Abstract
• Background and Aims The annual Lesquerella fendleri, native to the south‐western desert of United States and Mexico, and the perennial L. mendocina, native to Argentina, may have potential as new crops for cold‐arid environments. The introduction of a new crop requires an understanding of environmental influences on growth and development, particularly temperature, which has been recognized as the main factor affecting the rate of development in crops. The objective of this study was to examine differences in the phenology of L. fendleri and L. mendocina and in the response to temperature in both vegetative and reproductive phases.
• Methods Plants of each species were grown at a range of constant temperatures under controlled conditions and developmental responses were analysed and quantified.
• Key Results The rate of development of L. fendleri increased linearly with temperature in the phase from emergence (EM) to floral bud appearance (FBA) over the range 9–20 °C, and for the phase from FBA to first flower open (FL) over the range 9–24 °C. In contrast, the rate of development of L. mendocina was insensitive to temperature during the phase EM to FBA. In the phase FBA to FL, L. mendocina had a lower sensitivity to temperature than L. fendleri. In addition, L. fendleri exhibited a quantitative response to supra‐optimal temperatures (reducing rate of development with further increases in temperature) whereas L. mendocina showed a qualitative response, with development ceasing to progress at temperatures above the optimum.
• Conclusions This differential behaviour at high temperatures could explain the biennial habit found for L. mendocina sown during late spring under field conditions, whereas it behaves as an annual when sown in autumn–winter. The possibility is discussed of using this information for establishing the coincidence of critical stages with environmental conditions that can limit potential and actual yield through agronomic practices.
Key words: Lesquerella fendleri, Lesquerella mendocina, phenology, temperature
INTRODUCTION
In recent years, several new industrial crops have been developed for warm‐arid environments, but none of them has been adapted for cultivation in cold‐arid environments, such as extra‐Andean Patagonia (Zavala and Ravetta, 2000). These areas are characterized by long winters with freezing temperatures and high irradiance in summer. The Brassicaceae species Lesquerella fendleri and L. mendocina are among the most interesting species with potential as new crops for these regions (Ploschuk et al., 2003). Lesquerella fendleri is native to the arid and semi‐arid regions of the south‐western USA and its seeds contain significant amounts of hydroxy fatty acids (HFA; Roetheli et al., 1991), which are of industrial importance as chemical feedstock for the production of lubricants, plastics, protective coatings, surfactants and pharmaceuticals (Thompson, 1990). Lesquerella fendleri has been considered for domestication in the USA because of its high seed and oil yield, its low seed dormancy and its responsiveness to irrigation (Dierig et al., 1993).
Although L. fendleri yields are high when grown as a winter crop in Arizona, using a cropping system similar to that of wheat and other small grains (Dierig et al., 1993), under cooler climates it can be grown only as a spring crop, with lower yields compared with other Lesquerella species found in Arizona (Dierig et al., 1993). An alternative candidate for cropping in cold‐arid environments is L. mendocina, a perennial native to the ‘Monte’ region in Argentina (Correa, 1984). It also produces hydroxy fatty acids as the major oil constituent of the seeds but, unlike L. fendleri, it has only recently been considered for domestication (Ploschuk et al., 2001).
One of the first steps for the introduction of a wild species into cultivation is to understand the environmental control of its phenology. This is important in terms of the general adaptation of the species, but also in establishing the coincidence of critical stages with environmental conditions that can limit potential and actual yield (Richards, 1991). Study of two species of the same genera with contrasting life cycles offers a unique opportunity to investigate the ecophysiological responses leading to annual and perennial growth.
Little is known about the direct effects of temperature on the rate of development of Lesquerella species. In several crops it has been shown that base (Tb; Monteith, 1984) and optimum (To, Ritchie and NeSmith, 1991) temperatures for development processes can change during ontogeny, increasing with plant age (Hodgson, 1978; Angus et al., 1981; Hammer et al., 1982; Ellis et al., 1988; Slafer and Rawson, 1995). It is also unknown whether vernalization is required in the initiation of reproductive development of Lesquerella species. In a 2‐year field study, including six sowing dates (from mid‐March to mid‐November), it was found that vernalization had no effect on the temperature relations of the development of either species (Windauer, 2002). Lesquerella mendocina plants remained vegetative, showing a biennial habit, when sown in the field in late spring even when artificially vernalized (Windauer, 2002), or in a glasshouse at similar temperatures to those of late sowings in the field (Ploschuk et al., 2001).
The objectives of this work were (1) to examine differences in the phenological development behaviour of L. fendleri and L. mendocina and the relative sensitivities of vegetative and reproductive phases to temperature, and (2) to estimate Tb and To for these species, testing whether they change during plant ontogeny. To carry out this study, vernalized plants of the two species were grown under a long photoperiod at a wide range of temperatures.
MATERIALS AND METHODS
Seeds of L. fendleri (A. Gray) S. Watson were provided by D. Dierig (USDA, WCL Phoenix, AZ) and came from multiplication plots in Phoenix, Arizona, established from seed originally collected from native stands. Seeds of L. mendocina (Phil.) Kurtz were collected from a native stand at Lihuel Calel, La Pampa, Argentina (37°57′S, 65°33′W). All the seeds received a vernalization pre‐treatment (imbibed seeds at 4 °C in darkness for 14 d). Both the period and the temperature chosen for vernalization have been shown to be adequate to remove the low requirements (if any) displayed by these species (Windauer, 2002). At the beginning of the experiment, immediately after the end of the vernalization pre‐treatment, all plants were transferred to growth chambers.
Plants of both species were grown from seedling emergence to flowering in growth chambers at five constant temperatures (9, 12, 16, 20 and 24 °C ± 0·5 °C) and a constant 18 h photoperiod. Photon irradiance inside the growth chambers was 700 µmol m–2 s–1, enough to ensure normal growth of the plants (Taiz and Zeiger, 2003). Plants were grown in 1 L pots containing a mixture of moss peat and vermiculite (1 : 1). Ten pots (one plant per pot) were used per treatment. During the experiment the plants were watered daily with a complete Hoagland’s solution.
Observations of phenological development were made daily. Dates of floral bud appearance (FBA, floral buds within the same inflorescence joined, still covered by the terminal leaves) and the beginning of flowering (FL, the first flower opened on the floral stem) were recorded and corresponding rates of development for the vegetative (before FBA) and reproductive (FBA to FL) were calculated as the reciprocal of the duration of each phase (Monteith, 1984). The phenological observations finished when 100 % of plants had reached the floral stage (FL). When no switch to reproductive stage was observed, observations finished at 180 d after the beginning of the experiment.
Regression analysis was used to estimate the effect of temperature on the rate of development (the reciprocal of the duration of the phase in days: 1/d). The abscissa of the regression was taken to be Tb and the duration in thermal time (TT) of the phase was calculated as the inverse of the slope of the relationship between development and temperature in the sub‐optimal range (Garcia Huidobro et al., 1982).
To determine whether the optimum temperature was within the range of temperatures used, the rates of development at 24 and 20 °C were compared; if the former was not significantly faster than the latter, then the optimum temperature was assumed to be somewhere between these values. A similar comparison was made between the rate of development at 20 and 16 °C. A negative linear relationship was then drawn between the rate of development and temperature beyond the optimum and a positive relationship was fitted with the values below To (from where Tb and duration in TT were estimated ). The optimum temperature was taken to be the temperature corresponding to the two‐line intercept (according to Slafer and Rawson, 1995).
RESULTS
There were major differences between the species in their developmental responses to temperature. Whereas in L. fendleri 100 % of the plants switched to the reproductive stage and reached FBA under all temperatures regimes, in L. mendocina the response was different (Table 1): all plants reached FBA at the lowest temperature (9 °C), but none of the plants growing under the highest temperature (24 °C) did. At the intermediate temperatures, a large, though variable, proportion (60–80 %) of the plants reached FBA. The rate of development of L. mendocina at 24 °C was so slow that, by the time the experiment had finished, the apical meristem of the plants remained vegetative, as revealed by dissection.
Table 1.
Proportions of the population of L. fendleri and L. mendocina plants reaching reproductive stages (FBA, floral bud appearance) at each experimental temperature
| Proportion of plants reaching FBA (%) | ||
| Temperature (°C) | L. fendleri | L. mendocina |
| 9 | 100 | 100 |
| 12 | 100 | 80 |
| 16 | 100 | 60 |
| 20 | 100 | 80 |
| 24 | 100 | 0 |
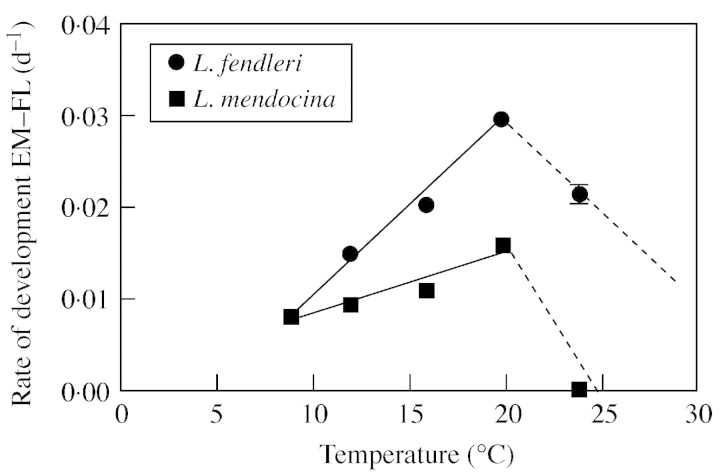
The analysis of the effect of temperature on development presented here is based on the behaviour of the plants that reached flowering. Considering the whole period from seedling emergence (EM) to FL, both species appeared to have a To between 20 and 24 °C, with a remarkable difference in sensitivity to sub‐optimum temperatures (Fig. 1). The development of L. fendleri was more responsive to temperature, with a slope almost three times higher than that for L mendocina (Table 2, Fig. 1). A linear model fitted the data well between 9 and 20 °C for both species (P < 0·001), showing that the rate of development increased linearly as temperature increased within this range (Fig. 1). The base temperature calculated for the emergence–first flower open phases using this model was 4·6 °C for L. fendleri and –2·9 °C for L. mendocina (Table 2). A bi‐linear model accurately described the observed variability in the rate of development for the whole thermal range examined, with an optimum temperature of around 20 °C for both species (Table 2).
Fig. 1. Relationships between rate of development (emergence to first flower open) and temperature for L. fendleri and L. mendocina. The solid lines were fitted by linear regression and broken lines were drawn by hand. Coefficients of determination are: L. fendleri, r2 = 0·95; L. mendocina, r2 = 0·78. Each point is the mean of ten plants for L. fendleri and of 6–10 plants for L. mendocina. Error bars indicate s.e. and are shown only when larger than the symbols.
Table 2.
Parameters of the linear regression of rate of development against temperature of vernalized plants of L. fendleri and L. mendocina (emergence to first flower open)
| Intercept ± s.e. | Slope ± s.e. | Temperature (°C) | Duration of development | |||||
| Species | (1/d ×10–3) | (1/°Cd ×10–3) | r 2 | n* | P* | Base | Optimum | (TT, °Cd) |
| L. fendleri | 8·6 ± 1·00 | 1·9 ± 0·06 | 0·952 | 10 | <0·001 | 4·6 | ∼20·0 | 536·6 |
| L. mendocina | 1·8 ± 0·93 | 0·6 ± 0·06 | 0·782 | † | <0·001 | –2·9 | ∼20·0 | 1566·0 |
Durations in thermal time were calculated as the reciprocal of the slopes.
* n is the sample size; P is the linear regression probability level.
† for 9 °C, n = 10; for 12 and 20 °C, n = 8; for 16 °C, n = 6.
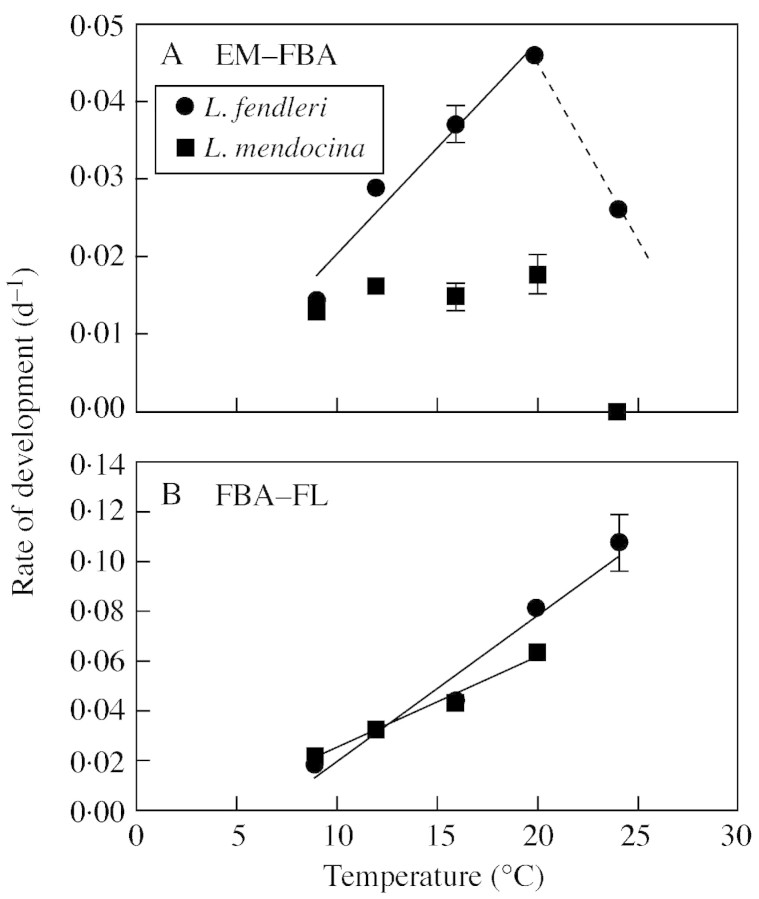
To find out whether the strong differential sensitivity to supra‐optimal temperatures was expressed throughout the whole period, the responses of the rates of development to temperature were analysed from EM to FBA and from FBA to FL. This showed that the linear model gave a good fit in the sub‐optimal temperature range in both phases for L. fendleri (Fig. 2A, B), although differences in sensitivity to temperature between phases were found: the slope for the phase FBA to FL was twice that of the earlier phase (Table 3). Results for L. mendocina, in contrast, showed that the rate of development from EM to FBA was little affected by temperature (Fig. 2A), suggesting that factor(s) other than temperature determined the rate of development during this early phase. Conversely, the linear model gave a good fit to the data for the later phase FBA to FL (Fig. 2B).
Fig. 2. Relationships between rate of development and temperature for L. fendleri and L. mendocina in two different phenophases: (A) emergence to floral bud appearance, and (B) floral bud appearanc to first flower open. The solid lines were fitted by linear regression and broken lines were drawn by hand. Each point is the mean of ten plants for L. fendleri and of 6–10 plants for L. mendocina. Error bars indicate s.e. and are shown only when larger than the symbols.
Table 3.
Parameters of the linear regression of rate of development against temperature of vernalized plants of L. fendleri and L. mendocina (floral bud appearance to first flower open)
| Intercept ± s.e. | Slope ± s.e. | Temperature (°C) | Duration of development | |||||
| Species | (1/d ×10–3) | (1/°Cd ×10–3) | r 2 | n* | P* | Base | Optimum | (TT, °Cd) |
| Emergence to floral bud appearance | ||||||||
| L. fendleri | –7·0 ± 2·71 | 2·7 ± 0·18 | 0·852 | 10 | <0·001 | 2·6 | ∼20·0 | 370·7 |
| L.mendocina | 10·2 ± 2·31 | 0·4 ± 0·16 | 0·130 | † | 0·04 | – | – | – |
| Floral bud appearance to first flower open | ||||||||
| L. fendleri | –41·2 ± 6·94 | 6·1 ± 0·41 | 0·853 | 10 | <0·001 | 6·1 | >24·0 | 165·2 |
| L.mendocina | –13·7 ± 3·53 | 4·0 ± 0·24 | 0·891 | † | <0·001 | 3·6 | >20·0 | 263·4 |
Durations in thermal time were calculated as the reciprocal of the slopes
* n is the sample size; P is the linear regression probability level.
† for 9 °C, n = 10; for 12 and 20 °C, n = 8; for 16 °C, n = 6.
Estimated base and optimum temperatures varied between species and phases. The base temperature calculated for the EM to FBA phase in L. fendleri was 2·6 °C, but it could not be estimated for L. mendocina due to the lack of a significant relationship between development rate for this phase and temperature. For the reproductive phase, from FBA to FL, Tb was 6·1 and 3·6 °C for L fendleri and L. mendocina, respectively (Table 3).
The optimum temperature also appeared to increase with ontogeny. While it was estimated to be close to 20 °C for EM to FBA for L. fendleri (Fig. 2A), for the FBA to FL phase it appeared to be greater than 24 °C (Fig. 2B), as the rate of development continued to increase with temperature across the whole experimental temperature range. In L. mendocina, the optimum temperature could not be estimated for the EM to FBA phase. As was previously pointed out, floral buds could not be recorded when plants were subjected to the highest temperature and, for the FBA to FL phase, To was only estimated to be >20 °C for the FBA to FL phase (Fig. 2B).
DISCUSSION
Phenological responses to temperature were different for the two species. Whereas L. fendleri showed linear responses to both sub‐ and supra‐optimal temperatures, L. mendocina plants did not respond to temperature before they had reached the FBA stage. Moreover, at temperatures higher than 20 °C, L. mendocina plants did not proceed to initiate reproduction. Ploschuk et al. (2001) reported that 90 % of L. mendocina plants remained vegetative when grown in a greenhouse at 25°C whereas all plants grown at 13 °C developed normally.
Interestingly, plants of L. mendocina, the native but undomesticated species, exhibited variability in their capacity to reach the reproductive stage at intermediate temperatures (12–20 °C). Although previous experiments indicated little or no vernalization requirement for this species (Windauer, 2002), plants not reaching FBA in the range 12–20 °C could have resulted from a subtle vernalization requirement that was not satisfied in this temperature range. Excluding this approx. 20–40 % of the population from further analysis should not preclude the possibility of analysing responses to temperature in the rest of the population. In any case, this response is different from that observed with temperatures above 20 °C where no L. mendocina plant reached FBA, possibly due to a qualitative response to supra‐optimal temperature. The present results suggest, then, that differences in growth‐habit between the Lesquerella species could be due to their different responses to temperature, especially at relatively high temperatures. This is the first time that different growth habits (i.e. annual and biennial) in two related species has been associated with a different phenological response to temperature.
When the effect of temperature on the rate of development to flowering was analysed, a linear model satisfactorily described the effect of temperatures below 20 °C (the optimum temperature estimated for both species). Within this thermal range, it was clear that sensitivity to temperature in L. fendleri was greater than that of L. mendocina. This is consistent with the finding that the thermal time required for phase completion was higher in L. mendocina than in L. fendleri when plants were sown in autumn–winter (Windauer and Ravetta, 1997; Windauer, 2002). The lower sensitivity to temperature found here in L. mendocina could be (at least partially) responsible for the longer duration of the cycle that this species displayed under field conditions compared with L. fendleri when the environment was supposed to be appropriate to trigger development (late sowings).
When the whole period to flowering (EM–FL) was divided into two phases (EM–FBA and FBA–FL), the rate of development of L. fendleri plants was linearly related to temperature in each phase; but the sensitivity to temperature differed (i.e. the slope of the relationship between rate of development and temperature was higher for the FBA–FL phase than for EM–FBA). Such differential responses to temperature for phases of development have been reported for other species (Hammer et al., 1989; Grimm et al., 1994; Yin et al., 1997). Moreover, both base and optimum temperature increased as development progressed towards flowering in this species, as also found in Brassica (Hodgson, 1978), wheat (Angus et al., 1981; Slafer and Rawson, 1995), rice (Yin et al., 1997), cotton (Roussopoulos et al., 1998), soybeans (Grimm et al., 1994) and Vicia faba (Ellis et al., 1988).
The development of L. mendocina was virtually unaffected by temperature during the EM–FBA phase, as expected in non‐domesticated species, and particularity in biennials or perennials, where other environmental signals might be necessary to trigger reproduction. Floral induction in some perennials is triggered only after a minimum plant size has been reached, and below that size no inductive signals can be effective (Klinkhamer et al., 1987a, b). This mechanism ensures the plant has accumulated enough resources so as to allow for successful reproduction. Therefore any environmental factor (e.g. nutrients, radiation, moisture or extreme temperatures) that affects the growth rate could also affect the time to flowering in these species (Reekie, 1997). In support of this idea, Ploschuk et al. (2001) found that the proportion of L. mendocina plants reaching FBA was lower when plants were exposed to water deficits than when grown in non‐stressed control conditions. Plants of L. mendocina would be induced to flower within the season only when the sowing was sufficiently early so as to allow enough growth to take place before the onset of high temperatures. This hypothesis needs to be thoroughly tested. The different reproductive responses displayed by these two Lesquerella species can hardly be regarded as the result of adaptations to particular features of their site of origin. Indeed, thermal and rainfall regimes in Reno, Arizona (where the L. fendleri population used in this study comes from) and in Lihue Calel, La Pampa, Argentina (where the L. mendocina population comes from) are very similar (Paruelo et al., 1995). Once plants are committed to reproductive development both the annual and the perennial species became highly responsive to temperature, but the relatively lower Tb of L. mendocina compared with that of L. fendleri may be a positive feature for a better performance under the conditions of cold‐arid regions.
The quantification of Tb and the thermal time required for the completion of the different phases in both species should facilitate the planning of sowing dates in order to avoid the coincidence of critical stages with environmental conditions that can limit yield. The information gathered for L. mendocina indicates that early sowings (i.e. April–May for Argentinean conditions) should be chosen if seed production is aimed for the first year. Under such conditions, the crop will presumably have time to accumulate enough biomass before winter and to respond quantitatively to temperature during spring.
ACKNOWLEDGEMENTS
This research was supported by grant PID 1/393 (BID 802‐OC/AR), Secretaría de Ciencia y Tegnología, Argentina. We thank D. Diering for providing the seeds of L. fendleri, Roberto Benech Arnold for thoughtful and useful comments on the manuscript, and Pilar Vilariño for correcting the English. L.B.W. held a UBA postgraduate scholarship. G.A.S. was working at the Universidad de Buenos Aires and CONICET during the experimental growing seasons, and at the ICREA/Universitat de Lleida during the final analysis of the results and the writing of the manuscript.
Received: 6 November 2003; Returned for revision: 24 February 2004; Accepted: 12 March 2004, Published electronically: 14 May 2004
References
- AngusJF, MacKenzie DH, Morton R, Schafer CA.1981. Phasic development in field crops. II. Thermal and photoperiodic responses of spring wheat. Field Crops Research 4: 269–283. [Google Scholar]
- CorreaM.1984.Flora patagónica IV – Dicotiledóneas dialipétalas “Salicaceae a Cruciferae” Buenos Aires: Colección Científica INTA. [Google Scholar]
- DierigDA, Thompson A, Nakayama F.1993.Lesquerella commercialization efforts in the United States. Industrial Crops and Products 1: 289–293. [Google Scholar]
- EllisRH, Roberts E, Summerfield R.1988. Variation in the optimum temperature for rates of seedling emergence and progress towards flowering amongst six genotypes of faba bean (Vicia faba). Annals of Botany 62: 119–126. [Google Scholar]
- Garcia HuidobroJ, Monteith JL, Squire GR.1982. Time, temperature and germination of pearl millet (Pennisetum typhoides S. & H.). 1. Constant temperature. Journal of Experimental Botany 33: 288–296. [Google Scholar]
- GrimmSS, Jones JW, Boote KJ, Herzog DC.1994. Modeling the occurrence of reproductive stages after flowering for four soybean cultivars. Agronomy Journal 86: 31–38. [Google Scholar]
- HammerGL, Goyne PJ, Woodruff DR.1982. Phenology of Sunflowers cultivars III. Models for prediction in field environments. Australian Journal Plant Physiology 33: 263–274. [Google Scholar]
- HammerGL, Vanderlip RL, Gibson G, Wade LJ, Henzell RG, Younger DR, Warren J, Dale AB.1989. Genotype‐by‐environment interaction in grain sorghum. II. Effects of temperature and photoperiod on ontogeny. Crop Science 29: 376–389 [Google Scholar]
- HodgsonAS.1978. Rapeseed adaptation in Northern New South Wales. I. Phenological responses to vernalisation, temperature and photoperiod by annual and biennial cultivars of Brassica campestris L. Brassica napus L. and wheat cv. Timgalen. Australian Journal Plant Physiology 29: 693–710. [Google Scholar]
- KlinkhamerP, de Jones T, Meelis E.1987a. Delay of floration in the biennial Cirsium vulgare Size effects and devernalisation. Oikos 49: 303–308. [Google Scholar]
- KlinkhamerP, de Jones T, Meelis E.1987b. Life history variation and the control of flowering in short‐lived monocarps. Oikos 49: 309–314. [Google Scholar]
- MonteithJL.1984. Consistency and convenience in the choice of units for agricultural science. Experimental Agriculture 20: 125–137. [Google Scholar]
- ParueloJM, Lauenroth WK, Epstein HE, Burke IC, Aguiar MR, Sala OE.1995. Regional climatic similarities in the temperate zones of North and South America. Journal of Biogeography 22: 915–925. [Google Scholar]
- PloschukEL, Windauer L, Ravetta DA.2001. Potential value of traits associated with perennial habit in the development of new oil‐seed crops for arid lands. A comparison of Lesquerella fendleri and L. mendocina subjected to water stress. Journal of Arid Environments 47: 373–386. [Google Scholar]
- PloschukEL, Cerdeiras G, Windauer L, Dierig DA, Ravetta, DA.2003. Development of alternative Lesquerella species in Patagonia (Argentina): potential of L. angustifolia Industrial Crops and Products 18: 1–6. [Google Scholar]
- ReekieE,1997. Trade‐off between reproduction and growth. Influence time of reproduction. In: Bazzaz, FA, Grace J, eds. Plant resource allocation San Diego, CA: Academic Press, 191–209. [Google Scholar]
- RichardsRA,1991. Crop improvement for temperate Australia: future opportunities. Field Crops Research 26: 141–169. [Google Scholar]
- RitchieJT, NeSmith DS.1991. Temperature of crop development. In Hanks J, Richie, JT, eds. Modeling plant and soil systems Madison, WI: American Society of Agronomy, 5–25. [Google Scholar]
- RoetheliJ, Carlson K, Kleiman R, Thompson A, Diering DA.1991.Lesquerella as a source of hydroxy fatty acids for industrial products. Washington DC: USDA–CSRS Office of Agricultural Materials Growing Industrial Series (unnm), 46. [Google Scholar]
- RoussopoulosA, Liakatas A, Whittington WJ.1998. Controlled‐temperature effects on cotton growth and development. Journal of Agricultural Science 130: 451–462. [Google Scholar]
- SlaferGA, Rawson HM.1995. Base and optimum temperatures vary with genotype and stage of development in wheat. Plant, Cell and Environment 18: 671–679. [Google Scholar]
- TaizL, Zeiger E.2003. Photosynthesis: physiological and ecological considerations. In: Taiz L, Zeiger E, eds. Plant physiology Sunderland, MA: Sinauer Associates, Inc, 227–249. [Google Scholar]
- ThompsonAE.1990. Arid‐land industrial crops. In: Janick J, Simon JE, eds. Advances in new crops Portland, OR: Timber Press, 232–241. [Google Scholar]
- WindauerL, Ravetta DA.1997. Influencia de algunos factores ambientales sobre la duración de la etapa emergencia‐ floración en L. fendleri In: Murphy G, ed. Proceedings of 7aReunión Argentina y 1 aLatinoamericana de Agrometereología, Buenos Aires, 41–42. [Google Scholar]
- WindauerL.2002.Puesta en cultivo de especies del género Lesquerella: Influencia de factores ambientales sobre el desarrollo. Thesis Magíster Scientiae, Facultad de Agronomía, Universidad de Buenos Aires, Argentina. [Google Scholar]
- YinX, Kropff M, Goudriaan J.1997. Changes in temperature sensitivity of development from sowing to flowering in rice. Crop Science 37: 1787–1794. [Google Scholar]
- ZavalaJ, Ravetta DA.2000. The effect of irrigation regime on biomass and resin production in Grindelia chiloensis Field Crops Research 9: 227–236. [Google Scholar]