Abstract
• Background and Aims The mangrove Rhizophora mucronata has previously been reported to lack annual growth rings, thus barring it from dendrochronological studies. In this study the reported absence of the growth rings was reconsidered and the periodic nature of light and dark brown layers visible on polished stem discs investigated. In addition, the formation of these layers in relation to prevailing environmental conditions, as well as their potential for age determination of the trees, was studied.
• Methods Trees of known age were collected and a 2·5‐year cambial marking experiment was conducted to determine the periodic nature of the visible growth layers.
• Key Results Annual indistinct growth rings were detected in R. mucronata and are defined by a low vessel density earlywood and a high vessel density latewood. The formation of these growth rings and their periodic nature was independent from site‐specific environmental conditions in two forests along the Kenyan coast. However, the periodic nature of the rings was seriously affected by slow growth rates, allowing accurate age determination only in trees with radial growth rates above 0·5 mm year–1. The onset of the formation of the low vessel density wood coincided with the onset of the long rainy season (April–May) and continues until the end of the short rainy season (November). The high vessel density wood is formed during the dry season (December–March). Age determination of the largest trees collected in the two studied forests revealed the relatively young age of these trees (±100 years).
• Conclusions This study reports, for the first time, the presence of annual growth rings in the mangrove R. mucronata, which offers further potential for dendrochronological and silvicultural applications.
Key words: Age determination, annual growth rings, cambial marking, dendrochronology, East Africa, mangroves, Rhizophora mucronata, wood anatomy
INTRODUCTION
Increasing deforestation and degradation of mangrove forests calls for the establishment of management plans integrating reforestation and sustainable wood production (Kairo et al., 2001). However, limited data are available on age, growth rate and age‐related yield of mangrove trees, necessary for the establishment of sustainable silvicultural practices (Devoe and Cole, 1998). Tree ring analysis is a potential tool for obtaining information on the aforementioned parameters. In addition, it may provide information about the relationship between growth and environmental variables (including climate) and allows the detection of past changes in environmental conditions, which may aid in understanding forest dynamics. However, dendrochronological techniques require the presence of annual growth rings, which are commonly said to be absent in mangrove trees, as in many tropical tree species (e.g. Détienne, 1989; Sass et al., 1995; Stahle, 1999). Among those, the mangrove Rhizophora mucronata, a dominant species in the Kenyan mangroves (UNEP, 2001), has been reported on several occasions to form wood which completely lacks growth rings (Panshin, 1932; Janssonius, 1950; van Vliet, 1976). Since samples we collected in Kenya displayed a clear alternation of dark brown and light brown layers, a reconsideration of the reported absence of growth rings in R. mucronata was necessary.
A simple method for determining the periodic nature of tree rings is counting rings (or in the case of this study, coloured layers) in trees of known age (Gourlay, 1995; Worbes, 1995; Eshete and Stahl, 1999). However, this method is usually limited to trees of which accurate information about the date of planting is available, such as is the case for trees from plantations, botanical gardens or, on rarer occasions, trees from the natural forest, if locals can provide reliable information (see Gourlay, 1995; Worbes, 1995). When the exact age of the tree is unknown, other methods must be utilized. Cambial marking is a method where a mechanical injury is inflicted to the cambium, causing a wound response in the tree. After a given time the tree is harvested and the generated callus tissue remains as an artificial and dateable scar in the wood. This time marker then allows the study of growth formations in the wood over a known time period (cf. Mariaux, 1967, 1968; Sass et al., 1995; Worbes, 1995). Cambial marking has been successfully applied to several species of the genus Rhizophora, including R. mucronata (Shiokura, 1989). The method gave satisfactory results in slow‐growing as well as in fast‐growing trees; however, data collected so far have been used only to evaluate growth rates, while the presence of dateable growth rings was not mentioned. In this study, the use of plantation trees with known age, as well as the results from a cambial marking experiment are combined to investigate the periodic nature of the light and dark layers, visible on the stem discs of R. mucronata. In addition, the timing of the formation of the coloured layers with respect to prevailing environmental conditions is estimated and the potential of the layers for age determination is discussed.
MATERIALS AND METHODS
Terminology
In this study, one coloured layer (dark or light) observed on the polished stem discs will be referred to as one growth layer. The term ‘growth ring’ will only be used when annual periodicity is proven (see Kaennel and Schweingruber, 1995). The term ‘growth phase’ will further be used to indicate whether the tree is in the process of producing a dark or a light coloured layer, as well as whether this growth layer is in an initial or a final stage. This term will be of importance when discussing the growth ring formation in relation to the climate conditions.
Study sites and sample collection
A total of 41 stem discs of Rhizophora mucronata Lam. were collected from two forests along the Kenyan coast: Gazi Bay (39°30′E, 4°25′S), located 40 km south of Mombasa, and Mida Creek (39°59′E, 3°21′S), located 80 km north of Mombasa. The stem discs were collected from a plantation, as well as from the natural forest, after a cambial marking experiment (see below). When selecting sampling sites, attention was given to differences in environmental conditions, such as inundation frequency and salinity, to test whether the possible periodic nature of the growth layers is site‐specific (Table 1). The samples are now stored in the xylarium of the Royal Museum for Central Africa (Tervuren Wood Collection, accession numbers: Tw55966, 90, 91, 58, 75, 78, 56700–04, 06, 07, 09–30, 33, 34, 36, 43, 44 and 45), Tervuren, Belgium.
Table 1.
Site specific environmental conditions for the different sampling sites in Gazi and Mida
| Site | Salinity* | Inundation class† | Forest type‡ | Dominant spp. |
| Gazi 1 | 49·2 | 2 | Basin | Rhizophora mucronata |
| Gazi 2 | 38·3 | 2 | Basin | R. mucronata |
| Gazi 3 | 37·6 | 4 | Fringing | R. mucronata, Ceriops tagal |
| Gazi 4 | 26·4 | 3 | Riverine | R. mucronata |
| Gazi 5 | 36·5 | 3 | Riverine | R. mucronata (plantation) |
| Gazi 6 | 31·9 | 3 | Riverine | R. mucronata |
| Mida | 30·6 | 4 | Riverine | R. mucronata, C. tagal |
* Soil water at 10 cm depth, measured with a conductivity meter (WTW Multiline P4).
† Inundated by (1) 100–76 %, (2) 75–51 %, (3) 50–26 %, (4) 25–6 % and (5) >5 % of the high tides, (1) and (5) were not recorded in this study.
‡Sensu Lugo and Snedaker (1974).
Climate description
The rainfall along the Kenyan coast shows a bimodal distribution, which is locally expressed in terms of the long rains (from April to July) and the short rains (from October to November) (McClanahan, 1988), with a mean annual precipitation of 1144 mm (Mombasa 1890–1985) (Lieth et al., 1999). The monthly average temperature ranges from 23·3 to 29·9 °C with a mean annual temperature of 26·4 °C (1931–1990) (Lieth et al., 1999). Precipitation data for the years in which the samples were collected (1999 and 2002) were obtained from the Mombasa meteorological station and from the NOAA Climate Data Library (NOAA, 2003).
Macroscopic and microscopic investigation
Stem discs were air‐dried and transverse sections were sanded (100–1200 grit). In addition, microscopic slides were prepared using conventional methods (see Jansen et al., 1998). Samples were investigated macroscopically, as well as microscopically to investigate which wood anatomical features are responsible for the visible coloured layers and to identify possible growth ring boundaries.
Cambial marking
Trees for the cambial marking experiment were marked on 18 and 19 Nov. 1999, using a hypodermic needle (18G; 1·2 mm diameter). Cambial marking was applied at the place where the diameter is measured in mangroves of the genus Rhizophora, which is at 130 cm above ground level, except when stilt roots are higher than 100 cm height. In this case the diameter is measured at 30 cm above the uppermost stilt roots (Cintrón and Novelli, 1984). A total of 34 stem discs were collected between 22 May and 9 June 2002. After drying, the stem discs were cut a few millimetres above the actual place of wounding and sanded until the full wound became visible. The wound tissue was carefully investigated to locate the position of the cambial initials at the time of pinning (see Results) and the number of growth layers formed since marking the cambium was determined. In addition, the average annual radial increment was obtained from measuring the distance between the cambial mark and the most recently formed wood (just underneath the bark) and dividing it by the number of years of the cambial marking experiment (approx. 2·5 years).
Plantation trees
Trees from a plantation were collected from the mangroves in Gazi Bay. This plantation is located on the eastern side of the bay, bordering the village of Kinondo. The trees were planted from propagules, in April 1994, in an overexploited clear‐cut area (Kairo, 1995). Six stem discs were collected in July 1999 (5‐year‐old trees) and six discs in May 2002 (8‐year‐old trees). The stem discs collected in 2002 were also part of the cambial marking experiment. The number of growth layers was counted on sanded stem discs and compared with the age of the trees. However, due to the presence of stilt roots, samples were not collected at the base of the tree, but at 130 cm height above ground level. Therefore, a discrepancy between the actual age of the tree and the number of observed growth layers is expected. In addition to the samples collected from the plantation and the cambial marking experiment, one sample was collected from a large recently fallen tree in Mida.
Timing of growth layer formation
The samples collected from the plantation, as well as those collected from the cambial marking experiment were used to estimate the growth phase at different collection times. More specifically, this part of the study investigates whether the onset of low‐ and high‐vessel density wood is linked to the seasonality of precipitation. In total, three time periods were investigated: July 1999, at the end of the long rainy season; November 1999, towards the end of the short rainy season (using the cambial mark); and May 2002, during the peak of the long rainy season. For the samples collected in July 1999 and May 2002, the growth phase was observed on the xylem just underneath the bark (which coincides with the position of secondary cell wall thickening). A more precise timing of the cambial activity was attempted for the samples marked in November 1999 by using the position of the cambial initials at the time of pinning as indicated by the cambial mark (see Results). For each sample the growth phase was quantified using one of the following possibilities: (a) growth layer in initial phase: when investigating the outermost xylem microscopically, a change in vessel density was observed, but the layer is not yet macroscopically visible; (b) growth layer halfway: in comparison to layers of former years (within one stem), the most recently formed layer seems halfway; or (c) completed growth layer: in comparison to layers of former years, the last formed layer seemed completed. Microscopic investigation did not yet reveal any change in vessel density.
RESULTS
Macroscopic and microscopic investigation
Macroscopic investigation of the sanded stem discs revealed a clear alternation of dark brown and light brown growth layers (Fig. 1A). Under low magnification, these coloured layers were found to be a reflection of changing vessel density, with light layers exhibiting a higher vessel density than dark layers (Fig. 1B). The lighter colour results from the higher number of vessels and is further enhanced by the polishing process, during which vessels fill up with wood dust. Microscopic investigation of the wood and the slides revealed no distinct boundary but rather a gradual transition in the vessel density between the light and dark layers (Fig. 1C).
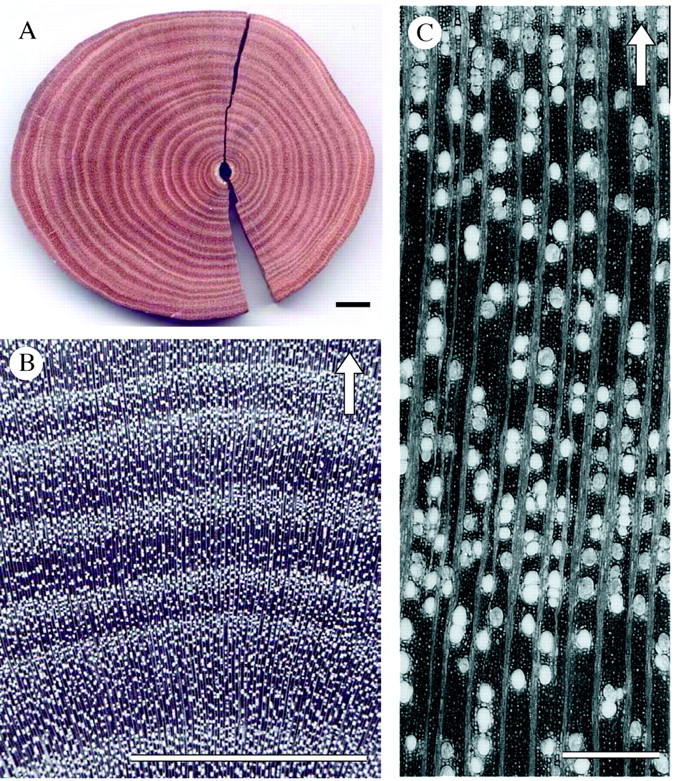
Fig. 1. (A) Macroscopic picture of a polished Rhizophora mucronata wood section showing a clear alternation of dark and light growth layers. (B) Magnified R. mucronata wood disc revealing the changing vessel density. (C) Microscopic photograph showing the gradual change in vessel density and the absence of distinct growth ring boundaries. Scale bars: A and B = 1 cm; C = 500 µm; the arrows indicate the direction of growth.
Cambial marking
Locations of the cambial marks were facilitated by a well‐defined reaction of the tree, resulting in a lenticel‐like structure visible on the outer bark. Within the xylem, the cambial mark appeared as a very local moderate reaction to the injury and could be observed best at the exact place of wounding. Of the 34 samples collected from the cambial marking experiment, only three were found to be unsuccessfully marked. Two of these samples did not show any mark in the xylem and therefore we believe the needle did not puncture the cambium. The third unsuccessfully marked tree had apparently undergone severe bark and cambial damage on part of the stem after November 1999, resulting in a premature cessation of growth. These three trees were discarded from further investigation.
Figure 2 shows a microscopic photograph of the wound tissue. At the exact position of needle insertion the puncture canal remains visible as an empty cone of 1·2 mm (the diameter of the needle), as a result of the removed piece of xylem by the needle (Fig. 2, 1). The puncture canal was surrounded by a larger cone of dark coloured, oxidized xylem, with unaffected wood anatomy (Fig. 2, 2), except for the part closest to the cambial wound, which shows fibres with incomplete cell wall thickening (Fig. 2, 3). A dark layer, formed by the residues of crushed cells, is visible above the empty cone, but also extends tangentially to about 500 µm on each side of the cone (Fig. 2, 4). This layer is usually referred to as the ‘stripes of cell wall residues’ (Kuroda, 1986; Nobuchi et al., 1995) and originates from crushed cambial cells and cambial derivatives on both sides of the cambium. Above the layer of crushed cells, a large area of callus‐like parenchymatous tissue indicates the actual wound response of the tree (Fig. 2, 5), after which wood production with recognizable fibres, rays and vessels was restored (Fig. 2, 6). However, the wood produced after the cambial wound showed smaller and fewer vessels and a higher ray density than the wood formed at a tangential distance from the cambial damage. Anticlinal division of the cambial cells induced by the wounding resulted in the formation of a parenchyma layer in the tangential continuation of the parenchymatous wound tissue (Fig. 2, 7), and indicates the position of the cambial initials at the time of pinning (P. Kitin, pers. comm.). It is this position that is used as the time marker. The growth layers appeared wavy at the location of the wounding, even those formed 2·5 years after the injury. Small differences in the morphology of the wound tissue occurred between trees, in particular the volume of parenchymatous wound tissue, as well as the radial length of the stripes of cell wall residues.
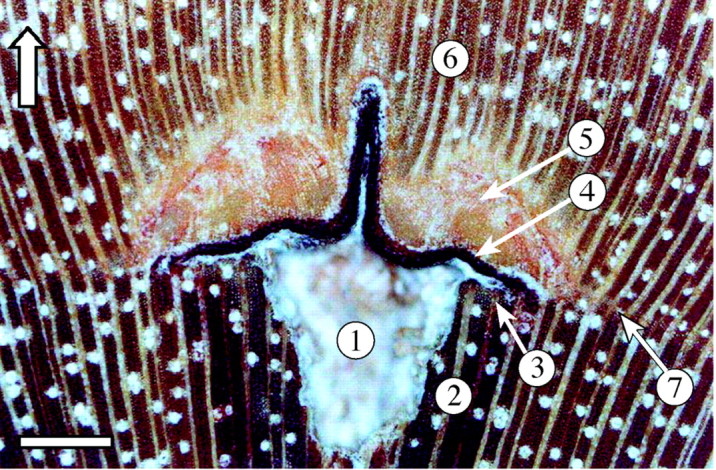
Fig. 2. Microphotograph of the cambial mark: 1, puncture canal; 2, oxidized wood; 3, fibres with incomplete cell wall thickening; 4, layer of crushed cambial derivatives; 5, parenchymatous wound tissue; 6, restored wood structure; 7, local parenchyma band indicating the position of cambial initials at the time of pinning (see text for more detailed explanation). Scale bar = 500 µm; the arrow indicates the direction of growth.
Periodic nature of the growth layers
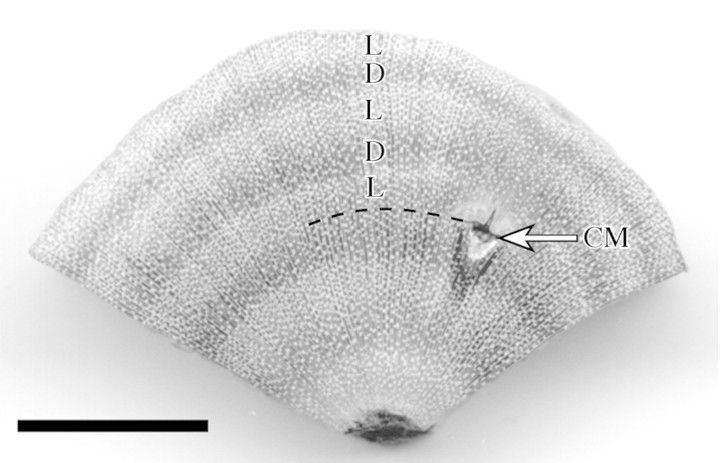
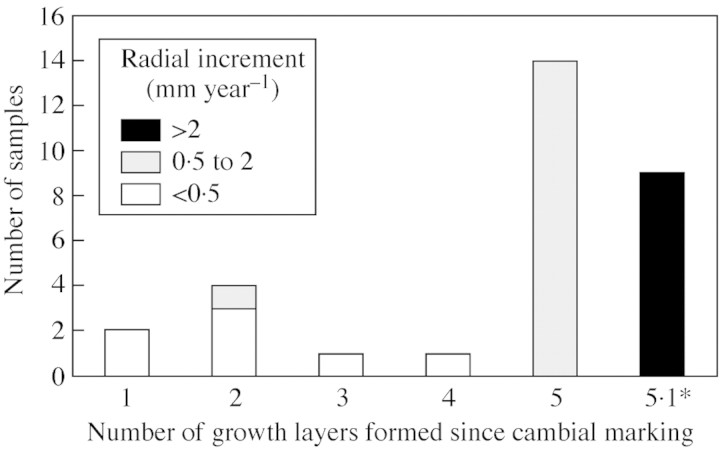
The number of growth layers formed since cambial marking ranged between one and five. Figure 3 displays a wood sample with five layers formed since the cambial mark (three light and two dark layers). Figure 4 shows the number of growth layers formed since cambial marking in relation to the annual radial increment of the trees. The category ‘5·1’ in Fig. 4 reflects the beginning of a new sixth layer, however, not yet macroscopically distinct.
Fig. 3. Polished R. mucronata wood section showing five growth layers (D = dark; L = light) produced since the cambial marking (CM) of November 1999. Scale bar = 1 cm.
Fig. 4. Number of growth layers formed since the cambial marking in relation to the average annual radial increment in 31 R. mucronata samples. * The category 5·1 indicates that these trees have started the formation of a sixth layer not yet macroscopically distinct.
Five‐year‐old trees (n = 6) collected from the plantation in Kinondo displayed 6·5 ± 0·5 (mean ± s.d.) growth layers, while 8‐year‐old trees (n = 6) displayed 11·5 ± 0·5 growth layers.
Timing of growth layer formation
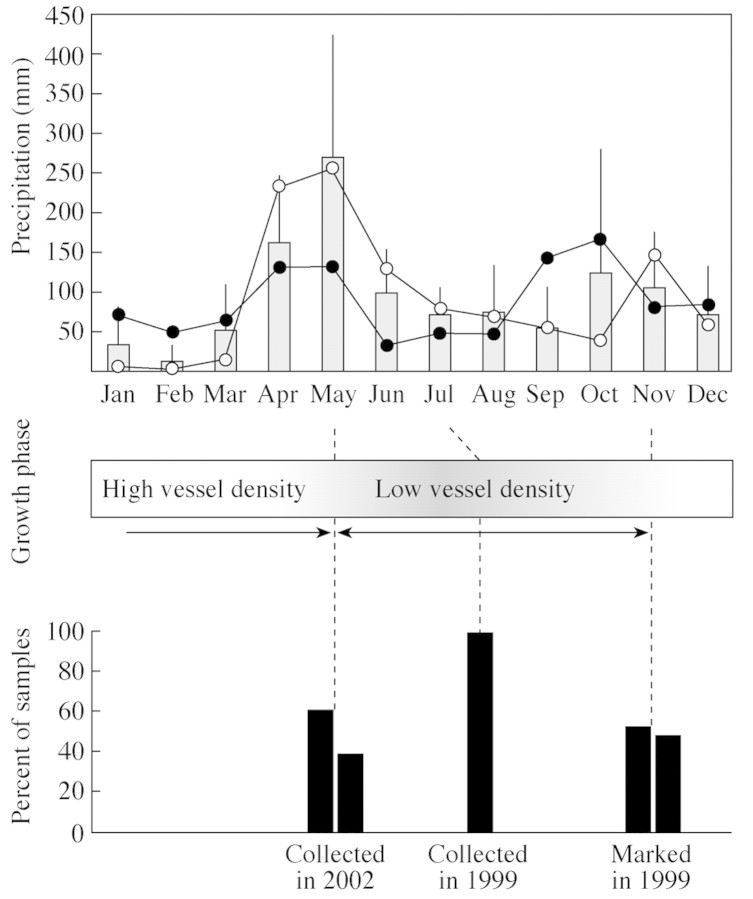
Of the samples collected in July 1999, all showed that the tree was producing low vessel density wood. In comparison to dark growth layers of other years (within each tree), this last‐formed layer seemed not to be completed, but rather halfway. Of the samples marked in November 1999, about half the samples had completed a dark layer, while in the other half a new layer with high vessel density (light) was in the process of formation. Of the samples collected in May–June 2002, 14 displayed the completion of a light layer, while nine had already started the formation of a new dark layer. These nine trees displayed a very high growth rate, and are the trees of category 5·1 in Fig. 4. Figure 5 shows the timing of the growth layer formation as observed from the three collection dates (July 1999, November 1999 and May 2002) in relation to a long‐term average of monthly precipitation (1890–1985; Lieth, 1999). However, year‐to‐year differences in the onset of the rainy seasons may quicken or delay the onset of change in vessel density. Therefore, the precipitation data for the two collection years are also shown in Fig. 5.
Fig. 5. Growth phase for the three collection dates (July 1999, November 1999 and May 2002) in relation to precipitation: 35‐year (1966–2001) monthly average (± s.d.; shaded bars; Mombasa Meteorological Department), precipitation for 1999 (open circles: Mombasa Meteorological Department) and precipitation for 2002 (solid circles; NOAA, 2003).
DISCUSSION
Detection of annual growth rings in R. mucronata
Of the 31 successfully marked trees, 14 trees developed five complete growth layers (three light and two dark) following the cambial marking in November 1999 (Figs 3 and 4), which corresponds to one light and one dark layer formed per year. Another nine trees also developed five growth layers but, in addition, displayed the beginning of a new sixth dark layer (Fig. 4). The development of this new dark layer was only observed in trees with average growth rates above 2 mm year–1 (radial increment) (Fig. 4), and its appearance points at the onset of wood formation with low vessel density (see below), without compromising the statement that one dark and one light layer is formed per year. Samples displaying the annual growth rings were found in all of the eight sampling sites investigated in this study (Table 2). The presence or absence of the annual growth rings could thus not be related to the site‐specific environmental conditions observed here (see Table 1). However, trees exhibiting a radial increment of <0·5 mm year–1 formed less than five growth layers (Fig. 4). A slow growth rate blurs the visual distinction between the high and low vessel densities due to the gradual transition of the vessel density from one layer to another. Age determination in slow‐growing trees is therefore prone to errors. However, it has to be noted that many wood samples showed asymmetric growth with the fastest‐growing radius developing easily recognizable annual growth rings, while the growth rings in the slow‐growing sections of the stem were blurred. Despite the small radial increment of the tree, age determination is still possible in slow‐growing trees when asymmetric growth is present. From the trees with a growth rate above 0·5 mm year–1, 96 % developed annual growth rings. This is a remarkable finding, considering that ‘missing’, ‘merging’ or ‘double’ rings often occur in temperate as well as tropical trees (e.g. Bertaudiere et al., 1999; Trouet et al., 2001; Rigling et al., 2002).
Table 2.
Number of samples (n) investigated in the different study sites and percentage of samples displaying annual growth rings
| Site | n | % with annual growth rings |
| Gazi 1 | 7 | 57 (4) |
| Gazi 2 | 5 | 60 (3) |
| Gazi 3 | 3 | 100 (3) |
| Gazi 4 | 3 | 100 (3) |
| Gazi 5 | 6 | 100 (6) |
| Gazi 6 | 5 | 40 (2) |
| Mida | 2 | 100 (2) |
| Total | 31 | 74 (23) |
Numbers in parentheses represent absolute counts.
Samples collected from the plantation offered further support for the annual periodic nature of the growth layers. Stem discs of plantation trees were collected at 130 cm height and therefore a time lag of the age of the samples with the actual age of the tree is expected and needs to be taken into account. Kairo (1995) measured a net vertical growth rate of the seedlings in this plantation of 20–40 cm year–1 (with a starting height of 40 cm for the propagule). Seedlings thus reach a height of 130 cm (our sampling height) after 2–4 years. Samples of 5‐year‐old trees showed an average number of 6·5 growth layers, which corresponds to three annual growth rings, while samples of 8‐year‐old trees showed an average number of 11·5 growth layers, corresponding to six annual growth rings. A time lag of 2 years thus appears between the actual age of the tree and the number of growth rings formed at 130 cm height, which is within the range of time needed for the seedlings to reach the sampling height, as reported by Kairo (1995). The results presented in this study offer strong evidence for the presence of annual growth rings (composed out of one dark and one light layer) in R. mucronata. Due to the absence of abrupt changes in the wood anatomy at the growth ring boundaries (see Fig. 1C), the growth rings will be defined as indistinct (see IAWA, 1989).
Timing of growth ring formation
The formation of low and high vessel density wood coincided with the seasonal rainfall distribution. The formation of wood with low vessel density started during the long rainy season and continued, despite the reduced rainfall, until the end of the short rainy season (Fig. 5). The wood with high vessel density was produced at the end of the short rainy season and continued during the dry season. The onset of high vessel density wood in November can be considered precise and representing the cambial activity, since the position of the cambial initials at the time of pinning was used (as indicated by the cambial mark; see results and Fig. 2). However, the onset of the low vessel density wood in May is only an approximation, as the position of secondary cell wall thickening (see Materials and methods) was used. It can therefore be assumed that the onset of the formation of low vessel density (dark) wood at the cambial initials started before May, and probably coincides with or starts shortly after the onset of the long rainy season in April. Furthermore, the year 2002 was characterized by a rather wet dry season and a below average precipitation in the first months of the rainy season, which may have delayed the onset of the low vessel density wood (Fig. 5). More research, using repeated cambial marking experiments is needed to test these hypotheses.
The difference in wood anatomy between rainy and dry season allows earlywood and latewood to be defined. The lack of a distinct growth ring boundary implies a rather continuous (not necessarily constant) radial growth and therefore the choice of what will be defined as the beginning of the growing season remains arbitrary. In a study on R. mucronata in Gazi, Mwangi Theuri et al. (1999) found an increased rate of photosynthesis and stomatal conductance during the rainy season. Therefore, the onset of the rainy season, which coincides with the onset of the low vessel density wood, was defined as the start of the growing season. The low vessel density wood is therefore considered earlywood, while the high vessel density wood is considered latewood.
How old are the Kenyan mangroves?
To determine how old R. mucronata trees become, the largest trees in a number of sites were sampled and their age was determined from counting the annual growth rings. As the samples are not collected at the base of the tree, an error on the tree’s age is introduced. Based on height increment data (obtained from Kairo, 1995), the number of years the trees needed to reach the sampling height was estimated for different inundation classes (Table 3) and was used to obtain the age of the trees. The ages of one of the largest trees from five sites are given in Table 4. The oldest sample was obtained from a recently fallen tree in the riverine forest in Mida. This tree displayed an age of 89 years with a height of 12 m and a diameter of 28·6 cm, and is also the largest stem disc of the investigated material. In Gazi and Mida, trees with similar diameters and slightly above are only encountered in the riverine forest type. In Gazi Bay, the riverine forest near Kinondo contains diameters up to 29 cm, while diameters of up to 34 cm were recorded in the riverine forest in Mida. From the diameter and the age of the stem disc, an average annual radial increment can be estimated assuming a constant growth rate over the years, which corresponds to 1·6 mm year–1. A tree of 34 cm diameter with an equal growth rate and a similar growth history would therefore be 106 years old. These data suggest that the mangrove trees in the two studied forests probably do not grow much older than 100 years. Both studied forests have a long history of human disturbance (Kairo, 2001), which may explain the relatively young age of the trees. However, sites with a certain degree of dwarf growth usually remain undisturbed, due to the low socio‐economical value of stunted stems. Therefore, these sites are expected to have older trees. In site 3 in Gazi, R. mucronata stems do not form straight poles and branching of the main stem occurs at 3 or 4 m height, reducing the socio‐economic value of these stems. However, the tree with the largest diameter (13·1 cm) in this site was only 71 years old. It is therefore suggested that the relatively young age of R. mucronata may result from the highly dynamic nature of the mangrove environment, which is constantly subjected to changes in water flow, sedimentation and/or erosion rates and periodic events of storms, resulting in an unstable geomorphic system. More research on the age of R. mucronata trees in other stable and unstable geomorphic areas as well as on the age of mangrove trees in general is necessary to confirm this hypothesis.
Table 3.
Age correction factor in number of years to add per 10‐cm sampling height, calculated from average annual height increment for the first 3 years (data obtained from Kairo, 1995) in different inundation classes in Gazi
| Inundation class* | Annual height increment (cm) | Age correction factor |
| 2 | 41·7 | 0·24 |
| 3 | 57·3 | 0·17 |
| 4 | 36·9 | 0·27 |
* See Table 1 for explanation.
Table 4.
Diameter (D), tree height (H), age of the sample (SA), sampling height (SH) and age of the tree (TA) of the biggest trees collected in four sites with different inundation classes (IC) in Gazi and one site in Mida
| Site | IC* | D (cm) | H (m) | SA (year) | SH (cm) | TA (year) |
| Gazi 1 | 2 | 7·2 | 5 | 37 | 130 | 40 |
| Gazi 2 | 2 | 10·6 | 8 | 29 | 145 | 32 |
| Gazi 3 | 4 | 13·1 | 6 | 67 | 130 | 71 |
| Gazi 4 | 3 | 11·1 | 5 | 15 | 130 | 17 |
| Mida | 3 | 28·6 | 12 | 85 | 250 | 89 |
The age of the sample was estimated by counting the annual growth rings; the age of the tree was obtained by correcting the age of the sample for sampling height, using the age correction factor of Table 3.
* See Table 1 for explanation.
CONCLUSIONS
Although it has been generally assumed that mangroves lack annual growth rings (see Tomlinson, 1986), very little research has been conducted on this subject. In a study on Avicennia germinans, Gill (1971) concluded that the rings of included phloem, clearly visible on sanded stem discs, are a result of endogenous control of the cambial activity and therefore do not indicate the age of the tree. Duke et al. (1981) reported the development of distinct growth rings in a mangrove associate of the genus Diospyros; however, the growth rings appeared to be non‐annual, allowing only a rough estimate of the trees age. Recently, another Rhizophora species, R. mangle, was suggested to produce annual growth rings based on 14C‐dating in two trees (Menezes et al., 2003). The data presented here, using cambial marking, as well as trees of known age, offer strong evidence for the presence of annual growth rings in the mangrove R. mucronata and give additional support to the work of Menezes et al. (2003) on R. mangle. Whether or not the presence of annual growth rings is a common characteristic of Rhizophora still needs to be determined. The presence of annual growth rings in R. mucronata offers great potential for further dendrochronological and silvicultural applications.
ACKNOWLEDGEMENTS
The authors thank D. P. Gillikin for his help in the field and for considerably improving this manuscript, the staff of KMFRI (Kenya Marine and Fisheries Research Institute), Mombasa for assistance in the field; P. Kitin and M. Worbes for helpful discussions and two anonymous reviewers for their useful comments on this manuscript. A.V. was financed by a PhD scholarship from the Fund for Scientific Research (FWO), Flanders, Belgium, a travel grant from the Commission for Research (OZR) of the Vrije Universiteit Brussel, Brussels, Belgium and an EC‐INCO project (IC18‐CT96‐0065).
Received: 27 October 2003; Returned for revision: 11 February 2004; Accepted: 5 March 2004. Published electronically: 14 May 2004
References
- BertaudiereV, Montes N, Gauquelin T, Edouard JL.1999. Dendroecology of thuriferous juniper (Juniperus thurifera L.): example from a French Pyrenean site at Rie mountain. Annals of Forest Science 56: 685–697. [Google Scholar]
- CintrónG, Novelli YS.1984. Methods for studying mangrove structure. In: Snedaker SC, Snedaker JG, eds. The mangrove ecosystem: research methods Paris: UNESCO, 91–113. [Google Scholar]
- DétienneP.1989. Appearance and periodicity of growth rings in some tropical woods. International Association of Wood Anatomists Bulletin 10: 123–132. [Google Scholar]
- DevoeNN, Cole TG.1998. Growth and yield in mangrove forests of the Federal States of Micronesia. Forest Ecology and Management 103: 33–48. [Google Scholar]
- DukeNC, Birch WR, Williams WT.1981. Growth rings and rainfall correlations in a mangrove tree of the genus Diospyros (Ebenaceae). Australian Journal of Botany 29: 135–142. [Google Scholar]
- EsheteG, Stahl G.1999. Tree rings as indicators of growth periodicity of acacias in the Rift Valley of Ethiopia. Forest Ecology and Management 116: 107–117. [Google Scholar]
- GillAM.1971. Endogenous control of growth ring development in Avicennia Forest Science 17: 462–465. [Google Scholar]
- GourlayID.1995. Growth ring characteristics of some African Acacia species. Journal of Tropical Ecology 11: 121–140. [Google Scholar]
- IAWA.1989. IAWA list of microscopic features for hardwood identification. International Association of Wood Anatomists Bulletin 10: 219–332. [Google Scholar]
- JansenS, Kitin P, De Pauw H, Idris M, Beeckman H, Smets E.1998. Preparation of wood specimens for transmitted light microscopy and scanning electron microscopy. Belgian Journal of Botany 131: 41–49. [Google Scholar]
- JanssoniusHH.1950. The vessels in the wood of Javan mangrove trees. Blumea 6: 465–469. [Google Scholar]
- KaennelM, Schweingruber FH.1995.Multilingual glossary of dendrochronology. Terms and definitions in English, German, French, Spanish, Italian, Portuguese and Russian. Bern: Paul Haupt Verlag. [Google Scholar]
- KairoJG.1995.Community participation forestry for rehabilitation of deforested mangrove areas of Gazi Bay (Kenya): ‘A first approach’. Final technical report, University of Nairobi, Nairobi, Kenya. [Google Scholar]
- KairoJG.2001.Ecology and restoration of mangrove systems in Kenya. PhD Thesis, Vrije Universiteit Brussel, Belgium. [Google Scholar]
- KairoJG, Dahdouh‐Guebas F, Bosire J, Koedam N.2001. Restoration and management of mangrove systems – a lesson for and from the East African region. South African Journal of Botany 67: 383–389. [Google Scholar]
- KurodaK.1986. Wound effects on cytodifferentiation in the secondary xylem of woody plants. Wood Research 72: 67–118. [Google Scholar]
- LiethH, Berlekamp J, Fuest S, Riediger S.1999.Climate diagrams of the world. CD‐Series: Climate and Biosphere. Leiden: Blackhuys Publishers. [Google Scholar]
- LugoAE, Snedaker SC.1974. The ecology of mangroves. Annual Review of Ecology and Systematics 5: 39–64. [Google Scholar]
- McClanahanTR.1988. Seasonality in East Africa’s coastal waters. Marine Ecology – Progress Series 44: 191–199. [Google Scholar]
- MariauxA.1967. Les cernes dans les bois tropicaux africain, nature et periodicité. Bois et Fôrets des Tropiques 113: 3–14. [Google Scholar]
- MariauxA.1968. Les cernes dans les bois tropicaux africain, nature et periodicité. Bois et Fôrets des Tropiques 114: 23–37. [Google Scholar]
- MenezesM, Berger U, Worbes M.2003. Annual growth rings and long‐term growth patterns of mangrove trees from the Bragança peninsula, North Brazil. Wetlands Ecology and Management 11: 233–242. [Google Scholar]
- Mwangi TheuriM, Kinyamario JI, Van Speybroeck D.1999. Photosynthesis and related physiological processes in two mangrove species, Rhizophora mucronata and Ceriops tagal, at Gazi Bay, Kenya. African Journal of Ecology 37: 180–193. [Google Scholar]
- NOAA(National Oceanic and Atmospheric Administration) 2003. IRI/LDEO Climate Data Library. http://iridl.ldeo.columbia. edu/SOURCES/.NOAA/.NCEP/.CPC/.EVE/IWMO/%2863820%29 VALUE/.total/.prcp/T/456./520./RANGEEDGES/, 14 Oct. 2003. [Google Scholar]
- NobuchiT, Ogata Y, Siripatanadilok S.1995. Seasonal characteristics of wood formation in Hopea odorata and Shorea henryana International Association of Wood Anatomists Journal 16: 361–369. [Google Scholar]
- PanshinAJ.1932. An anatomical study of the woods of the Philippine mangrove swamps. Phillippine Journal of Science 48: 143–205. [Google Scholar]
- RiglingA, Braker O, Schneiter G, Schweingruber F.2002. Intra‐annual tree‐ring parameters indicating differences in drought stress of Pinus sylvestris forests within the Erico‐Pinion in the Valais (Switzerland). Plant Ecology 163: 105–121. [Google Scholar]
- SassU, Killmann W, Eckstein D.1995. Wood formation in two species of Dipterocarpaceae in peninsular Malaysia. International Association of Wood Anatomists Journal 16: 371–384. [Google Scholar]
- ShiokuraT.1989. A method to measure radial increment in tropical trees. International Association of Wood Anatomists Bulletin 10: 147–154. [Google Scholar]
- StahleDW.1999. Useful strategies for the development of tropical tree‐ring chronologies. International Association of Wood Anatomists Journal 20: 249–253. [Google Scholar]
- TrouetV, Haneca K, Coppin P and Beeckman H.2001. Tree ring analysis of Brachystegia spiciformis and Isoberlina tomentosa: evaluation of the ENSO‐signal in the miombo woodland of Eastern Africa. International Association of Wood Anatomists Journal 22: 385–99. [Google Scholar]
- TomlinsonPB.1986.The botany of mangroves. London: Cambridge University Press. [Google Scholar]
- UNEP.2001.The Eastern African coastal resources atlas: KENYA. Eastern African Database and Atlas Project (EAF/14). [Google Scholar]
- van VlietGJCM.1976. Wood anatomy of the Rhizophoraceae. Leiden Botanical Series 3: 20–75. [Google Scholar]
- WorbesM.1995. How to measure growth dynamics in tropical trees – a review. International Association of Wood Anatomists Journal 16: 337–351. [Google Scholar]