural habitats (Schlesinger et al., 1990; Alpert and Mooney, 1996). Consequently, connected ramets can specialize to acquire different resources in complementary ways. Subsequent translocation between ramets can alleviate local resource shortages (Fig. 2). This type of behaviour (referred to as division of labour, Stuefer et al., 1996; Alpert and Stuefer, 1997; Hutchings and Wijesinghe, 1997) has been recorded in several clonal species.
Table 1.
Mean root/shoot ratios of whole clones and parts of clones of Glechoma hederacea when grown under homogeneous and heterogeneous conditions providing the same total nutrient supply
| Homogeneous | Heterogeneous | |
| Parts within central circle | 0·65a | 0·07c |
| Parts outside central circle | 0·03d | 0·08c |
| Whole clone | 0·15b | 0·08c |
The nutrient concentration in the heterogeneous treatment was higher within the central circle, and lower outside the central circle, than in equivalent areas in the homogeneous treatment. See text, and Birch and Hutchings (1994) for further details of treatments.
Means with different letters are significantly different (P < 0.05).
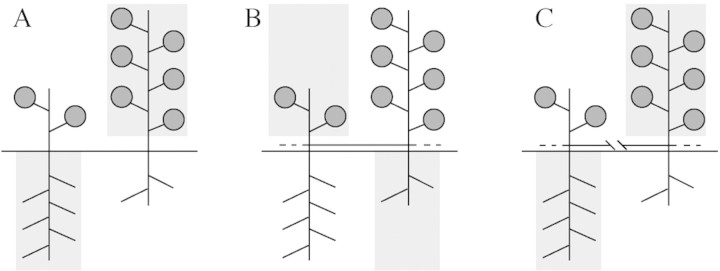
Fig. 2. Predicted patterns of adjustment of biomass allocation between roots and shoots for (A) non‐clonal plants growing in conditions of high light availability and low nutrient availability (left), and high nutrient availability and low light availability (right); (B) connected ramets of a clonal plant, of which one ramet (left) is growing in conditions of high nutrient availability and low light availability while the other (right) is growing in conditions of high light availability and low nutrient availability; and (C) separated ramets of a clonal plant, of which one ramet (left) is growing in conditions of high light availability and low nutrient availability while the other (right) is growing in conditions of high nutrient availability and low light availability. The diagrams show relative adjustments, rather than absolute growth of above‐ and below‐ground parts. The grey boxes indicate scarcity of either light or soil‐based resources. Diagram modified from Alpert and Stuefer (1997).
Wijesinghe and Hutchings’ (1999) study on G. hederacea also involved detailed analyses of the effects of heterogeneity on root growth and R/S ratio. As contrast between substrate patches increased, total clone root mass increased significantly in the larger patch treatments but did not change in the small patch treatments. Total root mass diverged significantly at the two patch scales as contrast increased, even though overall nutrient supply was the same, and this again had a significant effect on total growth. As contrast increased, R/S ratio increased within the good patches and decreased in the poor patches, and the difference at any level of contrast was greater in the larger scale treatments. These results reveal finely‐graded responses to local conditions, demonstrating that local biomass partitioning depends not only on patch quality and contrast, but also on patch scale. The changes in local R/S ratio were also the opposite of those predicted by optimization arguments to increase growth under homogeneous conditions: allocation to roots was greater where nutrients were more abundant. Unless nutrient concentration in the rich patches exceeds the species’ tolerance, such localized responses would be expected to promote resource acquisition. Thus, greater yield would be predicted, at least in the short term (Fransen and de Kroon 2001), than in homogeneous conditions with the same overall level of resource supply.
PREDICTING R/S RATIO IN HETEROGENEOUS ENVIRONMENTS
Clearly, when clonal plants grow in heterogeneous substrate, R/S ratio can be strongly modified by local conditions (Fig. 2). Thus, conditions experienced at the whole‐plant level do not determine biomass partitioning. This makes it extremely difficult to predict whole‐plant R/S ratio under heterogeneous conditions. As already stated, whole‐plant R/S ratio alters as growth proceeds, and different types of heterogeneity can modify this ontogenetic drift (Fig. 3A). Patch scale and contrast interact to modify whole‐plant (Fig. 3B) and local biomass partitioning (Fig. 3C).
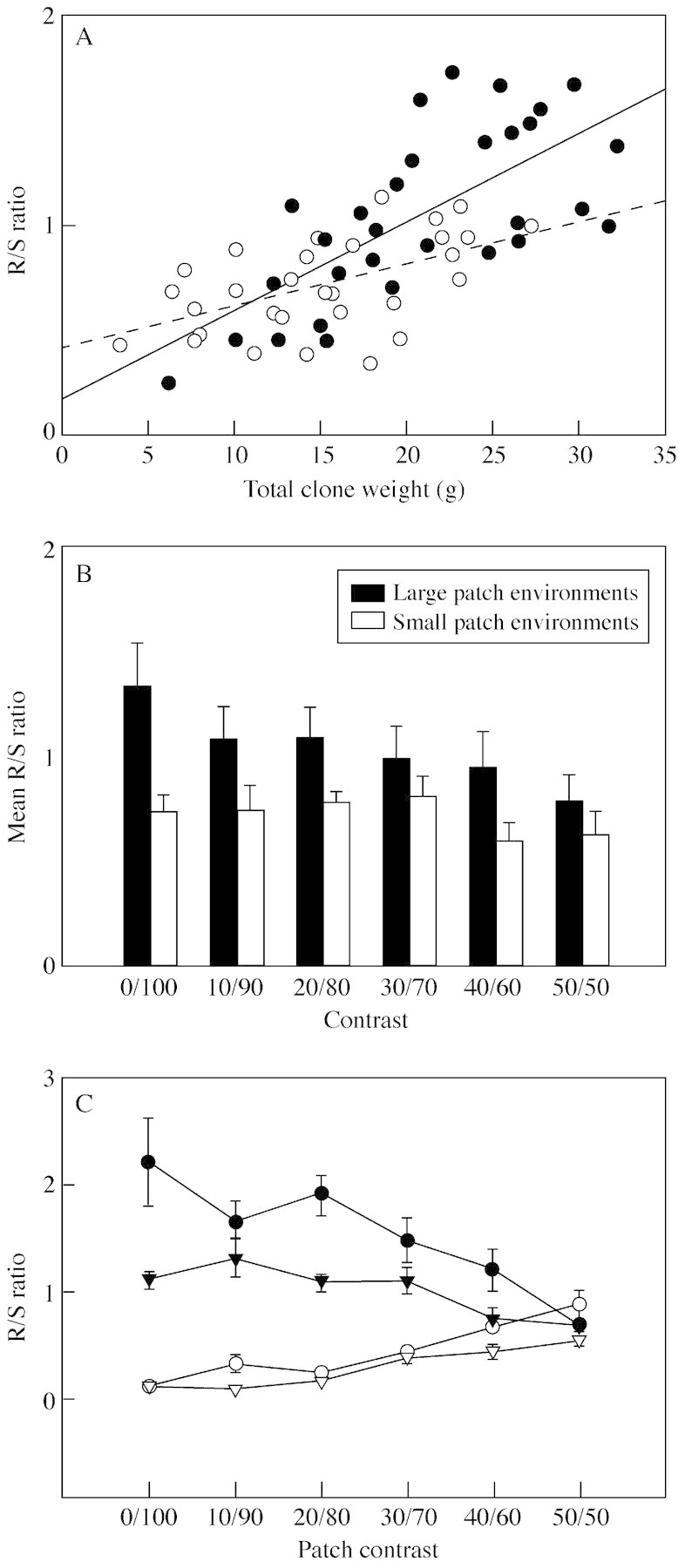
Fig. 3. (A) Relationship between root/shoot ratio and total clone weight for Glechoma hederacea grown in heterogeneous conditions with large scale patches (closed circles and solid line) and small scale patches (open circles and dashed line). Total nutrient supply is the same in each case. For clones in heterogeneous environments with large patches, y = 0·17 + 0·04x, r = 0·69, P < 0·001. For clones in heterogeneous environments with small patches, y = 0·41 + 0·02x, r = 0·54, P < 0·01. (B) Mean (± s.e.) root/shoot ratio for whole clones of Glechoma hederacea grown in large‐ and small‐patch environments with different degrees of contrast between patches. Patch scale had a significant effect on root/shoot ratio (F1,48 = 14·75, P = 0·0004), whereas neither contrast (F5,48 = 1·23, P = 0·31) nor the interaction between scale and contrast (F5,48 = 0·56, P = 0·73) significantly affected root/shoot ratio. Redrawn from Hutchings et al. (2003). (C) Mean (± s.e.) root/shoot ratio of clone parts in nutrient‐rich and nutrient‐poor patches in large‐ and small‐patch treatments at different patch contrasts. Closed circles = rich patches in large‐scale environments, closed inverted triangles = rich patches in small‐scale environments, open circles = poor patches in large‐scale environments, open inverted triangles = poor patches in small‐scale environments. Re‐drawn from Wijesinghe and Hutchings (1999).
Because of their spreading nature, and the ease with which they can be divided into ramets rooted in locations of different quality, within‐plant variation in R/S ratio is readily analysed in clonal species. In contrast, the effects of heterogeneity on R/S ratio in non‐clonal species can only be analysed at the whole‐plant level. Reviewing the data, Robinson (1994) found that localized nutrient applications to plant root systems increased R/S ratio in 50 % of cases, had no effect in 45 % and caused a reduction in 5 %. Robinson suggested that R/S ratio may be relatively unresponsive to localized nutrient patches because increased root growth within nutrient patches is accompanied by compensatory decreases in root growth elsewhere. In a later analysis, Robinson and van Vuuren (1998) reported that, in comparison with plants in uniformly nutrient‐rich conditions, R/S ratio increased by an average 30 % in grasses (including some clonal species) supplied with nutrient patches, but decreased by an average of 30 % in forbs. The responses of these two groups of species were significantly different. In contrast, providing nutrient‐rich patches within a nutrient‐poor environment produced no change in R/S ratio of grasses or forbs when compared to uniformly nutrient‐poor conditions.
Johnson and Biondini (2001) compared several aspects of morphological plasticity in 59 grassland species in homogeneous and heterogeneous conditions. The results are difficult to interpret, because total nutrient supply was greater in the homogeneous treatment, confounding the effects of spatial pattern and nutrient supply. However, some patterns did emerge. Firstly, although most species showed uniform development of root surface area throughout the homogeneous treatment, 21 responded to local nutrient supply in the heterogeneous treatment, and all of these developed significantly more root surface area in nutrient‐rich patches. Fourteen species had significantly higher R/S ratios in the patchy treatment, with increases ranging from 18 % to 244 %. In another study, Wijesinghe et al. (2001) found that R/S ratio was significantly affected by the pattern of nutrient supply in only one of six herbaceous species. However, in this case (Papaver rhoeas), R/S ratio was significantly lower when nutrient patches were close to the plant than in homogeneous conditions with the same nutrient supply. Precision in locating roots in nutrient‐rich patches was also significantly affected by the pattern of nutrient supply. When nutrient patches were closer, root investment decreased and allocation to shoots and flowers increased. Thus, R/S ratio can also be affected by the proximity of nutrient patches and by the proportions of the root system accessing patches of different quality. Clearly, the effects of heterogeneity on root growth and R/S ratio are complex, and not easily predicted from behaviour under homogeneous conditions.
ROOT GROWTH AND R/S RATIO IN PLANT POPULATIONS AND PLANT COMMUNITIES
As heterogeneity in nutrient supply can exert powerful effects on root placement, root growth, R/S ratio and whole‐plant growth, it might also affect competitive relationships between plants, and the composition and yield of populations and communities. Evidence supporting these predictions is now appearing in the literature. There are strong parallels between the effects of heterogeneity on root growth, R/S ratio and total yield on individual plants and its effects on populations, although the latter are more complex. For example, Day et al. (2003b, c) analysed the effects of nutrient heterogeneity on populations of Cardamine hirsuta. In heterogeneous treatments, nutrient‐rich and nutrient‐poor habitat patches were set up in chequerboard patterns and plants were located on a square grid superimposed upon these patches.
After 1 month of growth, root, shoot and total yields were significantly greater (by approximately 40 % in all cases) in populations grown in heterogeneous conditions at three patch scales. R/S ratios of individual plants could not be determined because of extensive root system overlap, but whole population below/above‐ground biomass ratios were also greater (although not significantly so) under heterogeneous conditions. A second experiment was harvested after 2 months, when populations were suffering mortality. At this stage of growth, the pattern of nutrient supply no longer had a significant effect on total yield. However, total mortality was greater in treatments with higher nutrient supply, and significantly higher in homogeneous conditions than in heterogeneous conditions at the same nutrient supply (see also Casper and Cahill, 1996). Variation in root mass per unit of substrate was far less under homogeneous than heterogeneous conditions. Day et al. (2003c) interpreted this as the cause of higher mortality in homogeneous conditions. Relatively even root growth throughout the homogeneous substrate was presumed to indicate uniformly strong competition for soil‐based resources. In heterogeneous conditions, however, plants in nutrient‐poor patches were small, and root mass per unit volume of substrate was also low in these patches. Thus it was suggested that the impact of competition between plants in these patches was low. Interestingly, although nutrient concentration was greater in nutrient‐rich patches in the heterogeneous treatments than in equivalent substrate patches in the homogeneous treatment, both patch types contained similar root masses per unit of substrate. This suggests that, when nutrient concentration surpasses some threshold, an optimal root mass density for exploiting a given substrate volume is not exceeded (see also Fitter, 1976). Experimental study of community responses to heterogeneity has also demonstrated significant elevations in community root and total biomass when nutrient supply is heterogeneous (Wijesinghe et al., 2004).
CONCLUSIONS
We believe that the tradition of studying plants under spatially homogeneous conditions has left serious gaps in our understanding of their natural behaviour. Natural environments are heterogeneous in ways that plants can detect and to which they can respond. Spatial heterogeneity in resource supply provides opportunities for plants to select more suitable habitat patches for the placement of resource‐acquiring structures, but we know very little about the ways in which plants measure local environmental quality and perceive different facets of heterogeneity, such as scale of patches and patch contrast. Likewise, we do not know how perception of heterogeneity is translated into responses, or how responses are modulated. More work is required in these areas. Although explicit study of plant foraging strategies (which acknowledges that the heterogeneity of their habitats is important) and their consequences for plant growth have only recently become active topics of research, much has already been learnt. Plants may acquire resources more efficiently from heterogeneous conditions than from equivalent homogeneous conditions, and this can have significant impacts on growth and yield. Physiologists and ecologists should devote much more effort to documentation, and especially to analysis, of the behaviour of single plants, competing plants, plant populations and plant communities, under patchy conditions. Ignoring the fact that heterogeneity is a constant presence in the natural world, and its potential influence upon plants at so many levels, perpetuates significant weaknesses in our understanding. Equally importantly, it prevents us from fully exploiting aspects of plant behaviour that are only revealed under patchy conditions.
ACKNOWLEDGEMENTS
Many of the results and ideas in this paper were developed during the tenure of UK Natural Environment Research Grant GR3/8843(ML4). We thank David Hutchings for drawing Fig. 2, and two anonymous referees for helpful comments.
Received: 11 November 2003; Returned for revision: 26 November 2003; Accepted: 3 March 2004. Published electronically: 20 May 2004
References
- AckerlyD.1997. Allocation, leaf display, and growth in fluctuating environments. In: Bazzaz FA, Grace J, eds. Plant resource allocation San Diego: Academic Press, 231–264. [Google Scholar]
- AlpertP, Mooney HA.1996. Resource heterogeneity generated by shrubs and topography on coastal sand dunes. Vegetatio 122: 83–93. [Google Scholar]
- AlpertP, Stuefer JF.1997. Division of labour in clonal plants. In: de Kroon H, van Groenendael J, eds. The ecology and evolution of clonal plants Leiden: Backhuys Publishers, 137–154. [Google Scholar]
- BirchCPD, Hutchings MJ.1994. Exploitation of patchily distributed soil resources by the clonal plant Glechoma hederacea Journal of Ecology 82: 653–664. [Google Scholar]
- BloomAJ, Chapin FS, Mooney HA.1985. Resource limitation in plants – an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- CahillJF.2003. Lack of relationship between below‐ground competition and allocation to roots in 10 grassland species. Journal of Ecology 91: 532–540. [Google Scholar]
- CampbellBD, Grime JP, Mackey JML.1991. A trade‐off between scale and precision in resource foraging. Oecologia 87: 532–538. [DOI] [PubMed] [Google Scholar]
- CasperBB, Cahill JF.1996. Limited effects of soil nutrient heterogeneity on populations of Abutilon theophrasti (Malvaceae). American Journal of Botany 83: 333–341. [PubMed] [Google Scholar]
- ChapinFS, Bloom AJ, Field CB, Waring RH.1987. Plant responses to multiple environmental factors. BioScience 37: 49–57. [Google Scholar]
- CharnovEL.1976. Optimal foraging, the marginal value theorem. Theoretical Population Biology 9: 129–136. [DOI] [PubMed] [Google Scholar]
- ColemanJS, McConnaughay KDM.1995. A non‐functional interpretation of a classical optimal‐partitioning example. Functional Ecology 9: 951–954. [Google Scholar]
- DavyAJ, Taylor, K.1974. Seasonal patterns of nitrogen availability in contrasting soils in the Chiltern Hills. Journal of Ecology 62: 793–807. [Google Scholar]
- DayKJ, John EA, Hutchings MJ.2003a. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina Functional Ecology 17: 454–463. [Google Scholar]
- DayKJ, Hutchings MJ, John EA.2003b. The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. Journal of Ecology 91: 305–315. [Google Scholar]
- DayKJ, Hutchings MJ, John EA.2003c. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. Journal of Ecology 91: 541–553. [Google Scholar]
- DrewMC.1975. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barley. New Phytologist 75: 479–490. [Google Scholar]
- DrewMC, Saker LR.1975. Nutrient supply and the growth of the seminal root system in barley. II. Localized, compensatory increases in lateral root growth and rates of nitrate uptake when nitrate supply is restricted to only part of the root system. Journal of Experimental Botany 26: 79–90. [Google Scholar]
- DrewMC, Saker LR.1978. Nutrient supply and the growth of the seminal root system in barley. III. Compensatory increases in growth of lateral roots, and in rates of phosphate uptake, in response to a localised supply of phosphate. Journal of Experimental Botany 29: 435–451. [Google Scholar]
- DrewMC, Saker LR, Ashley TW.1973. Nutrient supply and the growth of the seminal root system in barley. I. The effect of nitrate concentration on the growth of axes and laterals. Journal of Experimental Botany 24: 1189–1202. [Google Scholar]
- EinsmannJC, Jones RH, Pu M, Mitchell RJ.1999. Nutrient foraging traits in 10 co‐occurring plant species of contrasting life forms. Journal of Ecology 87: 609–619. [Google Scholar]
- EissenstatDM, Caldwell MM.1988. Seasonal timing of root growth in favourable microsites. Ecology 69: 870–873. [Google Scholar]
- EvansGC.1972.The quantitative analysis of plant growth. Oxford: Blackwell Scientific Publications. [Google Scholar]
- FacelliE, Facelli JM.2002. Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra‐specific competition and size distribution. Oecologia 133: 54–61. [DOI] [PubMed] [Google Scholar]
- FalikO, Reides P, Gersani M, Novoplansky A.2003. Self/non‐self discrimination in roots. Journal of Ecology 91: 525–531. [Google Scholar]
- FarleyRA, Fitter AH.1999. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology 87: 688–696. [Google Scholar]
- FitterAH.1976. Effects of nutrient supply and competition from other species on root growth of Lolium perenne in soil. Plant and Soil 45: 177–189. [Google Scholar]
- FranklandJC, Ovington JD, Macrae C.1963. Spatial and seasonal variations in soil, litter and ground vegetation in some Lake District woodlands. Journal of Ecology 51: 97–112. [Google Scholar]
- FransenB, de Kroon H.2001. Long‐term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2‐year experiment. Journal of Ecology 89: 711–722. [Google Scholar]
- FransenB, de Kroon H, Berendse F.2001. Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82: 2534–2546. [Google Scholar]
- FransenB, de Kroon H, de Kovel CGF, van den Bosch F.1999. Disentangling the effects of root foraging and inherent growth rate on plant biomass accumulation in heterogeneous environments: a modelling study. Annals of Botany 84: 305–311. [Google Scholar]
- GedrocJJ, McConnaughay KDM, Coleman JS.1996. Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Functional Ecology 10: 44–50. [Google Scholar]
- GersaniM, Abramsky Z, Falik O.1998. Density‐dependent habitat selection in plants. Evolutionary Ecology 12: 223–234. [Google Scholar]
- GersaniM, Brown JS, O’Brien E, Maina GG, Abramsky Z.2001. Tragedy of the commons as a result of root competition. Journal of Ecology 89: 660–669. [Google Scholar]
- GersaniM, Sachs T.1992. Developmental correlations between roots in heterogeneous environments. Plant, Cell and Environment 15: 463–469. [Google Scholar]
- GrossKL, Pregitzer KS, Burton AJ.1995. Spatial variation in nitrogen availability in three successional plant communities. Journal of Ecology 83: 357–368. [Google Scholar]
- HardinG.1968. The tragedy of the commons. Science 162: 1243–1248. [PubMed] [Google Scholar]
- HodgeA, Robinson D, Griffiths BS, Fitter AH.1999. Nitrogen capture by plants grown in N‐rich organic patches of contrasting size and strength. Journal of Experimental Botany 50: 1243–1252. [Google Scholar]
- HodgeA, Stewart J, Robinson D, Griffiths BS, Fitter AH.2000a. Competition between roots and soil micro‐organisms for nutrients from nitrogen‐rich patches of varying complexity. Journal of Ecology 88: 150–164. [Google Scholar]
- HodgeA, Stewart J, Robinson D, Griffiths BS, Fitter AH.2000b. Spatial and physical heterogeneity of N supply from soil does not influence N capture by two grass species. Functional Ecology 14: 645–653. [Google Scholar]
- HodgeA, Robinson D, Fitter AH.2000c. An arbuscular mycorrhizal inoculum enhances root proliferation in, but not nitrogen capture from, nutrient‐rich patches in soil. New Phytologist 145: 575–584. [DOI] [PubMed] [Google Scholar]
- HutchingsMJ, de Kroon H.1994. Foraging in plants: the role of morphological plasticity in resource acquisition. Advances in Ecological Research 25: 159–238. [Google Scholar]
- HutchingsMJ, John EA, Wijesinghe DK.2000. The effects of heterogeneous nutrient supply on plant performance: a survey of responses, with special reference to clonal herbs. In: Hutchings MJ, John EA, Stewart AJA, eds. The ecological consequences of environmental heterogeneity Oxford: Blackwell Science, 91–110. [Google Scholar]
- HutchingsMJ, John EA, Wijesinghe DK.2003. Towards an understanding of the consequences of patchy distribution of soil‐based resources for populations and communities of plants. Ecology 84: 2322–2334. [Google Scholar]
- HutchingsMJ, Wijesinghe DK.1997. Patchy habitats, division of labour and growth dividends in clonal plants. Trends in Ecology and Evolution 12: 390–394. [DOI] [PubMed] [Google Scholar]
- JacksonRB, Caldwell MM.1993. Geostatistical patterns of soil heterogeneity around individual perennial plants. Journal of Ecology 81: 683–692 [Google Scholar]
- JacksonRB, Caldwell MM.1996. Integrating resource heterogeneity and plant plasticity: modelling nitrate and phosphate uptake in a patchy soil environment. Journal of Ecology 84: 891–903. [Google Scholar]
- JacksonRB, Manwaring JH, Caldwell MM.1990. Rapid physiological adjustment of roots to localised soil enrichment. Nature 344: 58–60. [DOI] [PubMed] [Google Scholar]
- JohnsonHA, Biondini ME.2001. Root morphological plasticity and nitrogen uptake of 59 plant species from the Great Plains grasslands, U.S.A. Basic and Applied Ecology 2: 127–143. [Google Scholar]
- KovarJL, Barber SA.1988. Phosphorus supply characteristics of 33 soils as influenced by seven rates of phosphorus addition. Soil Science Society of America Journal 52: 160–165. [Google Scholar]
- KovarJL, Barber SA.1989. Reasons for differences among soils in placement of phosphorus for maximum predicted uptake. Soil Science Society of America Journal 53: 1733–1736. [Google Scholar]
- LechowiczMJ, Bell G.1991. The ecology and genetics of fitness in forest plants. II. Microspatial heterogeneity of the edaphic environment. Journal of Ecology 79: 687–696. [Google Scholar]
- MainaGG, Brown JS, Gersani M.2002. Intra‐plant versus inter‐plant root competition in beans: avoidance, resource matching or tragedy of the commons. Plant Ecology 160: 235–247. [Google Scholar]
- MüllerI, Weiner J, Schmid B.2000. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspectives in Plant Ecology, Evolution and Systematics 3: 115–127. [Google Scholar]
- ObornyB.1994. Growth rules in clonal plants and predictability of the environment: a simulation study. Journal of Ecology 82: 341–352. [Google Scholar]
- RobinsonD.1994. Tansley Review No. 73. The response of plants to non‐uniform supplies of nutrients. New Phytologist 127: 635–674. [DOI] [PubMed] [Google Scholar]
- RobinsonD, van Vuuren MMI.1998. Responses of wild plants to nutrient patches in relation to growth rate and life‐form. In: Lambers H, Poorter H, van Vuuren MMI, eds. Inherent variation in plant growth. physiological mechanisms and ecological consequences Leiden: Backhuys Publishers, 237–257. [Google Scholar]
- SchlesingerWH, Reynolds JF, Cunningham GL, Huenneke LF, Jarrell WM, Virginia RA, Whitford WG.1990. Biological feedbacks in global desertification. Science 247: 1043–1048. [DOI] [PubMed] [Google Scholar]
- ShipleyB, Meziane D.2002. The balanced‐growth hypothesis and the allometry of leaf and root biomass allocation. Functional Ecology 16: 326–331. [Google Scholar]
- SladeAJ, Hutchings MJ.1987. Clonal integration and plasticity in foraging behaviour in Glechoma hederacea Journal of Ecology 75: 1023–1036. [Google Scholar]
- StueferJF.1996. Potential and limitations of current concepts regarding the response of clonal plants to environmental heterogeneity. Vegetatio 127: 55–70. [Google Scholar]
- StueferJF, During HJ, de Kroon H.1994. High benefits of clonal integration in two stoloniferous species, in response to heterogeneous light environments. Journal of Ecology 82: 511–518. [Google Scholar]
- StueferJF, de Kroon H, During H.1996. Exploitation of environmental heterogeneity by spatial division of labour in a clonal plant. Functional Ecology 10: 328–334. [Google Scholar]
- van WijkMT, Williams M, Gough L, Hobbie SE, Shaver GR.2003. Luxury consumption of soil nutrients: a possible competitive strategy in above‐ground and below‐ground biomass allocation and root morphology for slow‐growing arctic vegetation. Journal of Ecology 91: 664–676. [Google Scholar]
- WijesingheDK, Hutchings MJ.1997. The effects of spatial scale of environmental heterogeneity on the growth of a clonal plant: an experimental study with Glechoma hederacea Journal of Ecology 85: 17–28. [Google Scholar]
- WijesingheDK, Hutchings MJ.1999. The effects of environmental heterogeneity on the performance of Glechoma hederacea: the interactions between patch contrast and patch scale. Journal of Ecology 87: 860–872. [Google Scholar]
- WijesingheDK, John EA, Beurskens S, Hutchings MJ.2001. Root system size and precision in nutrient foraging: responses to spatial pattern of nutrient supply in six herbaceous species. Journal of Ecology 89: 972–983. [Google Scholar]
- WijesingheDK, John EA, Hutchings MJ.2004. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology, in press. [Google Scholar]