Abstract
• Background and Aims Illicium floridanum, a species belonging to the basal extant angiosperm taxon Illiciaceae, reportedly exhibits self‐incompatibility (SI). To date, the site and timing of SI within the carpel of this species remains unidentified. Thus, the objective of this research was to determine the cellular and temporal aspects of SI in I. floridanum.
• Methods Following controlled application of cross‐ and self‐pollen in natural populations of I. floridanum, embryo sac development and temporal aspects of stigma receptivity, as well as pollen tube growth, fertilization, and embryo and endosperm development, were investigated with the aid of light and fluorescence microscopy.
• Key Results Flowers of I. floridanum exhibited complete dichogamy whereby stigmas only supported cross‐ and self‐pollen tube growth prior to anther dehiscence. In contrast to earlier reports of SI in this species, a prezygotic SI resulting in rejection of self‐pollen tube growth at the stigma was absent and there were no significant differences between cross‐ versus self‐pollen germination and pollen tube growth within the style and ovary during the first 5 d after pollination. Structural development of the four‐celled embryo sac was not differentially influenced by pollen type as noted to occur in other angiosperms with late‐acting ovarian SI. The ovule micropyle and embryo sac were penetrated equally by cross‐ and self‐pollen tubes. In addition, there were no statistically significant differences in cross‐ versus self‐fertilization. A resting zygote and multicellular endosperm at a variety of developmental stages was present by 30 d after application of cross‐ or self‐pollen.
• Conclusions In the clear absence of a prezygotic SI that was previously reported to result in differential self‐pollen tube growth at the stigma, self‐ sterility in I. floridanum is likely due to early‐acting inbreeding depression, although late‐acting post‐zygotic ovarian SI cannot be ruled out.
Key words: Illicium floridanum, self‐incompatibility, self‐sterility, complete dichogamy, early‐acting inbreeding depression, Illiciaceae, Austrobaileyales
INTRODUCTION
The Illiciaceae is comprised of a single genus of approx. 40 species distributed in eastern Asia and North and Central America (Smith, 1947; Saunders, 1995). As well as having a fossil record extending back to the Early Cretaceous (Friis et al., 2000), the taxon is a member of the paraphyletic group of angiosperms comprising the first three branches of angiosperm phylogeny [Amborellaceae, Nymphaceae and Austrobaileyales (Austrobaileyaceae, Illiciaceae, Schisandraceae and Trimeniaceae); Angiosperm Phylogeny Group (APG), 1998; Mathews and Donoghue, 1999; Parkinson et al., 1999; Qiu et al., 1999, 2001; Barkman et al., 2000; Graham and Olmstead, 2000; Soltis et al., 2000; Zanis et al., 2002].
Illicium floridanum, one of two North American species of the Illiciaceae (Hao et al., 2000), is a small evergreen shrub restricted to the floodplains of small streams in the Gulf States of the south‐eastern United States (Smith, 1947). The flowers of the species are bisexual and protogynous, producing on average 13 unfused uniovulate carpels (Robertson and Tucker, 1979; Thien et al., 1983). Each carpel has a stigmatic crest that lies on the same plane as undehisced stamens but which subsequently moves 90° to stand upright at the time of anther dehiscence (Thien et al., 1983). An external compitum is present in I. floridanum whereby pollen tubes germinating on the stigmatic crest of one carpel can exit the carpel and grow to an adjacent carpel via growth around a centrally located tissue (Williams et al., 1993) termed the apical residuum (Robertson and Tucker, 1979). Pollination in I. floridanum is affected by litter‐dwelling insects to include Diptera and Coleoptera (Thien et al., 1983).
Application of self‐pollen to stigmas of I. floridanum reportedly results in the absence of fruit production leading to the conclusion that the species exhibits self‐incompatibility (SI) (Thien et al., 1983). SI has been noted to occur on the stigma or within the substigmatic transmitting cells of the stigmatic crest, since self‐pollen tubes appear to stop growing after reaching a few millimetres in length (Thien et al., 1983). Ovules from self‐pollinated carpels appear to degenerate early in development (Thien et al., 1983). Using traditional classification schemes (de Nettancourt, 1977, 1997), SI has been classified as gametophytic because I. floridanum has bicellular pollen and the stigma has been interpreted as wet (Thien et al., 1983).
Recently, however, the stigma of I. floridanum has been demonstrated to be of the dry versus wet type (Koehl, 2002). These results are significant for two reasons. First of all, SI in combination with bicellular pollen and a dry‐type stigma was once considered anomalous (de Nettancourt, 1977). If I. floridanum exhibits SI in association with these stigmatic cellular characteristics, it would join a growing number of species with such features reinforcing the view that these character traits are in fact quite common (Franklin et al., 1995; Pontieri and Sage, 1999; Sage et al., 2000, 2001). The cellular nature of pollen and stigmatic epidermal cells involved in SI will likely have important implications for the type of self‐recognition and rejection mechanisms that are operating (Franklin et al., 1995; Franklin‐Tong and Franklin, 2003; Stone et al., 2003). Secondly, a recent study has shown the presence of stigmatic SI in association with a dry‐type stigma and bicellular pollen in Trimenia moorei (Trimeniaceae; Bernhardt et al., 2003), another species rooted at the base of the angiosperm phylogeny and occupying the same branch as the Illiciaceae. As well, a potential stigmatic SI has been reported in the third member of the branch, Austrobaileya scandens (Austrobaileyaceae; Prakash and Alexander, 1984). If SI in I. floridanum, T. moorei and subsequently A. scandens can be demonstrated to share similar cellular, molecular and genetic mechanisms, then it may have important implications for the reconstruction of relictual forms of SI, a long‐standing issue of interest (Whitehouse, 1950; Barrett, 1988; Olmstead, 1989; Bell, 1995; Read et al., 1995; Weller et al., 1995; Sage et al., 2000; Igic and Kohn, 2001; Steinbachs and Holsinger, 2002). The purpose of the present study is to characterize the exact site and timing of self‐pollen recognition and rejection by assessing cross‐ and self‐pollen tube growth and early stages of ovule/seed development following self‐ and cross‐pollen application in naturally occurring populations of I. floridanum.
MATERIALS AND METHODS
Study populations
Experimental material of Illicium floridanum Ellis used in this study was located in three populations in the southern United States. Population A was located adjacent to an oxbow of the Bowie River, in Forest County, Mississippi (31.37688, –89.35886). Population B was west of Rawls Springs, Forest County, near Hattiesburg (31.37286, –89.42064), and population C was located on Morgan’s Bluff along the West Pearl River, near Slidell, Louisiana (30·32899, –89·71267). Cross‐ and self‐ (inter‐ and intra‐population) pollinations were conducted in the field using randomly selected flowering shrubs. To exclude pollination by insects, floral buds were enclosed in light‐weight pollination bags constructed from bridal veil (mesh gauge approx. 100 µm) approx. 24 h prior to floral anthesis. Flowers were cross‐ and self‐pollinated by tapping freshly dehisced anthers just above receptive stigmas. Flowers were then re‐bagged until harvest. Self‐pollinations were conducted with pollen of the same plant. Intra‐populational crosses were conducted for the three populations in addition to inter‐populational crosses in all combinations.
Cross‐ versus self‐pollen tube growth in I. floridanum
Prior to conducting cross‐ and self‐pollinations, the timing of stigma receptivity in I. floridanum was assessed by applying pollen to stigmas when stigmas were (a) in the plane of undehisced anthers (female phase; Thien et al., 1983; n = 39) and (b) when stigmas had moved 90° to an upright position coinciding with anther dehiscence (male phase; Thien et al., 1983; n = 39). From these controlled pollinations, it was determined that stigmas were not receptive during the male phase as they did not support pollen germination. Therefore, all cross‐ and self‐pollinations were subsequently conducted during the female phase of floral development.
Qualitative and quantitative comparisons of cross‐ and self‐pollen tube growth prior to embryo sac penetration were characterized using aniline blue fluorescence microscopy (Martin, 1959; Sage et al., 1999) and light microscopy (Sage et al., 1999). Self‐ and intra‐population cross‐pollinations within populations A and B were conducted on five to ten plants per population and harvested at 48 h (n = 6–15 flowers/treatment/plant/population). Self‐ and intra‐population cross‐pollinations were performed on five to ten plants within population C and harvested at 1 h, 3 h, 6 h, 9 h, 15 h, 24 h, 72 h, 5 d and 10 d (n = 6–15 flowers/treatment/plant/harvest time). Inter‐population cross‐pollinations between the same plants used for intra‐population pollinations were conducted between B × C and A × C. These samples were harvested at 6 h, 9 h, 15 h, 24 h, and 72 h (n = 3 flowers/treatment/plant/harvest time) and A × B and B × A were harvested at 48 h (n = 7–10/flowers/treatment/plant). Quantitative characterization of self‐ and cross‐pollen tube growth was performed by measuring the following parameters at each harvest time for each treatment: (1) the number of germinated pollen grains; (2) the number of pollen tubes in the substigmatic transmitting tissue (SST); (3) the number of pollen tubes entering the ovary locule; and (4) the number of pollen tubes within the ovule micropyle. The data obtained for each of the four parameters was converted into a percentage of the total number of grains that landed on the stigmatic crest for that particular gynoecial unit. Initially, ANOVA was performed to determine if there were differences between data from intra‐ and interpopulation with respect to each parameter at each harvest time for each pollination treatment. No differences were detected in these analyses. Therefore, data from all cross‐pollination treatments were pooled as were data from all self‐pollinations. Two‐way ANOVAs (SigmaStat 2.0) were then used to examine differences over time between cross‐ and self‐pollen tube growth for each of the four quantified parameters.
Embryo sac development, fertilization, and embryo and endosperm development following cross‐ versus self‐pollination in I. floridanum
Embryo sac development was characterized prior to pollination to first assess whether or not an ovule contained a mature embryo sac at anthesis, and hence whether cross‐ versus self‐pollen influenced embryo sac maturation as reported for other angiosperms (Waser and Price, 1991; Sage et al., 1999). Ovules for characterization of embryo sac development were harvested from developing carpels and prepared using cryofixation/freeze substitution as described by Lam et al. (2001). To determine the success of fertilization, ovules/seeds from flowers in populations A, B and C were harvested 5 d, 10 d and 30 d following cross‐ and self‐pollination (n = 12–30 flowers/harvest time/treatment), fixed in formalin–acetic acid–ethanol (FAA), acetic acid–ethanol (1 : 3) or glutaraldehyde and prepared for light microscopy as described by Sage and Williams (1995). Serial sections were scored for fertilization as described by Sage et al. (1998) and Sage and Sampson (2003). Fertilization was indicated by: (a) the absence of unfused sperm nuclei within embryo sacs penetrated by a pollen tube; (b) the presence of a resting zygote and/or embryo; and (c) the presence of one or more endosperm nuclei. Unpollinated ovules were also harvested at 5 d, 10 d and 30 d post‐anthesis to compare embryo sac development with that from cross‐ and self‐pollinated ovules. Initally, ANOVA was performed to determine if there were differences between data from intra‐ and/or inter‐population with respect to presence or absence of fertilization at each harvest time for each pollination treatment. No differences were detected in these analyses. Therefore, data from all cross‐pollination treatments were pooled as were data from all self‐pollinations. Student’s t‐tests were then employed to determine whether significant differences existed between cross‐ and self‐embryo sac penetration as well as cross‐ and self‐fertilization.
RESULTS
Cross‐ and self‐pollen tube growth in I. floridanum
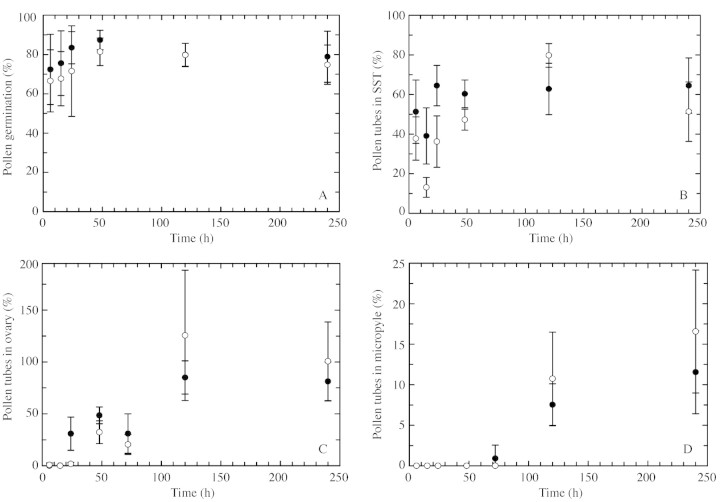
There were no qualitative differences at the structural level between cross‐ and self‐pollen tube growth from the time of pollen grain germination to ovule and embryo sac penetration. Cross‐ and self‐pollen tubes germinated within 1 h of pollination and grew to the base of the stigma papillae by 3 h. Pollen tube entry into the SST occurred by 6 h (Fig. 1A) and pollen tubes subsequently reached the ovary locule by 24 h and entered the micropyle within 48–72 h post‐pollination (Fig. 1B). Statistical analyses comparing cross‐ and self‐pollen tube growth over time revealed few quantitative differences. A two‐way ANOVA revealed no significant differences between cross‐ and self‐pollen grain germination over time (Fig. 2A; d.f. = 1, F = 0·217, P = 0·955). Similarly, there were no significant differences between cross‐ and self‐pollen tube growth over time within the SST (Fig. 2B; d.f. = 1, F = 0·776, P = 0·38), although pairwise comparisons showed significant differences within the SST at 15 h (d.f. = 1, H = 4.563, P = 0·033) and 24 h (d.f. = 1, F = 11·846, P = 0·001). No significant differences between cross‐ and self‐pollen tube growth entry into the ovary (Fig. 2C; d.f. = 1, F = 0·274, P = 0·601) were noted, although pairwise comparisons again revealed significant differences between cross‐ and self‐pollen tube growth in this instance at 24 h (d.f. = 1, H = 5·185, P = 0·023). Finally, there were no significant differences between cross‐ and self‐pollen tube entry into the micropyle during the first 5 d after pollination (Fig. 2D; d.f. = 1, F = 2·345, P = 0·126).
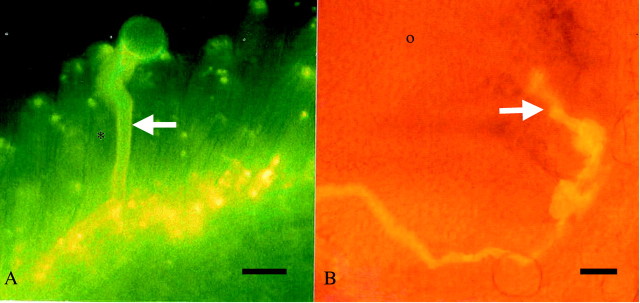
Fig. 1. Aniline blue fluorescence of self‐pollen tube (arrow) growth on a stigmatic cell (*) at 6‐h post‐pollination (A) and within the ovule micropyle (arrow) at 72 h following pollination (B). Bars: A = 25 µm; B = 50 µm. O, Ovule.
Fig. 2. Changes in cross‐ (open circles) and self‐ (filled circles) pollen germination (A) and cross‐ and self‐pollen tube growth within transmitting tissue (B–D) of Illicium floridanum carpels. Data are mean values ± 95 % confidence interval. See Results for details of statistics. SST, Substigmatic transmiting tissue.
Embryo sac development, embryo sac penetration, fertilization, and embryo/endosperm development following cross‐ versus self‐pollination in I. floridanum
Embryo sac development in I. floridanum from a chalazal spore of a T‐shaped tetrad of megaspores conformed to the Schizandra type (as defined by Battaglia, 1986) whereby the mature embryo sac of ovules from unpollinated ovaries contained a partially cellularized egg (Fig. 3A), two partially cellularized synergids and a single polar nucleus (Fig. 3B) as has been described for other members of the Austrobaileyales (Swamy, 1964; Friedman et al., 2003) but which is in variance with some species of the Illiciaceae wherein the embryo sac development is reported to be of the Polygonum type (Hayashi, 1963). Unpollinated embryo sacs were still four‐celled with intact synergids at 30 d post‐anthesis. Initial embryo sac penetration (cross, x̄ = 2. 4 % ± 6·4; self, x̄ = 0·0 % ± 0·0; d.f. = 1; P = 0·731) was observed at 5 d following cross‐ and self‐pollination (Fig. 3C). A resting zygote and cellular endosperm was present by 30 d (Fig. 3D) following both pollination treatments. In each case, cellular endosperm development was variable ranging from four to 300 cells. The percentages of cross‐ and self‐fertilization were statistically indistinguishable at 10 d (cross, x̄ = 62·33 % ± 48·6 %; self, x̄ = 64·2 % ± 41·6 %; d.f. = 9, P = 0·948) and 30 d (cross, x̄ = 88·8 % ± 20·2 %; self, x̄ = 66·7 % ± 57·7 %; d.f. = 7, P = 0·404).
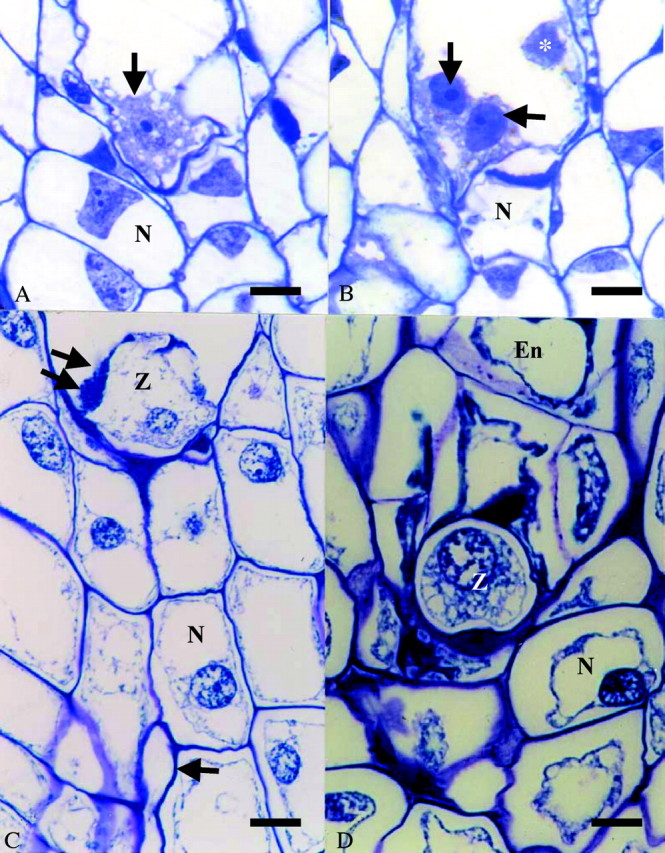
Fig. 3. Embryo sac contents at anthesis prior to pollination (A and B) and 10 d (C) and 30 d (D) post‐pollination. (A) Arrow denotes partially cellularized egg cell; (B) arrows mark partially cellularized synergids. *, Polar nucleus. (C) Single arrow marks pollen tube in nucellus; double arrow delineates penetrated synergid. Bars: A–C = 100 µm; D = 50 µm. En, Cellular endosperm; N, nucellus; Z, zygote.
DISCUSSION
Results from the present study examining cross‐ and self‐pollen tube growth are at odds with a previous interpretation of data that concluded SI was present within the stigmatic crest of I. floridanum (Thien et al., 1983). In contrast, the present study indicates that self‐pollen tubes did not cease growth at the stigma and there were no significant differences over time between cross‐ and self‐pollen tube germination and growth to the ovary. Furthermore, there was no differential cross‐ versus self‐pollen tube entry of micropyles and embryo sacs. Development of the embryo sac did not appear to be influenced by pollen type and percentages of cross‐ and self‐fertilization, although quite variable, were not significantly different such that a multicellular endosperm and a zygote were present 30 d following pollination. The absence of differences in ovule entry and fertilization between pollination treatments was observed in three distinct natural populations. In addition to these observations, novel information on one other feature of the reproductive biology of I. floridanum, that is important for intrafloral geitenogamy, is also provided. It is reported that stigmas support pollen germination only during the female phase of floral ontogeny. The discussion starts by reviewing other work on self‐sterility in angiosperms and then addresses potential mechanisms that could account for the previous report of a lack of self‐seed set in I. floridanum in the absence of an apparent prezygotic SI, as observed in the present study. Since these studies on the timing of stigma receptivity have important implications for the success of self‐pollen after anther dehiscence within a flower, this issue is then addressed. In conclusion, the results on stigma receptivity and compatibility status of I. floridanum are placed in the context of hypotheses on the roles of these two features in the reproduction of early angiosperms.
Mechanisms of self‐sterility in angiosperms
Self‐sterility in angiosperms results in reduced fruit/seed set following self‐pollination as compared with cross‐pollination and is principally achieved through two mechanisms: SI and early‐acting inbreeding depression. Self‐incompatibility is a genetically controlled mechanism functioning to enhance heterozygosity (Lundqvist, 1964; de Nettancourt, 1977, 1997). In some species, SI results in the prezygotic failure of self‐pollen tube growth at the stigma, style or ovary (Kenrick et al., 1986; Franklin et al., 1995; de Nettancourt, 1997; Sage et al., 2001). In other species, SI fails to result in differential prezygotic self‐pollen tube growth. Rather, self‐pollen tube growth in the gynoecium induces failed embryo sac development apparently via long‐distance signalling resulting in the elimination of ovules available for double fertilization (Sage et al., 1999). SI in such cases has been termed late‐acting ovarian SI (OSI) or just late‐acting SI. In addition, OSI in some species has also been posited to act after fertilization through the abortion of zygotes prior to division (Seavey and Bawa, 1986; Gibbs and Bianchi, 1993, 1999; Sage et al., 1994; Gibbs et al., 1999; Bittencourt et al., 2003; Sage and Sampson, 2003). The presence of SI operating after gamete fusion remains controversial and SI in such instances may be due to as of yet undetermined mechanisms of pre‐zygotic self‐ recognition (Sage and Sampson, 2003). In contrast to SI, early‐acting inbreeding depression occurs only post‐ zygotically and results in abortion of offspring homozygous for deleterious recessive alleles (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1996). Abortion due to inbreeding occurs at a variety of developmental stages as opposed to a single stage as observed in post‐zygotic SI (Seavey and Bawa, 1986; Sage et al., 1994; Seavey and Carter, 1994).
In the absence of differential ovule entry and fertilization of the four‐nucleate embryo sac of I. floridanum following cross‐ versus self‐pollination, self‐sterility can be attributed to either early‐acting inbreeding depression or post‐zygotic OSI. Notably, results from the present study indicate that endosperm development is not uniform but is quite variable following both cross‐ and self‐pollination. As well, fruits of I. floridanum abscise at various times after receiving cross‐ and self‐pollen and fruit size can be quite variable after either pollination treatment (Thien et al., 1983; T. L. Sage, pers. comm.). Although variation in endosperm and fruit development as well as the timing of fruit abscission during the reproductive phase is a common, well‐documented phenomenon in angiosperms attributed to a variety of features, including plant hormonal and nutrient status (Stephenson, 1981; Sage and Webster, 1987, 1990; Korbecka et al., 2002), such factors are also indicative of early‐acting inbreeding depression (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1996). Alternatively, the present study on I. floridanum notes that, 30 d after receiving cross‐ and self‐pollen, seeds from both pollination treatments contained embryos that had not yet proceeded beyond the zygotic stage of development. Post‐zygotic OSI following fertilization whereby the zygote fails to divide but endosperm development proceeds has been documented to occur in the relictual species, Pseudowintera axillaris (Sage and Sampson, 2003) and phylogenetically younger species of Asclepias (Sparrow and Pearson, 1948; Sage and Williams, 1991). Hence, in the absence of differential embryo development following cross‐ and self‐pollination beyond the zygote stage of ontogeny, developmental data from the present study cannot entirely eliminate the possibility of some form of OSI. Long‐term studies comparing seed development following the application of cross‐ and self‐pollen will be required to determine whether early‐acting inbreeding depression or post‐zygotic SI is operating in I. floridanum.
Temporal aspects of stigma receptivity in I. floridanum
A significant finding of this study is the presence of complete dichogamy in I. floridanum. The timing of stigma receptivity coincided with the previously defined female phase of floral ontogeny (Thien et al., 1983). Notably, the cessation of stigma receptivity is not only temporally associated with anther dehiscence and stigmatic movement, but also with changes in composition of esterified/unesterified pectins and arabinogalactan/arabinogalactan proteins in the extracellular matrix of the receptive region of the stigmatic epidermal cells (Koehl, 2002). While I. floridanum has previously been characterized as protogynous (Thien et al., 1983), this report of complete dichogamy is novel for the species. These results are noteworthy because they indicate that, although a pre‐zygotic SI is absent in I. floridanum, temporal features of stigma receptivity will prevent self‐fertlization within a flower. However, successful geitonogamy likely occurs because flowers at different stages of development are present at any one time on a plant and pollinators appear to move pollen effectively between such flowers (Thien et al., 1983). The extent to which geitonogamy is effected by pollinators and the subsequent affects on reproductive success and inbreeding within populations of I. floridanum remains to be determined.
CONCLUSIONS
The well‐resolved picture of basal angiosperm relationships has been noted to provide new opportunities to examine a suite of vegetative and reproductive characters potentially present in early angiosperms through comparative work on extant basal taxa (Friis et al., 2000; Endress, 2001; Feild et al., 2001). When considering protogyny as a breeding system character trait, this feature is widespread within basal angiosperms (Bernhardt and Thien, 1987; Ervik et al., 1995; Endress, 2001). And, amongst other members of core angiosperms at the base of the phylogeny, complete dichogamy at the floral level, as observed here for I. floridanum, occurs within Cabombaceae and Nymphaeaceae (Schneider and Jeter, 1982; Capperino and Schneider, 1985; Osborn and Schneider, 1988). The predominance of protogyny in basal angiosperms has lead to the speculation that the first angiosperms displayed this temporal feature (Bernhardt and Thien, 1987), although such functional character traits have not been subject explicitly to phylogenetic analysis.
Along similar lines, SI, a phenomenon estimated to be present in over one‐half of angiosperms (East, 1940; Brewbaker 1957; Arasu, 1968; Barrett, 1988) and unique to the group (de Nettancourt, 1977; although see Runions and Owens, 1998), has been posited by some to have played an important role in early angiosperm success (Whitehouse, 1950; Bell, 1995). In contrast, others have suggested that SI was not important in the early history of angiosperms noting that self‐compatibility is more common than SI amongst basal extant taxa angiosperms (Weller et al., 1995). Conclusions regarding the compatibility status of basal extant angiosperms are restricted due to the limited number of studies examining cross‐ and self‐pollen tube growth in these taxa at the microscopic level. Amongst the families at the base of the phylogeny, such studies are available only for Trimenia moorei (Bernhardt et al., 2003), three of five species comprising the Saururaceae (Pontieri and Sage, 1997, 1999) and two species within the Winteraceae, Drimys winteri and Pseudowintera axillaris (Sage et al., 1998; Sage and Sampson, 2003) whereby stigmatic SI has been demonstrated in the first two taxa and OSI in the latter. The remaining classification of the presence or absence of SI in other species are based on analysis of seed production (Prakash and Alexander, 1984; von Balthazar and Endress, 1999; Tosaki et al., 2001). Significantly, self‐seed set can occur in the presence of leaky SI systems (Stephenson et al., 2000; Sage et al., 2001), thereby leading to erroneous conclusions regarding the presence/absence of SI and necessitating the need to critically examine cross‐ and self‐pollen tube growth as well as ovule/seed development on an individual species basis. Thus, inferences from seed‐set data regarding the presence or absence of SI in basal angiosperms and its potential role in the early history of angiosperms are inconclusive. Through a critical analysis of cross‐ and self‐pollen tube growth, the present study has confirmed the absence of a prezygotic SI in I. floridanum, a species once considered to exhibit SI at the stigma (Thien et al., 1983).
ACKNOWLEDGEMENTS
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to T.L.S. The authors thank Peter Endress and Edward L. Schneider for comments on the final manuscript, Peter Weston for valuable discussions, Lorraine Thien and Sam and Linda Rosso for assistance with field work, and Eric Dunbar for technical assistance.
Supplementary Material
Received: 10 November 2003; Returned for revision: 12 February 2004; Accepted: 5 March 2004. Published electronically: 20 May 2004
References
- Angiosperm Phylogeny Group.1998. An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden 85: 531–553. [Google Scholar]
- ArasuNN.1968. Self‐incompatibility in angiosperms: a review. Genetica 39: 1–24. [Google Scholar]
- BarkmanTJ, Chenery G, McNeal JR, Lyons‐Weiler J, Ellisens WJ, Moore G., Wolfe AD, dePhamphilis CW.2000. Independent and combined analyses of sequences from all three genomic compartments converge on the root of flowering plant phylogeny. Proceedings of the National Academy of Sciences 97: 13166–13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BarrettSCH.1988. The evolution, maintenance, and loss of self‐incompatibility systems. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive ecology patterns and strategies Oxford: Oxford University Press, 98–124. [Google Scholar]
- BattagliaE.1986. Embryological questions: 7. Do new types of embryo sac occur in Schisandra? Annali di Botanica. [Google Scholar]
- BellPR.1995. Incompatibility in flowering plants: adaptation of an ancient response. Plant Cell 7: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BernhardtP, Thien L.1987. Self‐isolation and insect pollination in the primitive angiosperms: new evaluations of older hypotheses. Plant Systematics and Evolution 156: 159–176. [Google Scholar]
- BernhardtP, Sage TL, Weston P, Azuma H, Lam M, Thien LB, Bruhl J.2003. The pollination of Trimenia moorei (Trimeniaceae): floral volatiles, insect/wind pollen vectors, and stigmatic self‐incompatibility in a basal angiosperm. Annals of Botany 92: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BittencourtJrNS, Gibbs PE, Semir J.2003. Histological study of post‐pollination events in Spathodea campanulata Beauv. (Bignoniaceae), a species with late‐acting self‐incompatibility. Annals of Botany 91: 827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrewbakerJL.1957. Pollen cytology and self‐incompatibility systems in plants. Journal of Heredity 48: 217–277. [Google Scholar]
- CapperinoME, Schneider EL.1985. Floral biology of Nymphaea mexicana Zucc. (Nymphaeaceae). Aquatic Botany 23: 83–93. [Google Scholar]
- CharlesworthD, Charlesworth B.1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. [Google Scholar]
- deNettancourtD.1977.Incompatibility in angiosperms. Berlin: Springer‐Verlag. [Google Scholar]
- deNettancourtD.1997. Incompatibility in angiosperms. Sexual Plant Reproduction 10: 185–199. [Google Scholar]
- EastEM.1940. The distribution of self‐sterility in flowering plants. Proceedings of the American Philosophical Society 82: 449–518. [Google Scholar]
- EndressPK.2001. The flowers in extant basal angiosperms and inferences on ancestral flowers. International Journal of Plant Sciences 162: 1111–1140. [Google Scholar]
- ErvikF,Renner S, Johanson KA1995. Breeding system and pollination of Nuphar luteum (L.) Smith (Nymphaeaceae) in Norway. Flora 190: 109–113. [Google Scholar]
- FeildT, Brodribb T, Jaffre T, Holbrook NM.2001. Acclimation of leaf anatomy, photosynthetic light use, and xylem hydraulics to light in Amborella trichopoda (Amborellaceae). International Journal of Plant Sciences 162: 999–1008. [Google Scholar]
- FranklinFCH, Lawrence MJ, Franklin‐Tong VE.1995. Cell and molecular biology of self‐incompatibility in flowering plants. International Review of Cytology 158: 1–64. [Google Scholar]
- Franklin‐TongVE, Franklin FCH.2003. The different mechanisms of gametophytic self‐incompatibility. Philosophical Transactions of the Royal Society B 358: 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FriedmanWE, Gallup WN, Williams JH.2003. Female gametophyte development in Kadsura: implications for Schisandraceae, Austrobaileyales, and the early evolution of flowering plants. International Journal of Plant Sciences 164: S293–S305 [Google Scholar]
- FriisEM, Pedersen KR, Crane PR.2000. Reproductive structure and organization of basal angiosperms from the early Cretaceous (Barremian or Aptian) of western Portugal. International Journal of Plant Sciences 161: S169–S182. [DOI] [PubMed] [Google Scholar]
- GibbsPE, Bianchi M.1993. Post‐pollination events in the species of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with late‐acting self‐incompatiblity. Botanica Acta 106: 64–71. [Google Scholar]
- GibbsPE, Bianchi M.1999. Does late‐acting self‐incompatiblity (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa Annals of Botany 84: 449–457. [Google Scholar]
- GibbsPE, Oliveria PE, Bianchi M.1999. Postzygotic control of selfing in Hymenaea stigonocarpa (Leguminosae–Caesalpinioideae), a bat‐pollinated tree of the Brazilian cerrados. International Journal of Plant Sciences 160: 72–78. [Google Scholar]
- GrahamSW, Olmstead RG.2000. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. American Journal of Botany 87: 1712–1730. [PubMed] [Google Scholar]
- HaoG, Saunders RMK, Chye ML.2000. A phylogenetic analysis of the Illiciaceae based on sequences of internal transcribed spacers (ITS) of nuclear ribosomal DNA. Plant Systematics and Evolution 223: 81–90. [Google Scholar]
- HayashiY.1963. The embryology of the family Magnoliaceae sens. lat. I. Megasporogenesis, female gametophyte and embryogeny of Illicium anisatum L. Science Reports Tohoku University Series IV (Biology) 29: 27–33. [Google Scholar]
- HusbandBC, Schemske DW.1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54–70. [DOI] [PubMed] [Google Scholar]
- IgicB, Kohn JR.2001. Evolutionary relationships among self‐incompatibility RNases. Proceedings of the National Academy of Sciences 87: 9732–9735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KenrickJ, Kaul V, Williams EG.1986. Self‐incompatibility in Acacia retinoides: site of pollen‐tube arrest is the nucellus. Planta 169: 245–250. [DOI] [PubMed] [Google Scholar]
- KoehlV.2002.Functional reproductive biology of Illicium floridanum (Illiciaceae). MSc Thesis, University of Toronto. [Google Scholar]
- KorbeckaG,Klinkhamer PGL, Vrieling K.2002. Selective embryo abortion hypothesis revisited – a molecular approach. Plant Biology 4: 298–310. [Google Scholar]
- LamBCH, Sage, TL, Bianchi F, Blumwald E.2001. Role of SH3 domain‐containing proteins in clathrin‐mediated vesicle trafficking in Arabidopsis Plant Cell 13: 2499–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LundqvistA.1964. The nature of the two‐loci incompatibility system in grasses. IV. Interaction between the loci in relation to pseudocompatibility in Festuca pratensis Huds. Hereditas 52: 221–234. [Google Scholar]
- MartinFM.1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 436–437. [DOI] [PubMed] [Google Scholar]
- MathewsS, Donoghue MJ.1999. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286: 947–950. [DOI] [PubMed] [Google Scholar]
- OlmsteadRG.1989. The origin and function of self‐incompatibility in flowering plants. Sexual Plant Reproduction 2: 127–136. [Google Scholar]
- OsbornJM, Schneider EL.1988. Morphological studies of the Nymphaeaceae sensu lato. XVI. The floral biology of Brasenia schreberi Annals of the Missouri Botanical Garden 75: 778–794. [Google Scholar]
- ParkinsonCL, Adams KL, Palmer JD.1999. Multigene analyses identify the three earliest lineages of extant flowering plants. Current Biology 9: 1485–1488. [DOI] [PubMed] [Google Scholar]
- PontieriV, Sage TL.1997. Characterization of pollen/carpel interactions following self‐ and cross‐pollination in the paleoherb family, Saururaceae. American Journal of Botany 84: 65 (Abstract). [Google Scholar]
- PontieriV, Sage TL.1999. Evidence for stigmatic self‐incompatibility, pollination induced ovule enlargement, and transmitting tissue exudates in the paleoherb, Saururus cernuus L. (Saururaceae). Annals of Botany 84: 507–519. [Google Scholar]
- PrakashN, Alexander III JH.1984. Self‐incompatibility in Austrobaileya scandens In: Williams EG, Knox RB, eds. Pollination ’84 Melbourne: School of Botany, University of Melbourne, 214–216. [Google Scholar]
- QiuY‐L, Lee J, Bernasconi‐Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Savolainen V, Chase MW.1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404–407. [DOI] [PubMed] [Google Scholar]
- QiuYL, Lee JH, Whitlock BA, Bernasconi‐Quadroni F, Dombrovska O.2001. Was the ANITA rooting of the angiosperm phylogeny affected by long‐branch attraction? Molecular Biology and Evolution 18: 1745–1753. [DOI] [PubMed] [Google Scholar]
- ReadSM, Newbigin E, Clarke AE, McClure BA, Kao T.1995. Disputed ancestry: comments on the model for the origin of incompatibility in flowering plants. Plant Cell 7: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RobertsonRE, Tucker SC.1979. Floral ontogeny of Illicium floridanum, with emphasis on stamen and carpel development. American Journal of Botany 66: 605–617. [Google Scholar]
- RunionsJD, Owens J.1998. Evidence of prezygotic self‐incompatiblity in a conifer. In: Owens SJ, Rudall PJ, eds. Reproductive biology Kew: Royal Botanic Gardens, 55–264. [Google Scholar]
- SageTL, Sampson FB.2003. Evidence for ovarian self‐incompatibility as a cause of self‐sterility in the relictual woody angiosperm, Pseudowintera axillaris (Winteraceae). Annals of Botany 91: 807–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SageTL, Webster BD.1987. Flowering and fruiting patterns of Phaseolus vulgaris L. Botanical Gazette 148: 35–41. [Google Scholar]
- SageTL,Webster BD.1990. Development of aborting and nonaborting seeds of Phaseolus vulgaris L. Botanical Gazette 151: 167–175. [Google Scholar]
- SageTL, Williams EG.1991. Self‐incompatibility in Asclepias Plant Cell Incompatibility Newsletter 23: 55–57. [Google Scholar]
- SageTL, Williams EG.1995. Structure, ultrastructure, and histochemistry of the pollen tube pathway in the milkweed Asclepias exaltata L. Sexual Plant Reproduction 8: 257–265. [Google Scholar]
- SageTL, Bertin RI, Williams EG.1994. Ovarian and other late‐acting self‐incompatibility systems. In: Williams EG, Clarke AE, Knox RB, eds. Genetic control of self‐incompatibility and reproductive development in flowering plants Dordrecht: Kluwer, 116–140. [Google Scholar]
- SageTL, Griffin SR, Pontieri V, Drobac P, Cole WW, Barrett SCH. 2001. Stigmatic self‐incompatibility and mating patterns in Trillium grandiflorum and Trillium erectum (Melanthiaceae). Annals of Botany 88: 829–841. [Google Scholar]
- SageTL, Pontieri V, Christopher R.2000. Incompatibility and mate recognition in monocotyledons. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution Melbourne: CSIRO, 270–276. [Google Scholar]
- SageTL, Sampson FB, Bayliss P, Gordon MG, Heij EG.1998. Self‐sterility in the Winteraceae. In: Owens SJ, Rudall PJ, eds. Reproductive biology in systematics, conservation and economic botany Kew: Royal Botanic Gardens, 317–328. [Google Scholar]
- SageTL, Strumas F, Cole WW, Barrett SCH.1999. Differential ovule development following self‐ and cross‐pollination: the basis of self‐sterility in Narcissus triandrus (Amaryllidaceae). American Journal of Botany 86: 855–870. [PubMed] [Google Scholar]
- SaundersRMK.1995. Systematics of the genus Illicium L. (Illiciaceae) in Malesia. Botanical Journal of the Linnean Society 117: 333–352. [Google Scholar]
- SchneiderEL, Jeter J M.1982. Morphological studies of the Nymphaeaceae. XII. The floral biology of Cabomba caroliniana American Journal of Botany 69: 1410–1419. [Google Scholar]
- SeaveySR, Bawa KS.1986. Late‐acting self‐incompatibility in angiosperms. Botanical Review 52: 195–218. [Google Scholar]
- SeaveySR, Carter SK.1994. Self‐sterility in Epilobium obcordatum (Onagraceae). American Journal of Botany 81: 331–338. [PubMed] [Google Scholar]
- SmithAC.1947. The families Illiciaceae and Schisandraceae. Sargentia 7: 1–224. [Google Scholar]
- SoltisDE, Soltis PS, Chase MW, Mort ME, Albach DC, Zanis M, Savolainen V,et al.2000. Angiosperm phylogeny inferred from a combined data set of 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society 133: 381–461. [Google Scholar]
- SparrowIK, Pearson Nl.1948. Pollen compatibility in Asclepias syriaca Journal of Agricultural Research 77: 187–199. [Google Scholar]
- StephensonAG.1981. Flower and fruit abortion: proximate causes and ultimate functions. Annual Review of Ecology and Systematics 12: 253–279. [Google Scholar]
- StephensonAG, Good SV, Vogler DW.2000. Interrelationships among inbreeding depression, plasticity in the self‐incompatibility system, and the breeding system of Campanula rapunculoides L. (Campanulaceae). Annals of Botany 85: 211–219. [Google Scholar]
- SteinbachsJE, Holsinger KE.2002. S‐RNase‐mediated gametophytic self‐incompatibility is ancestral in eudicots. Molecular Biology and Evolution 19: 825–829. [DOI] [PubMed] [Google Scholar]
- StoneSL, Anderson EM, Mullen RT, Goring DR.2003. ARC1 is an E3 ubiquitin ligase and promotes the ubiquitination of proteins during the rejection of self‐incompatible Brassica pollen. Plant Cell 15: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SwamyBGL.1964. Macrogametophytic ontogeny in Schisandra chinensis Journal of Indian Botanical Society 43: 391–396. [Google Scholar]
- ThienLB, White DA, Yatsu LY.1983. The reproductive biology of a relict – Illicium floridanum Ellis. American Journal of Botany 70: 719–727. [Google Scholar]
- TosakiY, Renner SS, Takahashi H.2001. Pollination of Sarcandra glabra (Chloranthaceae) in natural populations in Japan. Journal of Plant Research 114: 423–427. [Google Scholar]
- von BalthazarM, Endress PK.1999. Floral bract function, flowering process and breeding systems of Sarcandra and Chloranthus (Chloranthaceae). Plant Systematics and Evolution 218: 161–178. [Google Scholar]
- WaserNM, Price MV.1991. Reproductive costs of self pollination in Ipomopsis aggregata (Polemoniaceae): are ovules usurped? American Journal of Botany 78: 1036–1043. [Google Scholar]
- WellerSG, Donoghue MJ, Charlesworth D.1995. The evolution of self‐incompatibility in flowering plants: a phylogenetic approach. In: Hoch PD, Stephenson AG, eds. Experimental and molecular approaches to plant biosystematics. St Louis: Missouri Botanical Garden, 355–382 [Google Scholar]
- WhitehouseHLK.1950. Multiple‐allelomorph incompatibility of pollen and style in the evolution of angiosperms. Annals of Botany 14: 198–216. [Google Scholar]
- WilliamsEG, Sage TL, Thien LB.1993. Functional syncarpy by intercarpellary growth of pollen tubes in a primitive apocarpous angiosperm, Illicium floridanum (Illiciaceae). American Journal of Botany 80: 137–142. [Google Scholar]
- ZanisMJ, Soltis DE, Soltis PS, Mathews S, Donoghue MJ 2002. The root of the angiosperms revisited. Proceedings of the National Academy of Sciences 99: 6848–6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.