Abstract
• Background and Aims This work has been conducted to assist theoretical modelling of the different stages of the blue light (BL)‐induced phototropic signalling pathway and ion transport activity across plant membranes. Ion fluxes (Ca2+, H+, K+ and Cl–) in etiolated oat coleoptiles have been measured continuously before and during unilateral BL exposure.
• Methods Changes in ion fluxes at the illuminated (light) and shadowed (dark) sides of etiolated oat coleoptiles (Avena sativa) were studied using a non‐invasive ion‐selective microelectrode technique (MIFE). The bending response was also measured continuously, and correlations between the changes in various ion fluxes and bending response have been investigated. For each ion the difference (Δ) between the magnitudes of flux at the light and dark sides of the coleoptile was calculated.
• Key results Plants that demonstrated a phototropic bending response also demonstrated Ca2+ influx into the light side approximately 20 min after the start of BL exposure. This is regarded as part of the perception and transduction stages of the BL‐induced signal cascade. The first 10 min of bending were associated with substantial influx of H+, K+ and Cl– into the light (concave) side of the coleoptiles.
• Conclusions The data suggest that Ca2+ participates in the signalling stage of the BL‐induced phototropism, whereas the phototropic bending response is linked to changes in the transport of H+, K+ and Cl–.
Key words: Avena sativa, blue light, signal transduction, ion fluxes, Ca2+, H+, K+, Cl–, membrane transport
INTRODUCTION
It has been known for more than a century that unilateral illumination induces phototropic bending in plants (Sachs, 1887). Like other interactions between organisms and their environments, plant phototropism can be thought of as a process involving three phases: perception, transduction and response.
Several recent models of these three phases during primary phototropic signalling conditions have attempted to combine the Cholodny–Went theory concerning differential auxin transport during phototropism with recent molecular biological studies (Liscum and Stowe‐Evans, 2000) and H+ and Ca2+ transport activity (Iino, 2001).
As a low‐fluence blue light (BL) perception target Liscum and Stowe‐Evans (2000) suggest a complex of PHOT1/NPH3 molecules. The transduction phase involves a phototropin‐activated phosphorelay, and the activation of Ca2+ channels located in the plasma membrane, which leads to elevation of [Ca2+]cyt (Baum et al., 1999; Babourina et al., 2002; Briggs and Christie, 2002; Babourina et al., 2003; Harada et al., 2003; Stoelzle et al., 2003). The subsequent activation of auxin transporters to cause the bending response is suggested to occur via two mechanisms: a direct phototropin‐dependent pathway and an indirect pathway involving an increase in [Ca2+]cyt.
Three models presented by Iino (2001) describe different sequences of events in the transduction phase after the BL signal is perceived by a photoreceptor system. In the first model, the first step after signal perception is the induction of lateral translocation of auxin, and a lateral asymmetry in Ca2+ distribution in the cytoplasm and the apoplast as a consequence of auxin asymmetry. In the second model, the first step after signal perception is the increase in [Ca2+]cyt and [H+]cyt on the concave side, and auxin is laterally translocated in response to the different Ca2+ concentrations in the apoplast. The third model incorporates the first two models. Two pathways generate the asymmetry of H+ concentration in the apoplast simultaneously. In all three models, the main factor proposed to induce the ultimate asymmetrical growth response is an increase in H+ in the apoplast of the convex side of the plant.
Although the hypotheses presented above are a good attempt to bring together many known components of the phototropic signal transduction and response, they do not incorporate some results available to date, especially those of electrophysiological origin. In addition to changed Ca2+ concentrations in the apoplast and the cytoplasm in etiolated seedlings during white light and BL illumination (Goswami and Audus, 1976; Gehring et al., 1990; Baum et al., 1999), which are incorporated in the models by both groups of authors, other membrane transport activities have been described in coleoptiles and hypocotyls during unilateral BL illumination. These activities include: (1) fast changes in membrane potential (Racusen and Galston, 1980; Spalding, 2000); (2) fast changes in surface potentials (Hartmann, 1975; Spalding and Cosgrove, 1989), and (3) changes in the activity of anion transport systems (Spalding, 2000).
One of the difficulties in creating models describing causal relationships during BL signal transduction and response is the absence of the assessment of changes in the ion transport activities (fluxes or concentrations) that are associated with the induction of bending. Few studies have been made using a long time scale to describe electrophysiological changes during bending itself. Schrank (1948) demonstrated some surface potential changes just before bending occurred. Newman (1963) reported that a wave of electric surface potential travelled at least 10 mm down the oat coleoptile after apical white‐light illumination. Bending was observed to start at each level at about the time of the fall from peak to trough in the electric wave. The low number of simultaneous electrophysiological and bending measurements does not mean that these experiments lacked interest or importance. In most cases, it was barely possible to perform such combined measurements. It is not feasible to measure membrane potential (Em) or to make voltage clamping in a vertical seedling during bending, or to make a patch clamp on an intact plantlet. To overcome some difficulties of making electrophysiological measurements on an intact moving object, the non‐invasive ion selective microelectrode technique (MIFE) can be applied (Newman, 2001). We have now measured H+, Ca2+, K+ and Cl– fluxes across the plasma membrane and cell wall of the parenchyma at both sides of the oat coleoptiles before and during BL exposure and during subsequent bending. Oat coleoptiles were used because their size allowed us to remove the cuticle from both dark and illuminated sides without damaging the parenchyma. To assist model development, two main questions were addressed. (1) Is there any difference between the light and dark side fluxes during perception and transduction of the BL phototropic signal? (2) What changes in ion fluxes accompany the bending response?
MATERIALS AND METHODS
Plant material and growing conditions
Seeds of oat (Avena sativa L. ‘Victory’, Svalof A.B., Svalof, Sweden) were planted in jars on 0·8 % (w/v) agar made up in 200 µm KCl and 100 µm CaCl2 solution. The jars were then placed in complete darkness at 22 ± 2 °C for 3 d.
Straight coleoptiles (about 1·5 cm high) were cut from the oat seedlings and placed into the experimental chamber after being gently abraded on each side of the coleoptile top using fine sand paper. This abrading was performed to remove the cuticle, which hinders the measurements of ion fluxes. Coleoptiles were floated for 3–4 h on the surface of an unbuffered solution of 1 mm KCl and 100 µm CaCl2 (pH 5·4) to recover from wounding (Hush et al., 1992). The coleoptiles were fixed vertically in the measuring chamber using silicon tube sections for support and immersed in a solution of the same composition as used for growing the seedlings. Ion fluxes were measured from both sides of the coleoptile as indicated in Fig. 1.
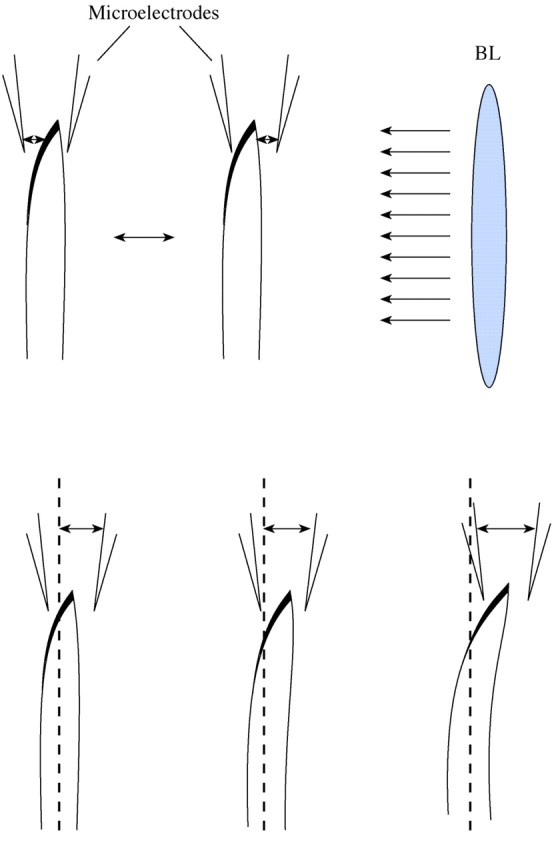
Fig. 1. Schematic diagram of experimental design for flux measurements from both sides of oat coleoptiles during unilateral BL illumination of the adaxial side. The plant was moved between two electrodes, with constant separation, in the two positions shown, 300 µm below the top, in a 10‐s ‘square wave’ cycle. The lower panel demonstrates position of the electrodes during bending.
All handling procedures were performed in approx. 5–10 min under ‘safe’ red light of 2·4 µmol m–2 s–1. The chamber was then placed in the Faraday cage under the microscope light (red plastic filter; 0·9 µmol m–2 s–1). Measurements started at least 60 min later, when the ion fluxes were stabilized, to reduce possible confounding effects of changes in the light conditions.
In all experiments, the source of monochromatic (468 nm) BL was a QBEAM projector (Quantum Devices, Inc., Barneveld WI, USA). In most experiments, the standard treatment was approx. 80 µmol m–2 given as a 60 min illumination of 0·023 µmol m–2 s–1. This is in the ‘first positive curvature’ range.
Ion flux measurements
The microelectrodes for flux measurements were made from borosilicate glass tubing (GC 150–10, Clark Electromedical Instruments, Pangbourne, Berks, UK).
Electrodes for flux measurements were pulled, dried in an oven at 200 °C for 5 h, and silanized with two drops (approx. 15 µg) of tributylchlorosilane (90796, Fluka Chemical, Buchs, Switzerland) for 10 min in the same oven under a steel cover. The cover was then removed, and the electrodes left to dry at 200 °C for another 30 min.
To fill the microelectrodes, silanized electrodes were broken back to get an external tip diameter of 3–5 µm. The electrode tips were then filled with commercially available ionophore cocktails (hydrogen 95297; calcium 21048; potassium 60031; chloride 24902; all from Fluka Chemical). The electrodes were calibrated in a known set of standards (pH from 4·6 to 7·8; Ca2+ from 0·1 to 1 mm; K+ and Cl– from 1 to 10 mm). Electrodes with responses less than 50 mV (H+, K+ and Cl–) and 25 mV (Ca2+) per decade were discarded.
Fluxes of H+, Cl–, K+ and Ca2+ were measured using the non‐invasive MIFE® (Microelectrode Ion Flux Estimation) system (University of Tasmania, Hobart, Australia) as described by Shabala et al. (1997). The theory of net ion flux measurements using the MIFE system has been recently reviewed (Newman 2001).
For ion flux measurements the microelectrodes filled with the ionophore were moved horizontally between two points from both sides of coleoptiles in a square‐wave manner with a 10 s cycle. The differences in the electrochemical potential were measured for a particular ion between these two positions, 50 and 85 µm from the tissue. From the electrochemical potential difference between the two positions the net flux was calculated for each ion, assuming a cylindrical geometry of ionic diffusion. The average concentration of the ion between the two positions was also calculated from the recorded data. We maintain our convention that net influx into tissue is positive for both cations and anions.
Net H+, Ca2+, K+ and Cl– fluxes, one ion at a time, were measured at 300 µm from the top on both sides simultaneously for 20–30 min prior to the BL treatment. The BL was switched on for 60 min and measurements continued for at least 30 min after the BL was switched off. We used 60 min illumination rather than a brief BL pulse to avoid any effect of a light–dark transition (Plieth et al., 1998) and to obtain a clear picture of BL signal perception and transduction. In order to prevent the distance between plant tissue and electrode tips changing due to growth and bending processes and to maintain constant flux‐measuring positions, the electrode position was adjusted using a three‐dimensional hydraulic manipulator. Growth was allowed for by manual corrections, transverse movement by computer‐controlled adjustments. Information about transverse adjustments was automatically recorded in a log file by the MIFE computer. This allowed us to create files describing transverse tip movements as an indicator of bending magnitude and rate.
Ten to 15 coleoptiles were measured for each ion studied. In some figures, representative examples are shown to illustrate the character of the ion flux responses. Statistical information on the magnitudes of ion flux changes (Δ, calculated as the difference between fluxes at the light and dark sides) is presented in Fig. 4. Pearson’s product–moment correlation coefficient r and regression coefficients were calculated using the Microsoft Excel package.
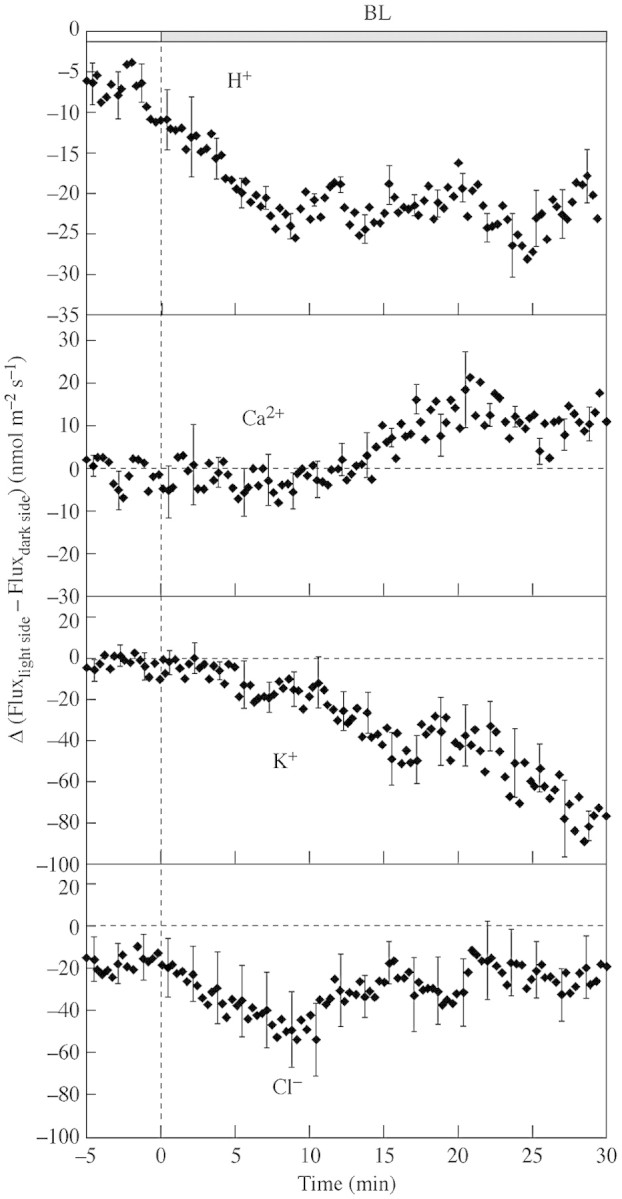
Fig. 4. The effect of BL on Δ, calculated as the difference between fluxes from the light side and the dark side, calculated from net H+, Ca2+, K+ and Cl– fluxes before BL onset and during BL illumination. The positive values indicate relatively higher influx into the light side than into the dark side. Representative error bars are ± s.e. (n = 6–10).
RESULTS
Initial flux response
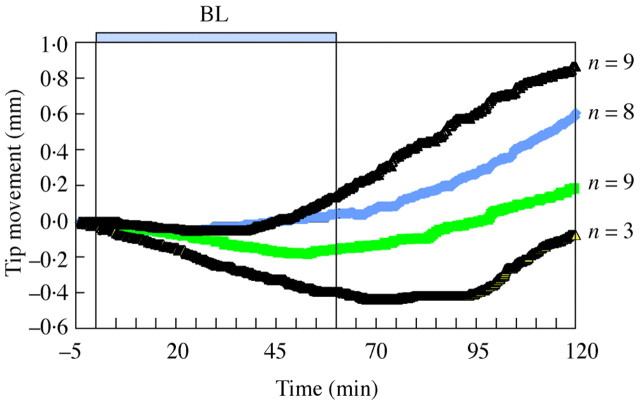
Of the plants studied, 83 % expressed positive phototropic bending, which could be first observed from 25 to 95 min after the BL onset (Fig. 2).
Fig. 2. Examples of oat coleoptile bending measured by lateral tip position in response to unilateral BL illumination of 82 µmol m–2: plants demonstrated immediate or delayed positive phototropism. Numbers indicate how many plants displayed movement similar to the lines shown.
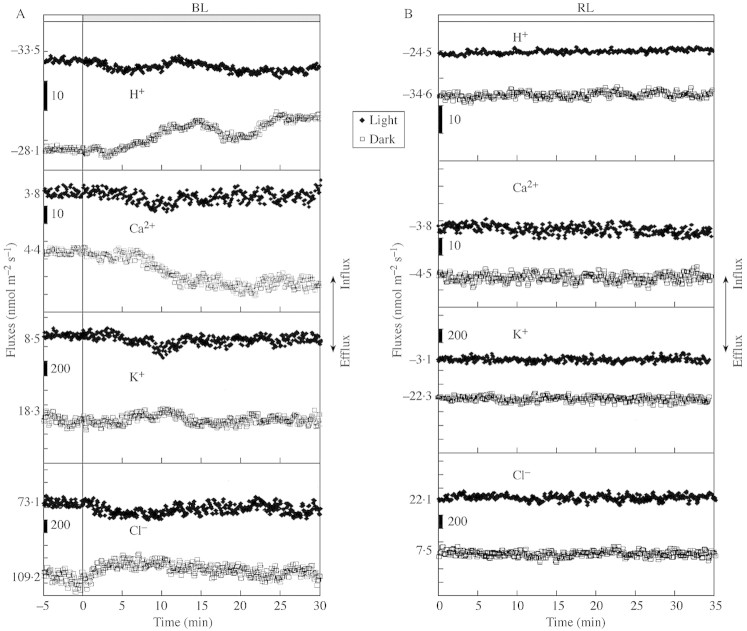
For all ions studied, an initial flux response was generally observed from both sides of the responding coleoptiles. Sometimes the response from the dark side was even greater than the response from the light side. During the first 30 min the flux response was in most cases multiphasic. No correlation was found between the magnitudes of the flux responses and the magnitude of subsequent bending. Figure 3A presents representative flux measurements for all ions studied for both sides (light and dark). Ion fluxes in control plants, not exposed to BL illumination, did not exhibit any fluctuations (Fig. 3B).
Fig. 3. (A) Typical examples (one out of six or ten) of initial BL‐induced changes in H+, Ca2+, K+, and Cl– fluxes, measured from both sides of oat coleoptiles. Shift to positive values in fluxes indicates ion influx from bathing medium to tissue. Each point is mean flux over 10 s. Initial ion flux values are indicated at the start of each line. (B) Typical examples for ion fluxes when plants were not exposed to BL.
The first H+ flux response was observed a few minutes after the BL onset. The shift in net H+ flux towards reduced efflux was greater for the dark side than for the light side of the coleoptiles (Fig. 3A).
To make the relation between fluxes from both sides more obvious, we calculated Δ as a difference between fluxes at the light and dark sides. This parameter allows the assessment of a gradient of transport activities for each ion. For H+, Δ indicates higher H+ efflux from the light side in comparison with the dark side (Fig. 4).
In plants with a bending response (67 % of all plants studied in experiments for Ca2+ measurements), Ca2+ efflux was observed from the dark side starting 10–15 min after the commencement of BL exposure, whereas the flux on the light side is relatively unchanged (Fig. 3A).
The calculated Δ for Ca2+ becomes positive 10–15 min after the BL onset (Fig. 4). The difference in the Ca2+ flux responses between the light and dark side may cause an internal Ca2+ gradient within the coleoptile, with higher Ca2+ concentration on the light side.
K+ fluxes were also affected during the first 20 min of BL illumination. The slight transient efflux observed from the coleoptile’s light side, with the simultaneous influx into the dark side (Fig. 3A), is better seen as an asymmetry in Δ between the two sides with a substantial efflux from the light side compared with the dark side (Fig. 4).
The response of Cl– fluxes showed sustained efflux from the light side and influx into the dark side (Fig. 3). The mean Δ calculated for the fluxes from both sides demonstrated that the Cl– efflux from the light side prevailed during the first 10 min (Fig. 4).
Flux changes during the bending response
Initial stages of positive bending were strongly linked with reduced efflux of H+, K+ and (less so) Cl– (Fig. 5 A–F) on the light side, whereas fluxes on the dark side changed little apart from the small K+ and Cl– efflux. These light‐side H+ and K+ flux changes were clearly observed during the first 10 min of bending and the fluxes stabilized after 15–20 min, whereas bending in many cases continued. The right‐hand charts in Fig. 5 (B, D, F, H) present flux values plotted against tip position for the 10‐min interval indicated by the arrow between the dotted lines in the left‐hand charts. The highest linear correlation coefficient between the tip position and ion fluxes was observed for H+ (r = 0·79, P < 0·05) (Table 1). The slope coefficient a quantifies how ion transport was changed for every bending distance unit. The mean of the regression coefficient a, calculated for every single plant with the bending response, is presented in Table 1. The magnitude of this coefficient was of the same order for K+ and Cl– fluxes (Table 1).
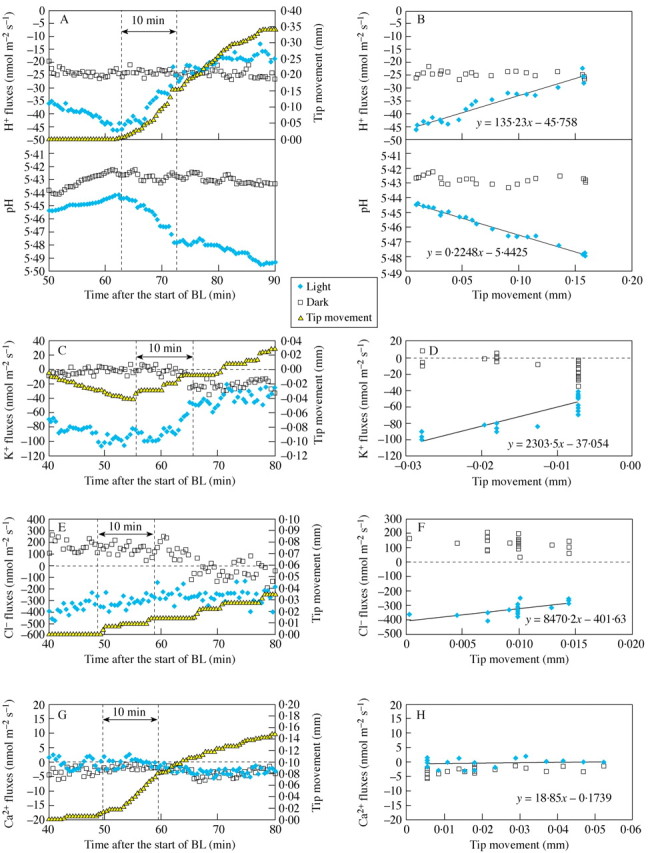
Fig. 5. Typical examples of changes in tip position and in pH and net H+ fluxes (A, B), K+ (C, D), Cl– (E, F) and Ca2+ (G, H); fluxes at 30‐s intervals for both sides of coleoptiles. Parts A, C, E and G present time courses of tip movement, pH and ion fluxes from both sides of the coleoptile. Parts B, D, F and H present the regression line between the tip position and the ion flux data for both sides during the first 10 min of bending, between two dotted lines as indicated in figures A, C, E, G. Filled symbols indicate pH and flux values measured at the light side, open symbols are for the dark side. For the same data points the regression lines were fitted using data analysis tools in the Microsoft Excel package.
Table 1.
Correlation between the tip position and ion fluxes from the light side during the first 10 min of positive bending
| Measured ion | a (n)* | r* |
| H+ | 151 ± 24 (7) | 0·79 ± 0·05 |
| K+ | 1216 ± 283 (6) | 0·74 ± 0·01 |
| Cl– | 4225 ± 802 (6) | 0·67 ± 0·03 |
| Ca2+ | –2480 ± 1466 (10) | –0·05 ± 0·04 |
For each coleoptile, a linear trend line (ax + b) was fitted for a 10‐min interval starting at the first movement towards light, as shown in Fig. 5. The correlation coefficient r was calculated for the same points using data analysis tools in Microsoft Excel.
* Tabulated coefficients a and r are the mean ± s.e. for the n plants for each ion
DISCUSSION
Changes in ion fluxes at the start of BL illumination
In plants with a bending response, 0·023 mmol m–2 s–1 BL caused a substantial shift in H+, K+, and Cl– fluxes towards influx (or reduced efflux) into the dark side and efflux from the light side during the first 30 min of BL exposure. The time scale for the changes in ion fluxes is comparable with the time 5–20 min when PHOT1 reaches maximum phosphorylation in soluble protein extracts prepared from insect cells and in wild‐type membranes (Christie et al., 1998). Also, this time scale is similar to the increase in [Ca2+]cyt and lowering of pHcyt observed at approx. 15 min on the dark side of corn coleoptiles after illumination by white light (Gehring et al., 1990), and plasma membrane depolarization on the dark side of corn coleoptiles (Racusen and Galston, 1980). The observed ion flux changes could be linked with the processes involved in the initial stages of the BL signal perception and transduction, following the activation of the BL target molecules, such as the PHOT1+NPH3 complex suggested by the Liscum and Stowe‐Evans (2000) model.
The observed net Cl– efflux from the light side (Figs 3A, 4) is most likely induced by the activation of anion channels, as was found in patch clamp experiments by Cho and Spalding (1996). As Spalding (2000) suggested, this activation of anion channels could lead to Em depolarization. This Em depolarization can in turn shift K+ fluxes to efflux, as has been shown recently for different plants (Babourina et al., 2001; Nocito et al., 2002). On the other hand, it is possible that our observed changes in K+ fluxes could be directly regulated by BL, as was shown for flexor cells from Samanea saman (Suh et al., 2000).
The most important finding from our study is that a bending response is accompanied by a high internal Ca2+ gradient in the light side (Fig. 4). This is evident from the fact that a Ca2+ efflux phase (from the dark side) was present in all plants for which a bending response was observed (n = 10). This efflux phase typically began 10–15 min after the commencement of BL exposure. However, the Ca2+ efflux phase was not observed in plants that demonstrated no phototropic bending (n = 5) (data not shown).
This observation is consistent with the suggestion made by Liscum and Stowe‐Evans (2000) that phosphorylation, via activated PHOT1, may lead to changes in Ca2+ channel activity. Some recent publications propose the activation of Ca2+ channels located at plasma membranes for the BL transduction stage in dicotyledons (Babourina et al., 2003; Stoelzle et al., 2003). Earlier studies on Ca2+ involvement in the bending response itself demonstrated that EGTA (Ca2+ chelator) and verapamil (Ca2+ blocker) decreased phototropic curvature when they were applied to the dark side of sunflower seedlings (Ma and Sun, 1997). However, our results indicate that monocotyledon species require Ca2+ redistribution within the parenchyma rather than uptake from the apoplast.
Changes in ion fluxes at the start of bending
To our knowledge, changes in ion fluxes at the start of bending have not been described before. Although the entire coleoptile could contribute to the movement of the tip, the dramatic flux changes were observed in the tip area. In contrast to the ‘perception–transduction’ phase of the BL signal, where changes in ion fluxes on the dark side were comparable with the light side, the changes during the beginning of the bending phase happened on the light side (Fig. 5). Fluxes of Cl–, K+ and H+ demonstrated a shift towards influx (or reduced efflux) on the light (future concave) side of the coleoptile. By contrast, Ca2+ fluxes were uncorrelated with bending movement, although they appear to be essential for the occurrence of bending (Figs. 4, 5H, Table 1).
Cl– and K+ are usually considered as the main components for regulation of turgor pressure, which is a central mechanism involved in bending. According to theory, an increase in influx of these ions should have been expected on the side growing more intensively, to become convex. In line with these expectations, Iino and Briggs (1984) found that during the phototropic bending, cells expanded more intensively on the dark (convex) side of the coleoptile. Also, in studies on the phototropic response in corn coleoptiles, Fuchs et al. (2003) demonstrated that the expression of ZMK1 (an inwardly rectifying K+ channel in non‐vascular tissue) started prevailing in the shaded side at 60 min after BL onset. Interestingly, this prevalence was rather due to a decrease in ZMK1 expression in the light side than to an increase of ZMK1 expression in the dark side. However, it seems that our observed changes of K+ and Cl– fluxes do not represent the processes needed for osmotic maintenance during cell expansion. Moreover, the correlation between ion flux and movement disappeared 10–15 min after bending started, while bending in most cases continued for a much longer period (Fig. 2). Probably the observed changes in ion fluxes are linked with some changes in Em (depolarization/hyperpolarization) transmitted from the zone where the real change in turgor occurred, in lower parts of the coleoptile, and charge‐balancing systems stabilized these changes after 10–15 min. It could also indicate some downstream change in Em because of auxin movement. Such ‘waves’ in surface potential were observed in earlier studies after auxin application to the top of the seedling (Newman, 1959) or after white light illumination of the intact seedling (Newman, 1963).
The influx of K+ and Cl– was accompanied with the reduced efflux of H+, which led to relative alkalinization of the light side apoplast during bending (Fig. 5). These data are in good agreement with the Iino’s (2001) model, which essentially incorporates the acid‐growth theory (Cleland et al., 1991; Rayle and Cleland, 1992; Van Volkenburgh, 1999; Cosgrove, 2000). Our data support the suggestion that the BL induced alkalinization of the apoplast, which in turn down‐regulates the activity of expansins (Cosgrove, 2000) and, therefore, reduces cell wall expansion (Spalding and Cosgrove, 1992).
In conclusion, our data suggest that Ca2+ participates in the signalling stage of BL‐induced phototropism, whereas phototropic bending itself is linked with changes in the transport of H+, K+ and Cl–.
ACKNOWLEDGEMENTS
The authors would like to thank Drs Newman, Shabala, Singh and Mapfumo and Mr Damon for critical reading of the manuscript.
Received: 24 October 2003; Returned for revision: 16 December 2004; Accepted: 25 March 2004, Published electronically: 20 May 2004
References
- BabourinaO, Hawkins B, Lew RR, Newman I, Shabala S.2001. K+ transport by Arabidopsis root hairs at low pH. Australian Journal of Plant Physiology 28: 635–641 [Google Scholar]
- BabourinaO, Newman IA, Shabala S.2002. Blue light induced kinetics of H+ and Ca2+ fluxes in etiolated wild type and phototropin‐mutant Arabidopsis seedlings. Proceedings of the National Academy of Sciences of the USA 99: 2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BabourinaOK, Newman IA, Shabala SN.2003. Electrophysiological localization of blue light sensory sites in etiolated dicotyledon seedlings. Plant Cell and Environment 26: 1505–14. [Google Scholar]
- BaumG, Long JC, Jenkins GI, Trewavas AJ.1999. Stimulation of the blue light phototropic receptor NPH1 causes a transient increase in cytosolic Ca2+ Proceedings of the National Academy of Sciences of the USA 96: 13554–13559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BriggsWR, Christie JM.2002. Phototropin 1 and 2: versatile plant blue‐light receptors. Trends in Plant Science 7: 204–210. [DOI] [PubMed] [Google Scholar]
- ChoMH, Spalding EP.1996. An Arabidopsis anion channel activated by blue light. Proceedings of the National Academy of Sciences of the USA 93: 8134–8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChristieJ, Reymond P, Powell GK, Bernasconi P, Raibekas AA, Liscum E, Briggs W.1998. Arabidopsis NPH1: a flavoprotein with properties of a photoreceptor for phototropism. Science 282: 1698–1701 [DOI] [PubMed] [Google Scholar]
- ClelandRE, Buckley G, Nowbar S, Lew NM, Stinemetz C, Evans ML, Rayle DL.1991. The pH profile for acid‐induced elongation of coleoptile and epicotyl sections is consistent with the acid‐growth theory. Planta 186: 70–74. [PubMed] [Google Scholar]
- CosgroveDJ.2000. Expansive growth of plant cell walls. Plant Physiology and Biochemistry 38: 109–124. [DOI] [PubMed] [Google Scholar]
- FuchsI, Philippar K, Ljung K, Sandberg G, Hedrich R.2003. Blue light regulates an auxin‐induced K+ channel gene in the maize coleoptile. Proceedings of the National Academy of Sciences of the USA 100: 11795–11800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GehringCA, Williams DA, Cody SH, Parish RW.1990. Phototropism and geotropism in maize coleoptiles are spatially correlated with increases in cytosolic free calcium. Nature 345: 528–530 [DOI] [PubMed] [Google Scholar]
- GoswamiKKA, Audus LJ.1976. Distribution of calcium, potassium and phosphorus in Helianthus annuus hypocotyls and Zea mays coleoptiles in relation to tropic stimuli and curvatures. Annals of Botany 40: 49–64. [Google Scholar]
- HaradaA, Sakai T, Okada K.2003. phot1 and phot2 mediate blue light‐induced transient increases in cytosolic Ca2+ differently in Arabidopsis leaves. Proceedings of the National Academy of Sciences of the USA 100: 8583–8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HartmannE.1975. Influence of light on the bioelectric potential of the bean. Phaseolus vulgaris hypocotyl hook. Physiologia Plantarum 33: 266–275. [Google Scholar]
- HushJM, Newman IA, Overall RL.1992. Utilization of the vibrating probe and ion‐selective microelectrode techniques to investigate electrophysiological responses to wounding in pea roots. Journal of Experimental Botany 43: 1251–1257. [Google Scholar]
- IinoM.2001. Phototropism in higher plants. In: Hader D‐P, Lebert M, eds. Photomovement. Amsterdam, New York: Elsevier Science, 659–811 [Google Scholar]
- IinoM, Briggs W.1984. Growth distribution during first positive phototropic curvature of maize coleoptiles Plant, Cell and Environment 7: 97–104. [Google Scholar]
- LiscumE, Stowe‐Evans EL.2000. Phototropism: a ‘simple’ physiological response modulated by multiple interacting photosensory‐response pathways. Photochemistry and Photobiology 72: 273–282. [DOI] [PubMed] [Google Scholar]
- MaL‐G, Sun D‐Y.1997. The involvement of calcium in light signal transduction chain for phototropism in sunflower seedlings Biologia Plantarum 39: 569–574. [Google Scholar]
- NewmanIA.1959. Electrical determination of transport of 3‐indol acetic acid in Avena Nature 184: 1728–1729. [Google Scholar]
- NewmanIA.1963. Electric potentials and auxin translocation in Avena Australian Journal of Biological Sciencess 16: 629–646. [Google Scholar]
- NewmanIA.2001. Ion transport in roots: measurement of fluxes using ion‐selective microelectrodes to characterize transporter function. Plant Cell and Environment 24: 1–14. [DOI] [PubMed] [Google Scholar]
- NocitoFF, Sacchi GA, Cocucci M.2002. Membrane depolarization induces K+ efflux from subapical maize root segments. New Phytologist 154: 45–51. [Google Scholar]
- PliethC, Sattelmacher B, Hansen UP.1998. Light‐induced cytosolic calcium transients in green plant cells. II. The effect on a K+ channel as studied by a kinetic analysis in Chara corallina Planta 207: 52–59. [Google Scholar]
- RacusenRH, Galston AW.1980. Phytochrome modifies blue‐light‐induced electrical changes in corn coleoptiles. Plant Physiology 66: 534–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RayleDL, Cleland RE.1992. The acid growth theory of auxin‐induced cell elongation is alive and well. Plant Physiology 99: 1271–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SachsJ.1887.Lectures on the physiology of plants. Oxford, UK: Clarendon. [Google Scholar]
- SchrankAR.1948. Note on the effect of unilateral illumination on the transverse electrical polarity in the Avena coleoptile. Plant Physiology 23: 362–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShabalaSN, Newman IA, Morris J.1997. Oscillations in H+ and Ca2+ ion fluxes around the elongation region of corn roots and effects of external pH. Plant Physiology 113: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SpaldingEP.2000. Ion channels and the transduction of light signals. Plant Cell and Environment 23: 665–674. [DOI] [PubMed] [Google Scholar]
- SpaldingEP, Cosgrove DJ.1989. Large plasma‐membrane depolarization precedes rapid blue‐light‐induced growth inhibition in cucumber Planta 178: 407–410. [PubMed] [Google Scholar]
- SpaldingEP, Cosgrove DJ.1992. Mechanism of blue‐light‐induced plasma‐membrane depolarization in etiolated cucumber hypocotyls. Planta 188: 199–205. [DOI] [PubMed] [Google Scholar]
- StoelzleS, Kagawa T, Wada M, Hedrich R, Dietrich P.2003. Blue light activates calcium‐permeable channels in Arabidopsis mesophyll cells via the phototropin signalling pathway. Proceedings of the National Academy of Sciences of the USA 100: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SuhS, Moran N, Lee Y.2000. Blue light activates potassium‐efflux channels in flexor cells from Samanea saman motor organs via two mechanisms. Plant Physiology 123: 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van VolkenburghE.1999. Leaf expansion – an integrating plant behaviour. Plant, Cell and Environment 22: 1463–1473. [Google Scholar]