Abstract
• Background and Aims Carbon gain depends on efficient photosynthesis and adequate respiration. The effect of temperature on photosynthetic efficiency is well understood. In contrast, the temperature response of respiration is based almost entirely on short‐term (hours) measurements in mature organisms to develop Q10 values for maintenance and whole‐plant respiration. These Q10 values are then used to extrapolate across whole life cycles to predict the influence of temperature on plant growth.
• Methods In this study, night temperature in young, rapidly growing plant communities was altered from 17 to 34 °C for up to 20 d. Day temperature was maintained at 25 °C. CO2 gas‐exchange was continuously monitored in ten separate chambers to quantify the effect of night‐temperature on respiration, photosynthesis and the efficiency of carbon gain (carbon use efficiency).
• Key Results Respiration increased only 20–46 % for each 10 °C rise in temperature (total respiratory Q10 of between 1·2 to about 1·5). This change resulted in only a 2–12 % change in carbon use efficiency, and there was no effect on cumulative carbon gain or dry mass. No acclimation of respiration was observed after 20 d of treatment.
• Conclusions These findings indicate that whole‐plant respiration of rapidly growing plants has a small sensitivity to temperature, and that the sensitivity does not change among the species tested, even after 20 d of treatment. Finally, the results support respiration models that separate respiration into growth and maintenance components.
Key words: Latuca sativa, Lycopersicum esculentum, Glycinemax, carbon use efficiency, R : P ratio, whole‐canopy CO2 gas‐exchange, Q10, respiration, night temperature, photosynthesis, growth and maintenance respiration
INTRODUCTION
There is a consensus that average global temperatures have warmed significantly in the last 100 years, and that this trend will continue, raising average global temperatures by 1–6 °C in the next 100 years (Sarmiento and Quere, 1996; Hansen et al., 1999). Much of this increase is the result of night temperatures rising faster than day temperatures due to less radiant heat loss because of increased cloudiness (Alward et al., 1999). It is therefore important to understand the influence of both day and night‐time warming on the carbon balance of plants, and thus on the carbon balance of the planet.
Plants have evolved in an environment that has cooler nights than days, but the impact of cool nights on carbon gain is not clear. In single leaves of cottonwood trees (Populus deltoides), the maximum potential photosynthetic rate increased for single, mid‐canopy‐level leaves after a single warmer night, which was attributed to less carbohydrate‐induced feedback inhibition of photosynthesis (Turnbull et al., 2002). McCree and Amthor (1982) found that a constant 20 °C day/night temperature slightly increased growth rate of a community of white clover (Trifolium repens) when compared to a community grown in 30 °C days and 10 °C nights. They suggested that the warm day temperature caused excess substrate use, while night respiration rates were not low enough to offset the daytime carbon loss.
Unfortunately, many conclusions about growth and respiration are based on measurements of single leaves, leaf disks or mature plant parts (Crawford and Huxter 1977; Labate and Leegood 1989; Bunce 1991; Roberts et al. 1992; Alexander et al., 1995; Maier et al., 1998). However, leaves typically have a photosynthetic rate that is 10–20 times their respiration rate (Björkman, 1981), and the photosynthetic rate of whole plants is four times their respiration rate (Gifford, 1994). If leaf mass is 30–70 % of total plant mass, 70–85 % of total respiration occurs in roots, stems and meristems. Therefore, even if a leaf was selected that represented the rest of the leaves, respiration rates derived from this leaf would poorly represent the whole plant.
Rarely, whole plants are measured, but even then the respiration measurements are done for only a few hours (Tjoelker et al., 1999). Teskey and Will (1999) and Percival et al. (1996) observed a lower temperature sensitivity for whole plants than for leaves, but Griffin et al. (2002) argue that temperature sensitivity should increase in whole plants because they would be less carbohydrate‐limited than single leaves during measurement at warm temperatures.
Classically, a functional model of respiration assigns the energy derived in respiration to a ‘growth’ component and a ‘maintenance’ component. While this model is only a functional model and the biochemistry to derive the energy in respiration for these components is the same, this model still provides one of the best ways to explain and model respiration. The theory behind this model has been supported in recent reviews (Amthor, 2000; Cannell and Thornley, 2000; Thornley and Cannell, 2000). The growth respiration coefficient (or efficiency of biosynthesis) has long been considered temperature insensitive (Penning de Vries et al., 1974), and maintenance respiration is considered temperature dependent (McCree, 1974). This is not to say that growth respiration is temperature insensitive; growth may increase with temperature and, as a result, growth respiration may increase. However, the cost of that growth is believed to remain constant, whereas the cost of the maintenance changes with temperature.
Short‐term studies on mature plant tissues typically indicate a respiratory Q10 of at least 2, which indicates that respiration doubles for every 10 °C rise in temperature (Burton et al., 1996; Bustan and Goldschmidt, 1998; Lomander et al., 1998). Ecological models and eco‐physiological models assign the temperature‐sensitive component only to the maintenance (or analogous) term, so that the total respiratory Q10 is less than 2·0, but there is a lack of whole‐plant measurements performed in long‐term studies to validate these ecological models. Still, reported Q10 values range from 1·2 to 4·0, and no mechanistic explanation for the variance is demonstrated (Larigauderie and Körner 1995; Boone et al., 1998; Chapman and Thurlow, 1998; Neven, 1998; Urmeneta et al., 1998; Nielsen et al., 1999; Quinlan and Lighton, 1999). Further confusing matters are researchers ‘correcting’ for temperature differences in their studies by assuming a Q10 of 2 for total respiration to calculate a basal respiration rate at a common temperature (Pearman et al., 1981; Wullschleger et al., 1992; Wullschleger and Norby, 1992; Witowski, 1997), which can mislead overall conclusions about the role of temperature on respiration.
Amthor (2000) suggested that low temperature sensitivity in long‐term measurements may be due to acclimation. Low Q10 values might be evidence for acclimation (Arnone and Körner, 1997). There is, however, great species variability in how much acclimation occurs. Larigauderie and Körner (1995) found that some species acclimated to some degree (long‐term acclimation ratio, LTR10 = 1·0–1·5), but others had LTR10 of between 2 to 5·5 indicating little to no acclimation. Root respiration was observed to acclimate based on the night temperature alone rather than daily mean or daytime high temperatures (Covey‐Crump et al., 2002), which makes studies of the influence of night temperature on whole‐plant respiration critical.
In addition to measuring respiration rates, it is important to measure whole‐plant respiratory efficiency and carbon conservation. A widely used parameter for this is the ratio of net carbon gain in a 24 h period to the total carbon fixed during the light period (Amthor, 1989). This ratio, called carbon use efficiency (CUE), is a measure of the efficiency of incorporation of fixed carbon into new biomass.
In this study, both the short‐term (days) and long‐term (weeks) effects of night temperature on respiration, net photosynthesis and CUE of plant communities were examined. We hypothesized that changing night temperature would exert a strong influence on night respiration and that change would result in differences in photosynthetic rates on the following day (Turnbull et al., 2002). Furthermore, we hypothesized that altered night respiration would change CUE, but that both respiration and CUE would acclimate to their pre‐treatment levels in a few days.
MATERIALS AND METHODS
Experimental set‐up and design
Three studies were conducted to compare night‐temperature effects across three crops: lettuce (Latuca sativa ‘Grand Rapids’), tomato (Lycopersicum esculentum ‘Red Robin’) and soybean (Glycine max ‘Hoyt’). These species were chosen to compare the temperature sensitivity of two starch accumulators (soybean and tomato) to a sucrose accumulator (lettuce). Seedlings were transplanted 4–7 d after imbibition into a ten‐chamber, computer‐controlled gas‐exchange system (Fig. 1). Temperature within each chamber was controlled with a chilled water coil and small heaters. System details have been described previously (van Iersel and Bugbee, 2000).
Fig. 1. The ten‐chamber gas‐exchange system. There are five chambers on each side of a walk‐in growth chamber. Each chamber has a reflective skirt wrapped around the outside to minimize side lighting.
Often, CO2 gas exchange systems are difficult to calibrate and are usually only tested with no plant material in a chamber. They are usually zeroed before measurement, ensuring that the gas analyser to be used has been properly zeroed and spanned. We zeroed each chamber, calibrated the gas analysers, and finally calibrated the entire system by slowly reacting dilute acid with a known mass of dried CaCO3. This caused CO2 to be evolved, which was measured with the gas analysers. The total moles of CO2 evolved was then compared to the initial moles of C in the CaCO3. This was repeated until the molar amount of CO2 evolved from the reaction equalled that which was initially placed into the chamber (100 ± 2 %) by repairing leaks in the system.
Lettuce and tomato were grown at a constant 25 °C, and soybeans were grown at a constant 20 °C until canopy closure. Daytime temperatures remained at these temperatures for the duration of the trial. The night temperatures were changed on Day 16 for lettuce and tomato and Day 17 for soybean. On that day (Day 1 after treatment on Fig. 3; there is no treatment Day 0), the night temperature set points were adjusted across a 17 °C range from 17 to 34 °C. Temperature treatments were maintained for 13 d until harvest on Day 29 for lettuce, for 20 d until Day 36 for tomato, and for 15 d until Day 34 for soybeans. Treatments extended into flowering for tomato and soybeans. Control plants had constant day/night temperature. Temperatures were measured with an aspirated, type‐E (0·5 mm diameter, 24‐AWG) thermocouple, and were maintained within ±0·2 °C of the set point. Groups of whole plants or plant communities were studied and arranged at the following densities: lettuce at 106 plants m–2, tomato at 80 plants m–2, and soybean at 35 plants m–2.
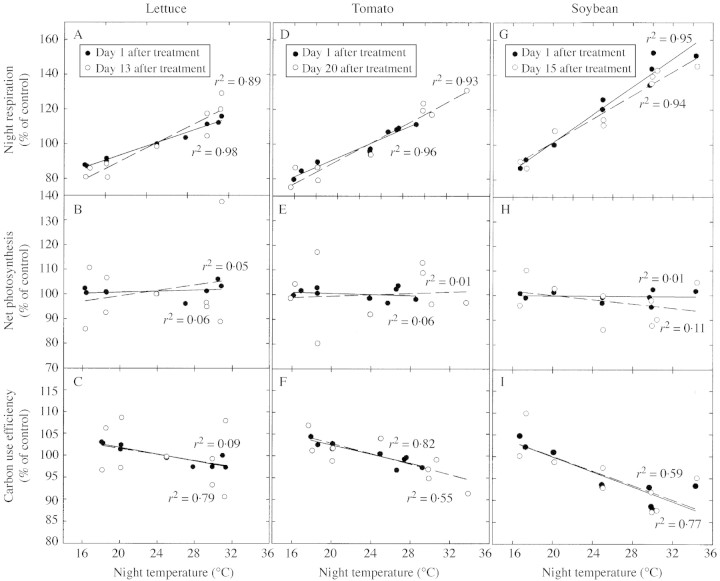
Fig. 3. Effect of night temperature on night respiration, photosynthesis and carbon use efficiency for lettuce (A, B, C), tomato (D, E, F), and soybean (G, H, I). Data are shown relative to the control for the first and last day of treatment. The slopes between the first and last treatment day are not statistically different from one another within a species and parameter (lowest P value = 0·29, d.f. = 8), which indicates that there was no acclimation to temperature.
Relative humidity was maintained between 60 and 80 %, but varied much less among the chambers for any given day because the daytime environments were similar (data not shown). A photosynthetic photon flux (PPF) of 600 µmol m–2 s–1 (±5 % among chambers) was provided by water‐filtered, high‐pressure sodium lamps. The photoperiod was 16 h for lettuce and tomato, and 12 h for soybeans. Reflective material was wrapped around each chamber and was adjusted daily to the top of the canopy to minimize side lighting (Fig. 1). CO2 was controlled at 1200 µmol mol–1 for the duration of all the studies. These studies were conducted at elevated CO2 for three reasons: (1) to increase photosynthesis and thus help ensure that the plants did not become carbohydrate‐limited at the higher temperatures; (2) to minimize the effect that vapour pressure deficit differences would have on photosynthesis if there were differences between chambers on a given day; and (3) to minimize possible temperature effects on photorespiration. Temperature responses most likely would be smaller if the plants were carbohydrate‐limited by ambient CO2. Separate hydroponic systems were enclosed in each chamber so that root respiration was included with the shoot. Hydroponic solution was bubbled with the same air as that used for the shoot environment.
Manual adjustment of pH on a daily basis resulted in a one pH unit day‐to‐day range. The pKa of carbonate is 6·2, which means that 50 % of the carbon dissolved in the water is in the carbonate form and 50 % is CO2. pH changes around pH 6 cause significant fluxes in and out of the nutrient solution. For this reason, the pH of the hydroponic solution was maintained between 4 and 5, which forces between 90 and 99 % of the CO2 out of solution. Previous studies indicate that healthy plants can be grown in pH of 4·0 (Monje and Bugbee, 1998).
Plant tissue analysis
Upon harvesting, plants were separated into shoots and roots (lettuce and tomato), or leaves, stems and roots (soybean). Tissue was weighed and dried at 80 °C for 72 h. Dry biomass was subsequently weighed, ground and sampled for analysis. Samples weighing 0·2 g were analysed for percent C, H and N with a LECO analyser (Model 1000; LECO Corporation, St Joseph, MI, USA) and samples of 1·0 g were used for analysis of other nutrients (ICP‐ES analysis, Utah State University Plant and Soil Analysis Laboratory). Nitrate was analysed with a 0·2 g sample placed in a 50 mL solution of 0·05 M Al2(SO4)3. The tissue and solution were shaken four times during the 1 h extraction period. The solution was measured with a NO3– selective electrode and an associated reference electrode (Models 930700 and 900200; Thermo Orion, Beverly, MA, USA). The readings were then converted from volts to NO3–‐N from a previous calibration curve. Assimilated N was calculated by subtracting NO3–‐N from total N.
Calculations
Carbon use efficiency (CUE) is a calculated term that measures how efficiently plants can incorporate the carbon fixed during the day into biomass gain. It is used instead of ‘percent respiration’ (100 × night respiration/net photosynthesis) because it incorporates differences in photoperiod automatically, whereas calculating a ratio of night respiration to net photosynthesis does not. While meaningful conclusions about a given species in a set of environmental conditions can be made, a ratio of night respiration to net photosynthesis precludes useful comparisons to be made across species and environmental conditions. Finally, CUE it is an efficiency parameter that can easily fit within models. Using Pnet (net photosynthesis, mol C m–2 d–1 based on ground area) and Rn (night‐time respiration, mol C m–2 night–1 based on ground area), daily carbon gain (DCG) can be calculated as:
DCG = Pnet – Rn
Cumulative carbon gain (CCG) is the running total of DCG.
Pgross is a calculated term that reflects the net C fixed (Pnet) and the amount of C that is simultaneously being respired. Because daytime respiration (Rd) can not be measured directly, Pgross is expressed as the sum of Pnet and some percentage of Rn. Some studies have indicated that Rd in leaves can be higher during the day due to their higher daytime carbohydrate content (Azcón‐Bieto et al., 1983; Azcón‐Bieto and Osmond, 1983). Other studies indicate daytime Rd is lower due to light‐inhibition of respiration (Sharp et al., 1984; Wang et al., 2001). Monje and Bugbee (1996) found that root respiration, at a constant temperature, is increased in the day presumably due to increased carbohydrate supply. The common approach for whole plants is to assume that the rate of Rd and Rn (µmol m–2 s–1 based on ground area) are equal when temperatures are constant. In a 12 h photoperiod, Rd (mol C m–2 d–1, ground area) then equals Rn. In a 16 h light/8 h dark photoperiod, Rd = Rn × 2. In these equations, respiration assumes a positive value (i.e. mass respired). Pgross (mol C m–2 d–1, ground area) can, therefore, be calculated as:
Pgross = Pnet + Rd
Carbon use efficiency can then be calculated as:
CUE = DCG/Pgross
Plants were grown in constant day/night temperatures until treatments were applied.
To compare our temperature sensitivities to the literature and to calculate the Rd from Rn to determine Pgross, Q10 values were calculated for the first treatment night. The Rn of plants in different temperatures was used to calculate two Q10 values (one from control temperature to coolest temperature and one from the control temperature to the warmest temperature) using the temperature response function:
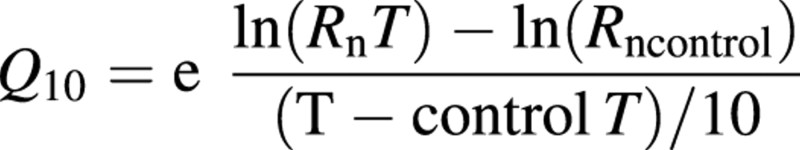
where RnT is the night respiration at temperature T and Rncontrol is the night respiration at the control temperature. An alternative method of Q10 calculation would be to calculate the slope of a respiration‐to‐temperature relationship. This would enable calculation of Q10 values at each temperature rather than rely on a reference temperature as in the first method, which could lead to different results if the control temperature is different. In order to obtain different Q10 values for different temperatures, a non‐linear relationship between respiration and temperature must exist. In our data, however, only linear relationships were statistically significant, based on linear regression ANOVA, so there was only a single Q10 value for each crop using this method. This value is in the middle of the range for Q10 values reported for this study in Table 1.
Table 1.
Final dry mass (g m–2 ground area), cumulative carbon gain (CCG) after treatment initiation, and respiration sensitivity to temperature, as measured by Q10 for each species
| Crop | Night temperature (C) | Final plant mass (g m–2) | CCG after treatment initiation (mol C m–2) | Sensitivity to temperature (Q10) |
| Lettuce | 18·2 | 476·8 | 13·8 | 1·22 to 1·19 |
| 20 | 466·7 | 14·3 | ||
| 25 | 483·7 | 13·5 | ||
| 30 | 493·0 | 13·9 | ||
| 31·3 | 490·5 | 13·8 | ||
| Tomato | 18 | 768·3 | 22·8 | 1·38 to 1·34 |
| 20 | 730·8 | 20·8 | ||
| 25 | 740·0 | 21·8 | ||
| 30 | 784·1 | 23·0 | ||
| 31·5 | 751·6 | 22·4 | ||
| Soybean | 17 | 901·0 | 22·8 | 1·61 to 1·40 |
| 20 | 881·3 | 23·2 | ||
| 25 | 927·5 | 23·0 | ||
| 30 | 842·8 | 23·6 | ||
| 32 | 879·8 | 23·1 |
Data are the average of two groups of plants in separate chambers. Q10 was higher at cooler temperatures and vice versa. There was no effect of temperature on final dry mass in any species (P > 0·32), nor on CCG after treatment initiation (P > 0·59).
Data analysis
Treatment effects were expressed as a percent of their initial value, then normalized to the control in the following manner:
[(post‐treatmenta dayb/pre‐treatmenta value)/(post‐treatmentcontrol dayb/pre‐treatment valuecontrol)] × 100
where ‘post‐treatmenta’ indicates the post‐treatment value of a parameter (i.e. CUE, Pnet, Rn) on ‘dayb’, ‘pre‐treatmenta value’ is the value of the parameter on the day before the night temperature was changed, ‘post‐treatmentcontrol dayb’ is the post‐treatment value of the parameter for the control on dayb, and ‘pre‐treatment valuecontrol’ is the pre‐treatment value of the parameter for the control the day before treatments began. This data treatment results in determining the temperature effects through time relative to the treatment plants in the numerator, and the effects through time relative to the control in the denominator.
Normalized data were analysed with linear regression to determine the night temperature effects on each parameter for each day after treatment began. Slopes of lines on different days were compared to see if their slopes were equal using the test statistic [(slope a – slope b)/(variance of slope a) = t(d.f. of slope a) (Neter et al., 1996). However, in the figures below, the Y‐intercept was adjusted so that the regression lines at the control point passed through 100 % on the Y‐axis in order to facilitate visual comparisons for the Results and Discussion.
RESULTS
Lettuce
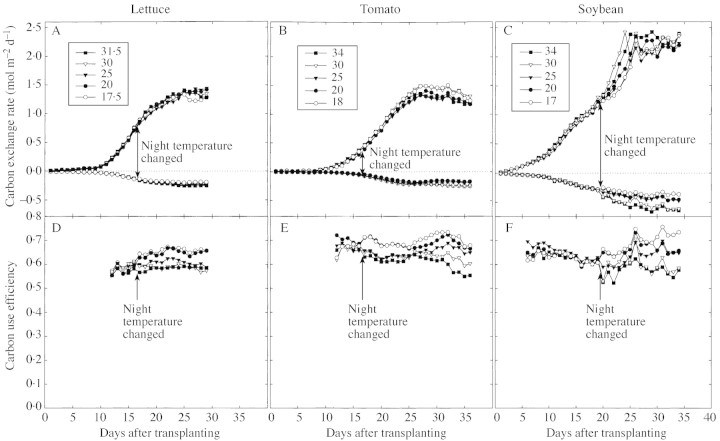
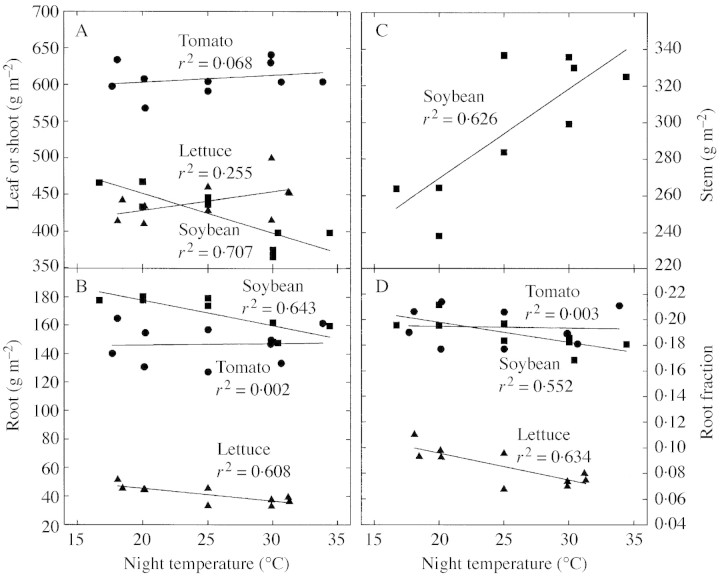
Night respiration increased 2·0 % per °C (Figs 2A, 3A). After 13 d of treatment, the slopes did not differ significantly from one another, indicating no acclimation to temperature. The average CUE was 0·62 the day before treatments were imposed (Fig. 2D). Net photosynthesis was not sensitive to night temperature (Fig. 3B). The CUE values for the coolest night temperatures were about 2 % higher than the control values and the warmest were about 2 % lower than the control values for all days after treatments were applied (Fig. 3C). The sensitivity of CUE to temperature did not change during the 13 d of treatment based on comparison of slopes (P = 0·35, d.f. = 8). There was no difference in final dry mass or in CCG after treatments were imposed (Table 1). Lettuce shoot mass increased significantly with temperature (Fig. 4A), while root biomass decreased (Fig. 4B). This caused the root fraction to decrease significantly with warmer temperatures (Fig. 4D). Leaves were not separated from the stem. Nitrate in both shoots and roots increased with increasing temperature (Table 2). There was no effect of night temperature on C, assimilated N, or K fractions in the shoot or root (Table 2).
Fig. 2. Net photosynthesis (daytime carbon exchange), night respiration (night respiration), and carbon use efficiency [(daily carbon gain)/(gross photosynthesis)] for lettuce (A and D), tomato (B and E), and soybean (C and F). Data points are the average of two chambers for a given temperature. Carbon exchange rates are per m2 of ground area. Temperature treatments were initiated on day 17 for lettuce and tomato, and day 18 for soybean.
Fig. 4. Effect of night temperature on shoot (A), root (B), and stem (C) mass, and root fraction (D). Lettuce shoot mass increased significantly with temperature, but soybean leaf mass decreased with temperature. Lettuce and soybean root mass decreased significantly with temperature, and soybean stem mass increased with temperature, possibly because height increased with temperature. These changes caused the root fraction to decrease with temperature in both lettuce and soybean. Night temperature did not influence any of the tomato variables.
Table 2.
Carbon, nitrate, assimilated N and K+ for the three species in the different temperatures
| C (%) | Nitrate (%) | Assimilated N (%) | K+ (%) | ||||||||||
| Crop | Night temperature (°C) | Shoot | Root | Stem | Shoot | Root | Stem | Shoot | Root | Stem | Shoot | Root | Stem |
| Lettuce | 18·2 | 40·2 | 39·8 | – | 0·55 | 0·10 | – | 1·8 | 2·6 | – | 5·7 | 2·9 | – |
| 20. | 38·6 | 41·4 | – | 0·77 | 0·09 | – | 2·4 | 2·4 | – | 4·8 | 2·3 | – | |
| 25. | 38·2 | 40·8 | – | 0·95 | 0·22 | – | 2·3 | 2·5 | – | 5·0 | 2·9 | – | |
| 30. | 38·3 | 41·0 | – | 0·66 | 0·20 | – | 2·5 | 2·6 | – | 5·2 | 2·9 | – | |
| 31·3 | 39·6 | 40·2 | – | 0·67 | 0·43 | – | 2·5 | 2·4 | – | 4·9 | 3·7 | – | |
| P value | 0·643 | 0·927 | NA | 0·029* | 0·019 | NA | 0·132 | 0·788 | NA | 0·458 | 0·142 | NA | |
| Tomato | 18. | 37·0 | 40·7 | – | 0·45 | 0·10 | – | 2·1 | 2·3 | – | 4·4 | 4·7 | – |
| 20. | 37·8 | 39·8 | – | 0·36 | 0·07 | – | 1·9 | 2·2 | – | 4·6 | 4·4 | – | |
| 25. | 37·6 | 39·3 | – | 0·38 | 0·09 | – | 2·0 | 2·1 | – | 4·6 | 4·4 | – | |
| 30. | 36·6 | 40·5 | – | 0·26 | 0·11 | – | 1·9 | 2·1 | – | 3·9 | 4·4 | – | |
| 31·5 | 36·7 | 40·5 | – | 0·17 | 0·12 | – | 2·2 | 2·1 | – | 5·3 | 3·3 | – | |
| P value | 0·942 | 0·795 | NA | 0·006 | 0·529 | NA | 0·841 | 0·014 | NA | 0·658 | 0·045 | NA | |
| Soybean | 17. | – | – | 40·2 | – | – | 0·14 | – | – | 1·1 | – | – | 2·4 |
| 20. | 41·1 | – | – | 0·22 | – | – | 3·2 | – | – | 2·4 | – | – | |
| 25. | 40·9 | 37·8 | 38·5 | 0·25 | 0·27 | 0·37 | 2·7 | 2·0 | 1·8 | 1·9 | 6·6 | 3·5 | |
| 30. | 39·9 | 38·1 | 40·1 | 0·40 | 0·27 | 0·27 | 3·1 | 2·2 | 1·3 | 2·6 | 5·5 | 3·0 | |
| P value | 0·049 | 0·878 | 0·577 | 0·03 | 0·875 | 0·340 | 0·566 | 0·478 | 0·455 | 0·712 | 0·511 | 0·404 | |
Some of the samples for soybean were lost. Assimilated N is calculated by subtracting NO3– from total N.
* indicates a quadratic fit. NA = not applicable.
Tomato
Night respiration increased 2·7 % per °C with warmer nights (Figs 2B and 3D). This caused the CUE to be slightly higher with cooler nights and lower with warmer nights (Figs 2E and 3F). After 20 d of temperature treatment, there was no difference in the sensitivity of CUE or respiration to altered night temperature (P = 0·48, d.f. = 8). Net photosynthesis was not affected by the altered night temperatures (Fig. 3E). There were no differences in the final dry mass of the treatments or in CCG after treatments began (Table 1). There was no effect of temperature on biomass partitioning for tomato (Fig. 4A–D). Leaves were not separated from the stem. There was no effect of night temperature on C and root NO3–, but NO3– decreased significantly in the shoot with increasing temperature (Table 2). There was a significant effect of temperature on K in the root (P = 0·045), but not in the shoot (Table 2). There was a small, but statistically significant effect of temperature on assimilated N in the roots.
Soybean
Soybean was the most sensitive of the three crops studied. Respiration increased 4·0 % per °C (Figs 2C and 3G). Carbon use efficiency changed after the night temperatures were changed because respiration changed (Figs 2F, 3I, G). With no change in photosynthesis (Fig. 3H), the decrease in respiration resulted in an increase of CUE of 3 % relative to the control and a decrease of 12 % relative to the control at the highest temperature (Fig. 3G). No acclimation occurred after 15 d of treatment (P = 0·29, d.f. = 8) (Fig. 3G). No differences in final dry mass were observed between treatments or in the CCG after treatments began (Table 1). Soybean leaf mass decreased significantly with temperature (Fig. 4A), while root biomass decreased (Fig. 4B). Stem biomass increased significantly by the same absolute amount as the leaves decreased (Fig. 4C). Together, these shifts caused the root fraction to decrease significantly with warmer temperatures (Fig. 4D). There was a significant effect of night temperature on C fraction of the leaves (P = 0·049), but not on stems or root (Table 2). Nitrate increased with temperature in shoots (Table 2). There was no effect of night temperature on assimilated N or K fractions in the shoot or root.
Differential temperature effects among species
The effect of temperature on respiration and CUE differed among species (Fig. 5A, B). Soybean respiration was significantly more sensitive than lettuce (t = 6·34, d.f. = 8, P < 0·001), and had more CUE sensitivity than lettuce (t = 2·91, d.f. = 8, P < 0·025), but all three species were much less sensitive than commonly believed. Lettuce respiration was significantly less sensitive than tomato (t = 3·418, d.f. = 8, P < 0·025) (Fig. 5A, 5B), and CUE was marginally significantly different (t = 1·64, d.f. = 8, P ≈ 0·08). The average Q10 for whole‐plant respiration of lettuce was 1·20, tomato was 1·36, and soybean was 1·46, with lower Q10 values for warmer temperatures (Table 1).
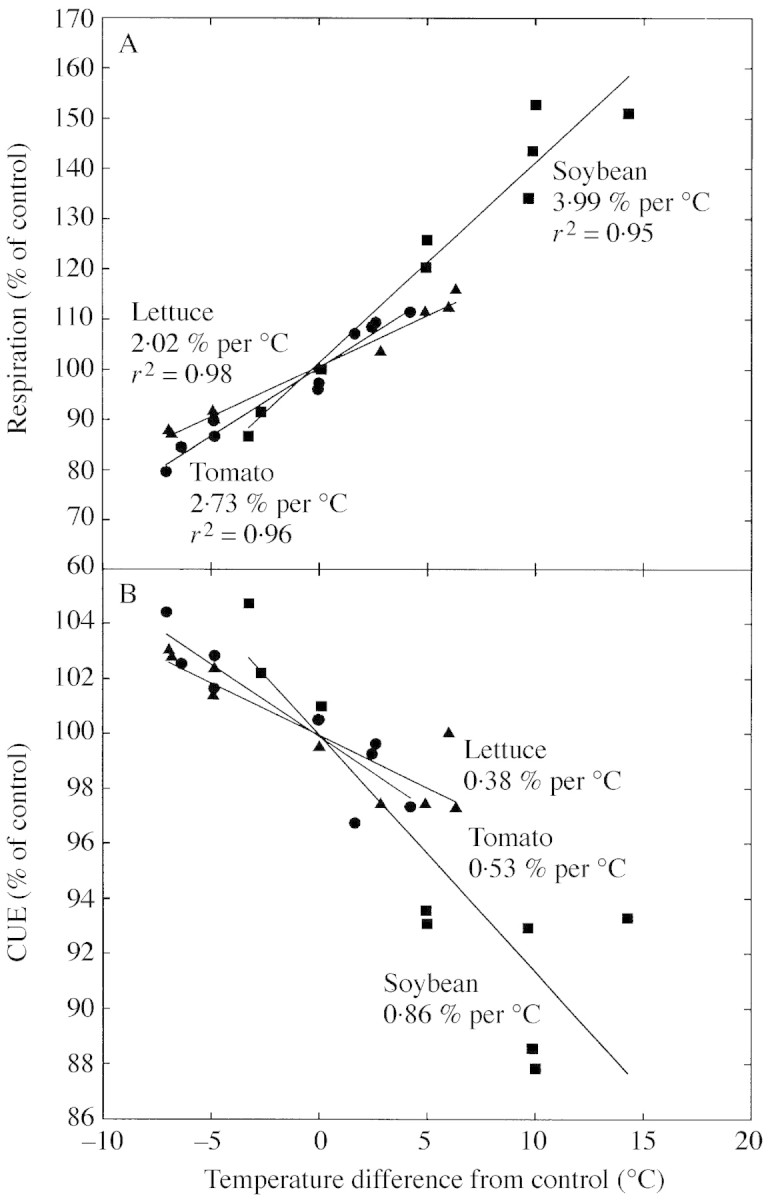
Fig. 5. Effect of night temperature on respiration (A) and carbon use efficiency (B) in lettuce, tomato and soybean relative to the control. Soybean respiration was significantly more temperature sensitive than tomato (t = 4·05, d.f. = 8, P < 0·005), and tomato respiration is significantly more sensitive than lettuce (t = 3·418, d.f. = 8, P < 0·025). Soybean CUE was significantly more sensitive to temperature than tomato (t = 2·04, d.f. = 8, P < 0·05), and tomato CUE was marginally more sensitive to temperature than lettuce (t = 1·64, d.f. = 8, P ≈ 0·08). Data are normalized to the control temperature (25 °C in lettuce and tomato, and 20 °C in soybean).
DISCUSSION
Current paradigm: respiration is highly sensitive to temperature
Most formal models do an excellent job of separating growth and maintenance respiration and assigning the temperature‐sensitive portion only to the maintenance fraction so that the net result is a Q10 for total respiration much less than 2. This is not to suggest that growth is not temperature sensitive; growth rates often increase with temperature. Assume the following conditions: total respiration is 10 µmol m–2 s–1; growth respiration accounts for 80 % of total (8 µmol m–2 s–1); maintenance respiration has a Q10 of 2·0; and growth increases 10 % with a 10 °C rise in temperature. If the temperature increases 10 °C, total respiration would only be 12·8 µmol m–2 s–1 for a Q10 for total respiration of only 1·28. Conversely, if growth respiration only accounts for 20 % of total respiration, a Q10 for total respiration of 1·82 would be predicted. This scenario is predicted by some current models, but there are no long‐term studies with rapidly growing plant communities to validate these models. Unfortunately, studies on the temperature sensitivity of respiration rarely divide respiration into its component parts, and often assume a Q10 of about 2 that is derived from short‐term studies on mature plant parts to adjust measured respiration values back to a reference temperature (Ruter and Ingram, 1991; Witowski, 1997).
Some researchers have speculated that the range in Q10 values is the result of acclimation to temperature, or simply a result of measurement temperature (Tjoelker et al., 2001). However, much of the range in values may be caused by differences in plant growth rate. Studies that report low values for Q10 have often been done with growing organisms. When environmental conditions were conducive to rapid growth rates, Percival et al. (1996) showed that Q10 values for respiration were very low (1·1–1·5). Urmeneta et al. (1998) also showed that rapidly growing photosynthetic algae had low Q10 values (1·4–1·6). Perhaps the most convincing study to show an inverse relationship with growth rate and Q10 values is from Ogawa and Takano (1997) as they tracked respiration sensitivity to temperature over the course of a growing season from mid‐July through to December. In this study, Q10 increased from about 1·6 in July (high growth) up to 2·7 in December.
Loveys et al. (2003) investigated the influence of relative growth rate on respiration rates by looking at a range of species with inherently high or low growth rates. They found no correlation between those species’ growth rates and single leaf and detached root‐system respiration for those plants. Poorter et al. (1991) found strong relationships between inherent growth rates and respiration rates in roots. In that study, the authors separated respiration into growth, maintenance and ion uptake, and attributed the apparent differences in respiratory requirements to ion uptake of the different species.
Neven (1998) suggests that it is not the growth rate of the plant but the measurement techniques that determine, at least in part, the apparent temperature sensitivity of respiration. He states that stress on metabolism from rapid heating or cooling will increase the apparent Q10 of respiration, whereas constant temperature models are poor estimators of variable temperature profiles because they lack the same stress. So by the nature of the measurement, short‐term temperature ramping of tissue may over‐estimate Q10 through stress on the respiratory system, and constant temperature systems, as in our study, may underestimate the sensitivity because temperature stress to the respiratory system is low. This is analogous to differences in temperature sensitivity of respiration observed in plant material developed in a variable environment compared to that developed in a stable environment and switched to variable environments (see discussion in Atkin and Tjoelker, 2003). Plants exposed to variable environments have the capacity to deal with temperature stress more than plants developed in constant environments.
Current paradigm: respiration and CUE acclimate to temperature changes
Although both respiration and CUE were influenced by temperature, neither returned to pre‐treatment levels after treatments began. Several studies have reported respiratory acclimation or adaptation to changes in temperature, and some back to pre‐treatment levels. For example, Illeperuma et al. (1998) reported a sharp rise in respiration of harvested potato tubers upon transfer from 1 to 10 °C, but after about 7 d, respiration begins to decline. Bryla et al. (1997) showed that citrus root respiration increased sharply upon exposure to warm temperatures but completely returned to previous rates after only 4 d. Gifford (1995) found that CUE returned to the pre‐treatment level within 4 d. The initial change in CUE in our study was similar to Gifford (1995), but he altered both day and night temperature and grew his plants in ambient CO2, so changes in photorespiration may have complicated the results.
Based on short‐term measurements of detached plants, Tjoelker et al. (1999) concluded that a lower Q10 than that obtained from short‐term measurements indicated acclimation to temperature. However, the temperature sensitivity in our study did not change during the entire treatment period (up to 20 d), so all of the acclimation would have had to occur during the first day. This seems unlikely.
Another possibility is that the low Q10 was due to substrate limitation; if there is no substrate to respire, then respiration can not increase with temperature. These plants were grown in 1200 µmol CO2 mol–1 with relatively high light (34·5 mol photons m–2 d–1). Previous studies done in similar environments indicated that these conditions provide an ample carbohydrate supply (Smart et al., 1994). The two starch‐accumulating species (tomato and soybean) were slightly more temperature sensitive than the sucrose‐accumulating species (lettuce). If anything, the plants in this study should have been more sensitive to temperature than plants grown at ambient CO2. Loveys et al. (2003) report a negative correlation between substrate content and respiration rate, so clearly substrate availability is not the only determining factor for driving respiration.
It is unlikely that decreases in daytime respiration compensated for increases in night respiration. For example, the night respiration of soybean increased from 1·0 to 1·4 mol C m–2 night–1 when the night temperature was increased from 20 to 30 °C. The daytime respiration would need to decrease by 75 % for CUE to remain the same. There was no effect of temperature on Pnet so this should result in a 12 % decrease in Pgross. Also, this type of change would indicate an uncoupling of temperature and respiration during the day as well as a decrease in respiration with an increase in carbohydrate supply, which is highly unlikely (Moser et al., 1982; Azcón‐Bieto et al., 1983; Azcón‐Bieto and Osmond, 1983; Monje and Bugbee, 1996). While a 75 % reduction in daytime respiration may be possible in single leaves, there is no evidence that this occurs on a whole‐plant level.
Current paradigm: allocational changes can help plants acclimate to temperature changes
Since the biochemistry of respiration is the same regardless of the ultimate use of the energy made during respiration, some argue that the growth and maintenance model may not be sufficient to explain these results. Altered night temperature may shift C allocation between roots and shoots, or alter plant composition. These shifts could alter the energy requirements for growth respiration. Lettuce and soybean had slightly less root biomass and more shoot biomass with warmer temperatures, but this pattern was not observed in tomato. In spite of this shift, the temperature sensitivity remained the same for the duration of treatment.
There were no consistent patterns across species in biomass allocation between roots, shoots and stems. There was also no consistent pattern in C fraction, assimilated N or K fraction. Nitrate was significantly higher in warmer temperatures for lettuce roots, but some of this increase at the highest temperature is partly explained by dilution effects of K (i.e. low %N when %K is high). Carbon was partitioned more to lettuce shoots and less to roots as temperature increased. If root tissue was easier to make than shoot tissue, CUE would have slightly decreased with increasing temperature. However, both the C fraction and the assimilated N fraction of roots and shoots were similar so it is not clear that the energy required for synthesis of root and shoot tissue would be significantly different. Tomatoes accumulated less nitrate and had less assimilated N as the temperature increased, so the CUE should have increased slightly with increasing temperature.
Soybeans had more stem tissue and less leaf tissue at higher temperatures. Although the C fraction in soybean stems and leaves is similar, the assimilated N is higher in leaves, so leaf tissue would most likely be more difficult to synthesize. This suggests that the CUE of soybeans should slightly increase with increasing temperature.
Overall, it is unlikely that changes in allocation patterns or composition had a significant effect on the small temperature sensitivities. If there were allocation changes that were not measured, those changes did not influence the temperature sensitivity in the long term.
Current paradigm: cool night temperatures improve growth
Turnbull et al. (2002) reported increased maximum leaf photosynthetic rates following a single warm night. They attributed this effect to less carbohydrate‐induced feedback inhibition of photosynthesis. They conclude by hinting that whole‐plant carbon gain may increase with warmer night temperatures because of less feedback inhibition. We did not see any evidence after 20 d of warmer nights of increased photosynthesis on a whole plant community basis that would support their claims.
McCree and Amthor (1982) found that growth rate was improved by 15 % at a constant 20 °C compared with 30/10 °C day/night due to improved carbon balance. They attributed the growth improvement to excessive dark respiration during the day and only slightly reduced night‐time respiration, but their studies were done at ambient CO2, so photorespiration at 30 °C would be significantly increased compared to 20 °C. This would decrease photosynthetic efficiency and reduce growth in the warmer day temperature treatment. They also used a 20 °C difference between day and night. This extreme change in temperature may have affected water relations, leaf expansion and chilling injury in addition to respiration, so the direct effect of night temperature on growth is not clear from their study.
Our studies do not indicate a statistically significant advantage of either warm or cool nights on final dry mass or on cumulative carbon gain after treatments were applied. There was a statistically significant effect of temperature on night respiration, as expected, but, surprisingly, there was no significant effect on Pnet. Because the carbon flux in Pnet is typically four to five times larger than dark respiration in growing plants, changes in dark respiration have only a small effect on CUE. The relatively small variation between replicate chambers in Pnet dominated the effect of the small, but statistically significant, differences in CUE on growth. If experimental error in Pnet was eliminated, the growth effects would have been determined by the night temperature effect on CUE, which was less than 0·5 % per °C in lettuce and tomatoes, and less than 1 % per °C in soybeans. These effects do suggest a small advantage of cool night temperatures that might be statistically significant in a study with many replicate chambers, but our data indicate that cool nights may not provide a large growth advantage (less than 1 % per °C).
One potential source of variation between chambers is variable side lighting that would have provided more or less light for a group of plants on any given day. Small differences in the height of the reflective curtain may have large effects in the amount of light a canopy receives, and therefore influence the amount of growth, especially in small canopies such as these. For example, these chambers had a growing area of 0·17 m2 (0·47 × 0·36 m). If the reflective curtain was only 1 cm lower than the height of the canopy, the effect is to increase the growing area by 10 % (Bugbee, 1994). This effect is larger than the effect of night temperature on CUE
Applicability to ambient CO2 environments
These studies were conducted at elevated CO2 to minimize the potential carbohydrate limitations to the temperature response. Some previous studies have suggested that elevated CO2 inhibits respiration. For example, Amthor et al. (1992) initially reported a 20–30 % reduction in dark respiration when CO2 was doubled. However, no theoretical basis for the effect of CO2 on dark respiration has been found. Recent studies indicated that the direct effect of CO2 on dark respiration is statistically insignificant. Amthor (2000) used an improved gas exchange chamber and found that the apparent effect of CO2 on respiration resulted from leaks in the original chamber. Indeed, using five gas exchange measurement approaches, he consistently found that respiration was insensitive to short‐term changes in CO2 concentration. Similarly, Burton et al. (1996) initially reported a significant inhibition of root respiration in elevated CO2, but later re‐did the tests and found that once leaks were sealed, no CO2 effect was observed (Burton and Pregitzer, 2002).
CONCLUSIONS
Respiration had a much lower Q10 than is commonly reported. Whole‐plant respiration is relatively insensitive to temperature in young plants because total respiration consists of a large fraction of growth respiration. In light of this, the present work validates models that divide respiration into a non‐temperature‐sensitive growth component and a temperature‐sensitive component.
No change in the sensitivity of respiration to temperature was observed even after 20 d of treatment. Little acclimation was observed in C, nitrate, assimilated N and K contents, and no trend was observed among tissues or species. Allocational shifts had minimal effects on the temperature sensitivity of respiration. Because respiration did not acclimate to temperature through time, CUE also did not acclimate. We believe this is the first long‐term study to demonstrate continuously altered CUE as a result of environmental changes. Although cooler night temperature decreased respiration and slightly increased CUE, night temperature had a statistically and biologically insignificant effect on growth.
ACKNOWLEDGMENTS
This research was supported by the National Aeronautics and Space Administration Advanced Life Support Program, the National Aeronautics and Space Administration Graduate Student Research Program, and by the Utah Agricultural Experiment Station, Utah State University. Approved by Utah Agricultural Experiment Station as journal paper no. 7510. We thank Catherine Billings for hydroponic system maintenance, James Cavazzoni for running simulations using modified CROPGRO models, and Marc van Iersel, David Tissue, and Julie Chard for helpful comments.
Received: 3 December 2003; Returned for revision: 9 February 2004; Accepted: 22 March 2004, Published electronically: 24 May 2004
References
- AlexanderJD, Donnelly JR, Shane JB.1995. Photosynthetic and transpirational responses of red spruce understory trees to light and temperature. Tree Physiology 15: 393–398. [DOI] [PubMed] [Google Scholar]
- AlwardRD, Detling JK, Milchunas DG.1999. Grassland vegetating changes and nocturnal global warming. Science 283: 229–231. [DOI] [PubMed] [Google Scholar]
- AmthorJS.1989.Respiration and crop productivity. New York: Springer‐Verlag. [Google Scholar]
- AmthorJS.2000. The McCree–de Wit–Penning de Vries–Thornley respiration paradigms: 30 years later. Annals of Botany 86: 1–20. [Google Scholar]
- AmthorJS,Bloom AJ, Koch GW.1992. CO2 inhibits respiration in leaves of Rumex crispus L. Plant Physiology 98: 757–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ArnoneJA, Korner C.1997. Temperature adaptation and acclimation potential of leaf dark respiration in two species of ranunculus from warm and cold habitats. Arctic and Alpine Research 29: 122–125. [Google Scholar]
- AtkinOK, Tjoelker MG.2003. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science 8: 343–351. [DOI] [PubMed] [Google Scholar]
- Azcón‐BietoJ, Osmond CB.1983. Relationship between photosynthesis and respiration. The effect of carbohydrate status on the rate of CO2 production by respiration in darkened and illuminated wheat leaves. Plant Physiology 71: 574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcón‐BietoJ, Lambers H, Day DD.1983. Effect of photosynthesis and carbohydrate status on respiratory rates and the involvement of the alternative pathway in leaf respiration. Plant Physiology 72: 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BjörkmanO.1981. Responses to different quantum flux densities: plants, light and its effects on photosynthesis. Encyclopedia of Plant Physiology New Series 12: 57–107. [Google Scholar]
- BooneRD, Nadelhoffer KJ, Canary JD, Kaye JP.1998. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 396: 570–572. [Google Scholar]
- BrylaDR, Bouma TJ, Eissenstat DM.1997. Root respiration in citrus acclimates to temperature and slows during drought. Plant Cell and Environment 20: 1411–1420. [Google Scholar]
- BugbeeB.1994.Effects of radiation quality, intensity, and duration on photosynthesis and growth. International Lighting on Controlled Environments Workshop. NASA‐CP‐95‐3309. [Google Scholar]
- BunceJA.1991. Control of the acclimation of photosynthesis to light and temperature in relation to partitioning of photosynthate in developing soybean leaves. Journal of Experimental Botany 42: 853–859. [Google Scholar]
- BurtonAJ, Pregitzer KS.2002. Measurement of carbon dioxide concentration does not affect root respiration of nine tree species in the field. Tree Physiology 22: 67. [DOI] [PubMed] [Google Scholar]
- BurtonAJ,Preigitzer KS, Zogg GP, Zak DR.1996. Latitudinal variation in sugar maple fine root respiration. Canadian Journal of Forestry Research 26: 1761–1768. [Google Scholar]
- BustanA, Goldschmidt EE.1998. Estimating the cost of flowering in a grapefruit tree. Plant Cell and Environment 21: 217–224. [Google Scholar]
- CannellMGR, Thornley JHM.2000. Modelling the components of plant respiration: some guiding principles. Annals of Botany 85: 45–54. [Google Scholar]
- ChapmanSJ,Thurlow M.1998. Peat respiration at low temperatures. Soil Biology and Biochemistry 30: 1013–1021. [Google Scholar]
- Covey‐CrumpEM, Attwood RG, Atkin OK.2002. Regulation of root respiration in two species of Plantago that differ in relative growth rate: the effect of short‐ and long‐term changes in temperature. Plant Cell and Environment 25: 1501–1513. [Google Scholar]
- CrawfordRMM, Huxter TJ.1977. Root growth and carbohydrate metabolism at low temperatures. Journal of Experimental Botany 28: 917–925. [Google Scholar]
- GiffordRM.1994. The global carbon cycle: a viewpoint on the missing sink. Australian Journal of Plant Physiology 21: 1–15. [Google Scholar]
- GiffordRM.1995. Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature: long‐term vs. short‐term distinctions for modeling. Global Change Biology 1: 385–396. [Google Scholar]
- GriffinKL, Turnbull M, Murthy R, Lin G, Adams J, Farnsworth B, Mahato T, Bazin G, Potasnak M, Berry JA.2002. Leaf respiration is differentially affected by leaf vs. stand‐level night‐time warming. Global Change Biology 8: 479–485. [Google Scholar]
- HansenJ,Ruedy R, Glascoe J, Sato M.1999. GISS analysis of surface temperature change. Journal of Geophysical Research 104: 30997–31022. [Google Scholar]
- van IerselMW, Bugbee B.2000. A multiple chamber, semicontinuous, crop carbon dioxide exchange system: design, calibration, and data interpretation. Journal of the American Society for Horticultural Science 125: 86–92. [PubMed] [Google Scholar]
- IlleperumaC,Schlimme D, Solomos T.1998. Changes in sugars and activities of sucrose phosphate synthase, sucrose synthase, and invertase during potato tuber (Russet Burbank) reconditioning at 10 °C in air and 2·53 kPa oxygen after storage for 28 days at 1C. Journal of the American Society for Horticultural Science 123: 311–316. [Google Scholar]
- LabateCA, Leegood RC.1989. Influence of low temperature on respiration and contents of phosphorylated intermediates in darkened barley leaves. Plant Physiology 91: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LarigauderieA,Körner C.1995. Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Annals of Botany 76: 245–252. [Google Scholar]
- LomanderA,Katterer T, Andren O.1998. Modelling the effects of temperature and moisture on CO2 evolution from top‐ and subsoil using a multi‐compartment approach. Soil Biology and Biochemistry 30: 2023–2030. [Google Scholar]
- LoveysBR,Atkinson LJ, Sherlock DJ, Roberts RL, Fitter AH, Atkin OK.2003. Thermal acclimation of leaf and root respiration: an investigation comparing inherently fast‐ and slow‐growing plant species. Global Change Biology 9: 895–910. [Google Scholar]
- MaierCA, Zarnoch SJ, Dougherty PM.1998. Effects of temperature and tissue nitrogen on dormant season stem and branch maintenance respiration in a young loblolly pine (Pinus taeda) plantation. Tree Physiology 18: 11–20. [DOI] [PubMed] [Google Scholar]
- McCreeKJ.1974. Equations for the rate of dark respiration of white clover and grain sorghum, as functions of dry weight, photosynthetic rate, and temperature. Crop Science 14: 509–514. [Google Scholar]
- McCreeKJ, Amthor ME.1982. Effects of diurnal variation in temperature on the carbon balances of white clover plants. Crop Science 22: 822–827. [Google Scholar]
- MonjeO,Bugbee B.1996. Characterizing photosynthesis and transpiration of plant communities in controlled environments. Acta Horticulture 440: 123–128. [DOI] [PubMed] [Google Scholar]
- MonjeO, Bugbee B.1998. Adaptation to high CO2 concentration in an optimal environment: radiation capture, canopy quantum yield, and carbon use efficiency. Plant Cell and Environment 21: 315–324. [DOI] [PubMed] [Google Scholar]
- MoserLE, Volenec JJ, Nelson CJ.1982. Respiration, carbohydrate content, and leaf growth of tall fescue. Crop Science 22: 781–786. [Google Scholar]
- NeterJ,Kutner MH, Nachsheim CJ, Wasserman W.1996.Applied linear statistical models, 4th edition. Chicago, IL: Irwin. [Google Scholar]
- NevenLG.1998. Respiratory response of fifth‐instar codling moth (Lepidoptera: Tortricidae) to rapidly changing temperatures. Journal of Economic Entomology 91: 302–308. [DOI] [PubMed] [Google Scholar]
- NielsenMG,Elmes GW, Kipyatkov VE.1999. Respiratory Q10 varies between populations of two species of Myrmica ants according to the latitude of their sites. Journal of Insect Physiology 45: 559–564. [DOI] [PubMed] [Google Scholar]
- OgawaK, Takano Y.1997. Seasonal courses of CO2 exchange and carbon balance in fruits of Cinnamomum camphora Tree Physiology 17: 415–420. [DOI] [PubMed] [Google Scholar]
- PearmanI,Thomas SM, Thorne GN.1981. Dark respiration of several varieties of winter wheat given different amounts of nitrogen fertilizer. Annals of Botany 47: 535–546. [Google Scholar]
- Penning de VriesFWT, Brunsting AHM, van Laar HH.1974. Products, requirements and efficiency of biosynthesis: a quantitative approach. Journal of Theoretical Biology 45: 339–377. [DOI] [PubMed] [Google Scholar]
- PercivalDC, Proctor JTA, Tsujita MJ.1996. Whole‐plant net CO2 exchange of raspberry as influenced by air and root‐zone temperature, CO2 concentration, irradiation, and humidity. Journal of the American Society for Horticultural Science 121: 838–845. [Google Scholar]
- PoorterH, van der Werf A, Atkin OK, Lambers H.1991. Respiratory energy requirements of roots vary with the potential growth rate of a plant species. Physiologia Plantarum 83: 469–475. [Google Scholar]
- QuinlanMC, Lighton JRB.1999. Respiratory physiology and water relations of three species of Pogonomyrmex harvester ants (Hymenoptera: Formicidae). Physiology of Entomology 24: 293–302. [Google Scholar]
- RobertsEM, Rao NR, Huang J, Trolinder NL, Haigler CH.1992. Effects of cycling temperatures on fiber metabolism in cultured cotton ovules. Plant Physiology 100: 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RuterJM, Ingram DL.1991. Root respiratory characteristics of ‘Rotundifolia’ holly under superoptimal temperatures. Journal of the American Society for Horticultural Science 116: 560–564. [Google Scholar]
- SarmientoJL, Le Quere C.1996. Oceanic carbon dioxide uptake in a model of century‐scale global warming. Science 274: 1346–1349. [DOI] [PubMed] [Google Scholar]
- SharpRE, Matthews MA, Boyer JS.1984. Kok effect and the quantum yield of photosynthesis. Light partially inhibits dark respiration. Plant Physiology 75: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SmartDR, Chatterton N. J. Bugbee B.1994. The influence of elevated CO2 on non‐structural carbohydrate distribution and fructan accumulation in wheat canopies. Plant Cell and Environment 17: 435–442. [DOI] [PubMed] [Google Scholar]
- TeskeyRO, Will RE.1999. Acclimation of loblolly pine (Pinus taeda) seedlings to high temperatures. Tree Physiology 19: 519–525. [DOI] [PubMed] [Google Scholar]
- ThornleyJHM, Cannell MGR.2000. Modelling the components of plant respiration: representation and realism. Annals of Botany 85: 55–67. [Google Scholar]
- TjoelkerMG, Oleksyn J, Reich PB.1999. Acclimation of respiration to temperature and CO2 in seedlings of boreal tree species in relation to plant size and relative growth rate. Global Change Biology 5: 679–691. [Google Scholar]
- TjoelkerMG, Oleksyn J, Reich PB.2001. Modeling respiration of vegetation: evidence for a general temperature‐dependent Q10 Global Change Biology 7: 223–230. [Google Scholar]
- TurnbullMH,Murthy R, Griffin KL.2002. The relative impacts of daytime and night‐time warming on photosynthetic capacity in Populus detoides Plant Cell and Environment 25: 1729–1737. [Google Scholar]
- UrmenetaJ,Alcoba O, Razquin E, Tarroja E, Navarrete A, Guerrero R.1998. Oxygenic photosynthesis and respiratory activity in microbial mats of the Ebro Delta, Spain, by oxygen exchange method. Current Microbiology 37: 151–155. [DOI] [PubMed] [Google Scholar]
- WangX,Lewis JD, Tissue DT, Seemann JR, Griffin KL.2001. Effects of elevated CO2 concentration on leaf dark respiration of Xanthium strumarium in light and in darkness. Proceedings of the National Academy of Science of the USA 98: 2479–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WitowskiJ.1997. Gas exchange of the lowest branches of young Scots pine: a cost‐benefit analysis of seasonal branch carbon budget. Tree Physiology 17: 757–765. [DOI] [PubMed] [Google Scholar]
- WullschlegerSD, Norby RJ.1992. Respiratory cost of leaf growth and maintenance in white oak saplings exposed to atmospheric CO2 enrichment. Canadian Journal of Forestry Research 22: 1717–1721. [Google Scholar]
- WullschlegerSD, Norby RJ, Gunderson CA.1992. Growth and maintenance respiration in leaves of Liriodendron tulipifera L. exposed to long‐term carbon dioxide enrichment in the field. New Phytologist 121: 515–523. [Google Scholar]