Abstract
• Background and Aims In 1987, Kjellsson and Rasmussen described the labellar trichomes of Dendrobium unicum Seidenf. and proposed that these hairs function as pseudopollen. Pseudopollen is a mealy material that superficially resembles pollen, is usually laden with food substances and is formed when labellar hairs either fragment into individual cells or become detached from the labellum. However, the trichomes of D. unicum are very different from pseudopollen‐forming hairs found in other orchid genera such as Maxillaria and Polystachya. Moreover, Kjellsson and Rasmussen were unable to demonstrate the presence of food substances within these trichomes and argued that even in the absence of food substances, the hairs, in that they superficially resemble pollen, can still attract insects by deceit. The aim of this paper is to investigate whether the labellar trichomes of D. unicum contain food reserves and thus reward potential pollinators or whether they are devoid of foods and attract insects solely by mimicry.
• Methods Light microscopy, histochemistry and transmission electron microscopy.
• Key Results Dendrobium unicum produces pseudopollen. Pseudopollen here, however, differs from that previously described for other orchid genera in that the pseudopollen‐forming trichomes consist of a stalk cell and a ‘head’ of component cells that separate at maturity, in contrast to Maxillaria and some Polystachya spp. where pseudopollen is formed by the fragmentation of moniliform hairs. Moreover, the pseudopollen of Maxillaria and Polystachya largely contains protein, whereas in D. unicum the main food substance is starch.
• Conclusions Flowers of D. unicum, rather than attracting insects solely by deceit may also reward potential pollinators.
Key words: Amyloplast, deception, histochemistry, mimicry, protein, pseudopollen, reward, starch, transmission electron microscopy, trichome
INTRODUCTION
Many epidendroid orchid species that reward visiting insects do so by means of nectar (van der Pijl and Dodson, 1969; Arditti, 1992; Dressler, 1993). However, some epidendroid species lack nectar and reward potential pollinators with pseudopollen instead. Pseudopollen is a mealy material, usually whitish or yellowish in colour, which superficially resembles pollen. It is usually laden with food materials and is formed when hairs either fragment into individual cells or become detached from the labellum. Genera that produce pseudopollen include Maxillaria Ruiz & Pav. (Janse, 1886; Porsch, 1905, van der Pijl and Dodson, 1969; Davies and Winters, 1998; Davies et al., 2000, 2003a), Polystachya Hook. (Porsch, 1905; Davies et al., 2002) and Eria Lindl. (Beck, 1914). However, not all members of these genera produce pseudopollen. For example, within Maxillaria, pseudopollen has hitherto only been observed for species assigned to the M. grandiflora (H.B.K.) Lindl., M. discolor (Lodd. ex Lindl.) Rchb.f., M. splendens Poepp. & Endl. and M. lepidota Lindl. alliances (Davies and Winters, 1998; Davies et al., 2000, 2003a; Davies and Turner, 2004). Similarly, within the genera Polystachya and Eria, pseudopollen seemingly occurs only in members of certain sections, e.g. Polystachya Rchb.f. and Mycaranthes Rchb.f., respectively.
In both Maxillaria and members of section Polystachya, pseudopollen or ‘farina’ is formed by the fragmentation of uniseriate moniliform hairs, resulting in the formation of individual cells or short chains of cells. These component cells are usually rounded, elliptic, lemon‐shaped or fusiform and generally contain protein, either as discrete protein bodies (Maxillaria) or distributed throughout the cytoplasm (Polystachya), and little or no lipid. The presence of starch, however, is more variable (Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004). Other Polystachya spp. produce bicellular trichomes that become detached from the labellum. These also contain food reserves and probably function as pseudopollen. Transmission electron microscopy (TEM) studies of pseudopollen of M. sanderiana Rchb.f. revealed that each component cell contains a single protein body and numerous small amyloplasts, each containing several starch grains. Lipid droplets in the cytoplasm, however, are comparatively rare (Davies et al., 2000). When starch is present in Polystachya spp. such as P. foliosa (Hook.f.) Rchb.f., it too occurs in small amyloplasts similar to those found in Maxillaria (Davies et al., 2002). Thus, the main food reserve in both these genera is considered to be protein. However, although the dimensions of the component cells of both genera are similar, the relative intensity of staining following the xanthoproteic test (Davies et al., 2000, 2002, 2003a) indicates that protein is present at lower concentrations in the pseudopollen of Polystachya spp. than in that of Maxillaria spp. (Davies et al., 2002).
Field observations of bees gathering pseudopollen from Maxillaria spp. are rare (Dodson and Frymire, 1961; Dodson, 1962) and in recent years there has been a tendency to dismiss these records or to doubt their validity (e.g. Roubik, 2000). However, Singer has reported Trigona spp. (Meliponini – stingless bees) gathering hairs, possibly pseudopollen, from the labella of M. ochroleuca Lodd. ex Lindl. and M. brasiliensis Brieger & Bicalho (Singer, 2003; Singer and Koehler, 2004; R. B. Singer, pers. comm.). Similarly, the halictid bee Dialictus aff. creberrimus has been seen gathering pseudopollen from Polystachya flavescens (Lindl.) J. J. Sm. (Goss, 1977).
In 1987, Kjellsson and Rasmussen proposed that spherical clusters of cells occurring on the labellum of Dendrobium unicum Seidenf., a species found in Northern Thailand and Laos, may also function as pseudopollen. Their work is noteworthy for two reasons: (1) it is the first account of pseudopollen for the genus Dendrobium Sw.; and (2) if these clusters of cells really are pseudopollen, they are morphologically remarkably different from the pseudopollen described for other orchid genera.
The flower of Dendrobium unicum is scented and has a non‐resupinate labellum with a longitudinal tripartite ridge‐like callus. The callus is coated with farinaceous granulae interpreted as 3–12‐celled trichomes (Kjellsson and Rasmussen, 1987). These clusters fall apart forming single cells or groups of four cells (‘tetrads’) that may function as pseudopollen. Although they did not observe pollination in the field, Kjellsson and Rasmussen (1987) speculated that D. unicum is pollinated by small bees or wasps that collect the granulae. Unfortunately, they were not able to verify whether the latter contained food reserves. Despite this, they argued that even in the absence of food substances, the pseudopollen, in that it resembles pollen, could still attract pollinators solely by mimicry as proposed by Vogel (1979). However, in the absence of both pollinator and food reserve data, they were not able to prove conclusively that pseudopollen occurs in this species, merely that the mealy covering on the callus disappeared and that this process coincided with the removal of pollinaria by insects. Thus, this paper is an attempt to resolve whether the granulae contain food substances and thus act as a reward or whether they are devoid of foods and attract pollinators solely by deceit.
MATERIALS AND METHODS
Labella of D. unicum Seidenf. were prepared for TEM as described in our previous papers. Samples of granulae were mounted in drops of water and tested for starch, lipid and protein using IKI, a saturated ethanolic solution of Sudan III and a modified xanthoproteic test, respectively. They were then examined microscopically for the presence of reaction products (Davies et al., 2000, 2002, 2003a, b; Davies and Turner, 2004).
RESULTS
Flowers of D. unicum lack a spur or mentum and do not produce nectar. The labellum is non‐resupinate and parenchymatous. Its cells are highly vacuolated with nuclei and a little peripheral cytoplasm. The labellum has a longitudinal tripartite ridge‐like callus whose ridges reach their maximum thickness half way along the labellum where the mid lobe is at its widest. Excellent photomicrographs showing the morphology of the pseudopollen‐forming trichomes of D. unicum have already been published (Kjellsson and Rasmussen, 1987) and further similar figures would be redundant. The ridges are farinaceous due to granulae (‘heads’ of trichomes comprising clusters of cells) formed of four to 13 component cells. Most granulae, however, consist of eight or nine cells and these ‘heads’ are attached to the labellar surface by means of a stalk cell. The granulae fragment to form individual cells or small groups of cells. The results presented here are almost identical to those obtained by Kjellsson and Rasmussen (1987) except that, in our specimens, the granulae tended to break up into individual cells or small groups of cells rather than the packets of four cells described as ‘tetrads’ by the earlier authors.
Histochemistry revealed that the component cells of granulae contain starch and protein but no detectable lipid. The intensity of staining following the xanthoproteic test indicated that aromatic amino acids are distributed throughout the cytoplasm at relatively low concentrations, whereas, following treatment with IKI, most of the cell contents stained a purple‐brown colour rather than the typical purple‐black colour one would expect were starch present. That the food reserve is indeed starch was confirmed by treating a control sample of granulae with 1 % (w/v) α‐amylase solution for 25 min at 25 °C prior to testing with IKI, whereupon the cell contents failed to stain purple‐brown.
Light microscopy coupled with histochemistry indicated that the component cells are relatively devoid of cytoplasmic organelles and that each cell contains a single starch grain. TEM (Fig. 1A and B) confirmed that the component cells possess nuclei and contain a little peripheral cytoplasm with relatively few organelles such as mitochondria and plastids. Moreover, TEM also confirmed that much of the cell is occupied by a single starch grain. The plastids contain numerous plastoglobuli but relatively few internal lamellae. Spherical osmiophilic bodies resembling plastoglobuli also occur in the cytoplasm. Identical osmiophilic bodies have been observed in labellar cells of Maxillaria cf. notylioglossa Rchb.f. (Davies et al., 2003b). By contrast, the stalk cell, which is also nucleated, tends to have dense cytoplasmic contents.
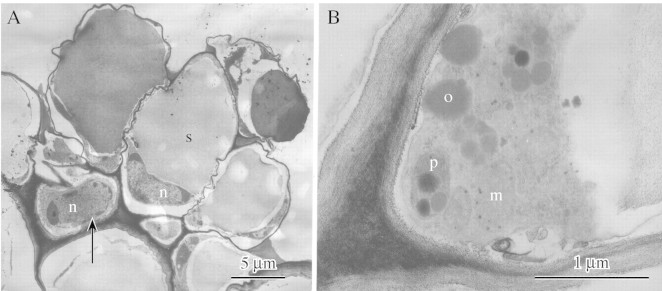
Fig. 1 (A) TEM of labellar trichome of D. unicum showing stalk cell with ‘head’ of component cells. Note that both cell types contain a nucleus (n) but whereas the stalk cell (arrow) contains dense cytoplasmic contents, the component cells have peripheral cytoplasm. Each component cell contains a single starch grain (s). Scale bar = 5 µm. (B) Detail of component cell showing peripheral cytoplasm with mitochondria (m) and plastids (p), the latter containing plastoglobuli. Spherical osmiophilic bodies (o) may also occur in the cytoplasm. Scale bar = 1 µm.
DISCUSSION
Our studies of granulae in D. unicum closely agree with those of Kjellsson and Rasmussen (1987). These peculiar trichomes contain food reserves and thus probably function as pseudopollen, rewarding potential pollinators rather than attracting them by deceit alone as has been proposed for a number of orchid species (Vogel, 1979; Roubik, 2000). Indeed, Kjellsson and Rasmussen (1987), too, believed that these hairs function as ‘food‐bodies’ even though they were not able to demonstrate that they contained any food materials.
The pseudopollen‐forming hairs of D. unicum differ from those of Maxillaria spp. and Polystachya spp. in that they comprise a stalk cell with an associated ‘head’ of component cells rather than the moniliform chains of cells or bicellular units often found in these two last genera (Davies and Winters, 1998; Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004). Moreover, the pseudopollen of D. unicum is remarkable in that the main food reserve here is starch not protein as in Maxillaria (Davies et al., 2000, 2003a; Davies and Turner 2004) and Polystachya (Davies et al., 2002). Nevertheless, protein does occur in the pseudopollen of D. unicum but this is distributed at relatively low concentrations throughout the cytoplasm as in Polystachya (Davies et al., 2002) rather than concentrated into a discrete protein body as in Maxillaria (Davies et al., 2000). Starch also occurs in the pseudopollen of some species of Maxillaria and Polystachya (Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004) where it is confined to small amyloplasts, each containing a number of starch grains rather than one relatively large starch grain as found in D. unicum. The presence of starch as the main food reserve would, in effect, contribute towards pollinator selection since any potential pollinator would need to be able to utilize this complex carbohydrate. This feature alone may prove useful in identifying possible pollinators for this species since starch is an inferior reward compared with protein or lipids and is acceptable only to eusocial bees that have other food stores. In contrast, starchy pseudopollen is insufficient reward for solitary bees (D. W. Roubik, pers. comm.). Moreover, the flower of D. unicum, in that it produces pseudopollen, is unlikely to attract wasps since these insects seemingly do not collect pollen.
In conclusion, the pseudopollen of D. unicum does indeed contain food materials and is remarkable in that it differs from that hitherto described for other orchid species both in morphological terms and in that the main food reserve here is starch not protein. As such, this type of pseudopollen, being atypical, may well have been overlooked in the past. It thus remains to be seen whether other species of Dendrobium, or indeed other orchid genera, produce pseudopollen of this kind. However, one caveat remains. To date, no insects have been observed gathering the labellar hairs of D. unicum. Although it is likely that these hairs indeed may function as pseudopollen, it must be remembered that the presence of starch alone is not sufficient evidence that this is the case, especially as the pollen of anemophilous flowers (e.g. grasses) is often rich in starch. Only when field studies have been undertaken can we be absolutely certain of the role of these unusual trichomes.
ACKNOWLEDGEMENTS
The authors are grateful to A. Gregg (Swansea Botanical Complex, Swansea, UK) and J. Davison for providing flowers for this study.
Received: 28 January 2004; Returned for revision: 1 March 2004; Accepted: 15 March 2004. Published electronically: 24 May 2004
References
- ArdittiJ.1992.Fundamentals of orchid biology. New York: John Wiley & Sons. [Google Scholar]
- BeckG.1914. Die Pollennachahmung in den Blüten der Orchideen‐Gattung Eria. Sitzungs Berichte Akadamie der Wissenschaften in Wien 123: 1033–1046. [Google Scholar]
- DaviesKL, Turner MP.2004. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 93: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DaviesKL, Roberts DL, Turner MP.2002. Pseudopollen and food‐hair diversity in Polystachya Hook. (Orchidaceae). Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003a. Atypical pseudopollen‐forming hairs in Maxillaria (Orchidaceae). Botanical Journal of the Linnean Society 143: 151–158. [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003b. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DodsonCH.1962. The importance of pollination in the evolution of the orchids of tropical America. American Orchid Society Bulletin 31: 525–534, 641,–649, 731–735. [Google Scholar]
- DodsonCH, Frymire GP.1961. Natural pollination of orchids. Missouri Botanical Garden Bulletin 49: 133–139. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the orchid family. Cambridge, MA: Dioscorides Press. [Google Scholar]
- GossGJ.1977. The reproductive biology of the epiphytic orchids of Florida. 6. Polystachya flavescens (Lindley) J.J. Smith. American Orchid Society Bulletin 46: 990–994. [Google Scholar]
- JanseJM.1886. Imitirte pollenkörner bei Maxillaria sp. Deutsche Botanische Gesellschaft Berichte 4: 277–283. [Google Scholar]
- KjellssonG, Rasmussen FN.1987. Does the pollination of Dendrobium unicum Seidenf. involve pseudopollen? Die Orchidee 38: 183–187. [Google Scholar]
- van der PijlL, Dodson CH.1969.Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press. [Google Scholar]
- PorschO.1905. Beiträge zur ‘histologischen’ Blütenbiologie I. Österreichische Botanische Zeitschrift 55: 253–260. [Google Scholar]
- RoubikDW.2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- SingerRB.2003. Orchid pollination: recent developments from Brazil. Lankesteriana 7: 111–114. [Google Scholar]
- SingerRB, Koehler S.2004. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). Annals of Botany 93: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VogelS.1979. Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ (ed.). The pollination of flowers by insects London: Academic Press, 89–96. [Google Scholar]


