Abstract
This study examined the influence of high light levels on antioxidant metabolism and the photosynthetic properties of Begonia × erythrophylla leaves. The pigment composition of shaded leaves and those developing in full sunlight was typical of shade‐ and sun‐leaves, respectively. After 28 d in full sunlight, the preformed leaves of shade plants transferred to full sunlight (transferred‐leaves) showed photo‐bleaching with lower Chl (a + b) content and Chl a : Chl b ratios than shade‐leaves, with Chl (a + b) : carotenoid ratios not significantly different. The variable/maximal fluorescence (Fv/Fm) of sun‐leaves was not significantly different from that of shade‐leaves, but transferred‐leaves had reduced Fv : Fm ratios. Light response curves for the electron transport rate (ETR), the oxidation state of photosystem II (qP) and non‐photochemical quenching (NPQ) showed significant differences between the three leaf types, with transferred‐leaves not able to acclimate completely to full sunlight, having lower ETR, qP and NPQ values at high light levels than sun‐leaves. Transfer to full sunlight caused a rapid increase in H2O2 and lipid hyperoxides, and a slight increase in protein oxidation. Ascorbate and glutathione levels decreased rapidly, as did the size of the total glutathione pool and, in addition to the general oxidation of proteins, rapid decreases in both the initial and total activities of chloroplastic fructose‐1,6‐bisphosphatase and glyceraldehyde‐3‐phosphate dehydrogenase were observed. These results suggest that a more oxidizing cellular environment is the likely cause of the photo‐bleaching observed upon transfer of shade‐leaves to full sunlight. Acclimation of transferred‐leaves to full sunlight involved gradual increases in the activities of enzymes involved in antioxidant metabolism, including superoxide dismutase, catalase, glutathione reductase, ascorbate peroxidase, dehydroascorbate reductase and monodehydroascorbate reductase, but the levels of these enzymes still remained at levels lower than those found in sun‐leaves.
Key words: Active oxygen species, antioxidants, Begonia × erythrophylla, light, oxidative stress, photosynthesis
INTRODUCTION
Antioxidant metabolism plays an important role in protecting plants from a wide variety of environmental stresses, such as drought, extreme temperatures, pollutants, ultraviolet radiation and high levels of light (Foyer et al., 1994; Smirnoff, 1995). Although light is required for plant growth and development, when exposed to photosynthetically active radiation (PAR) at photosynthetic photon flux densities (PPFD) greater than those required for CO2 assimilation, electron carriers can be over‐reduced and plants can suffer photo‐inhibition (Demmig‐Adams and Adams, 1992; Foyer et al., 1994).
Plants grown under higher PPFD usually have a decreased capacity to absorb incident radiation and an increased ability to dissipate excess excitation energy. Chlorophyll fluorescence has proved useful for measuring the photochemical and non‐radiative dissipation activities in leaves under different PPFD. In two recent studies, Greer and Jeffares (1998) and Laing et al. (2000) demonstrated that the quantum yield of photosystem II (PSII) and the oxidation state of PSII (qP) decline as PPFD increases, while electron transport rate (ETR) and non‐photochemical quenching (NPQ) increase.
Under conditions of excess photon energy the NPQ capacity of the photosynthetic apparatus can be exceeded and the photosynthetic electron transport system becomes a source of active oxygen species (AOS) and can generate singlet oxygen, 1O2, and superoxide, O2– (Asada, 1994). The half‐life of O2– in plant cells is relatively short. It can disproportionate spontaneously to form hydrogen peroxide (H2O2) and molecular oxygen (O2), or this reaction can be catalysed by the enzyme superoxide dismutase (SOD) (Noctor and Foyer, 1998). The H2O2 produced by these reactions is not as reactive as O2– and, by comparison, is a relatively long‐lived molecule. However, in cells under oxidative stress, O2– can reduce transition metals which can in turn reduce H2O2, generating highly reactive hydroxyl radicals (OH·). These can trigger the autocatalytic process of lipid peroxidation (Halliwell and Gutteridge, 1989).
The ability to withstand the oxidative stress imposed by AOS depends on the antioxidative capacity of the cell. Plant cells contain both enzymic and non‐enzymic antioxidants. SOD catalyses the disproportionation of H2O2 to O2, and the resultant H2O2 is decomposed by catalase (CAT) or ascorbate peroxidase (APOX), the latter enzyme requiring reduced ascorbate, provide by the ascorbate–glutathione pathway, for the reaction to occur. In the ascorbate–glutathione pathway, reduced glutathione (GSH) is needed for the reduction of dehydroascorbate (DHA), which is formed via monodehydroascorbate (MDHA) produced by the action of APOX or by non‐enzymatic reactions of ascorbate (ASA) with oxidants. In addition to playing roles in enzymic antioxidant metabolism, both ASA and GSH can directly scavenge AOS (Asada, 1994). GSH is especially important as it helps protects thiol‐containing enzymes such as the Calvin‐cycle enzymes, NADP+‐dependent glyceraldehyde‐3‐phosphate dehydrogenase (G3PDH) and chloroplast fructose‐1,6‐bisphosphatase (FBPase). The maintenance of a reduced pool of glutathione is mediated by the activity of glutathione reductase (GR) at the expense of NADPH oxidation (Hausladen and Alscher, 1993).
There are many reports in the literature detailing changes in the activities of enzymes involved in antioxidant metabolism in response to high‐light stress (Gillham and Dodge, 1987; Foyer et al., 1989; Mishra et al., 1995; Grace and Logan, 1996; Logan et al., 1998). From these and other studies it has become clear that plants grown under low PPFD are less able to protect themselves from AOS, due to a lower capacity to dissipate excess light energy and a lower capacity to scavenge AOS, compared with plants grown in full sun.
Begonias are popular ornamental plants, with many species grown indoors as potted plants. Numerous studies have shown light to be of great importance for the growth and development of begonia, and hence their commercial production (Nowak and Feild, 1993; Rudnicki et al., 1993; Myster, 1999). Many begonias are classified as shade‐demanding understorey species, and have leaves that are highly adapted to deep shade (Lee et al., 1990). As a result they are often propagated under very low light levels (Myster, 1999) and can suffer damage if transferred to full sunlight. Despite the importance of light for the development of begonias, very little is known about the physiology of how these commercially valuable plants respond to light.
Begonia × erythrophylla J. Neuman, also known as B. × Feistii Hort. ex L.H. Bailey, is a hybrid begonia (B. manicata × B. hydrocotylifolia) probably of garden origin and is a popular ornamental plant, mostly grown indoors as a pot plant. B. × erythrophylla plants have large well‐developed leaves to maximize light interception and, when placed in full sunlight, fully expanded leaves exhibit rapid photo‐bleaching.
There have been no reports in which the influence of light levels on the production of AOS and antioxidant metabolism in begonia plants has been considered. The objectives of this study were to determine the role that antioxidant metabolism plays in the acclimation of B. × erythrophylla plants following transfer of plants, grown in the shade, to full sunlight, and to test the hypotheses that pre‐existing foliage suffers oxidative damage, does not acclimate completely to full sunlight and that only new leaves are fully acclimated to full sunlight. Specifically, we monitored chlorophyll fluorescence parameters, the production of AOS, oxidative damage to cellular macromolecules and the development of the antioxidant systems following transfer of B. × erythrophylla plants from shade to full sunlight.
MATERIALS AND METHODS
Plant material and sampling protocols
Begonia × erythrophylla stock plants were grown in a glasshouse under shade‐cloth, which reduced PAR to approx. 10–15 % of ambient, with a mean midday PAR of 155 µmol m–2 s–1 during the experimental period. The photoperiod in the glasshouse was extended to 16 h by artificial illumination (Phillips SonT Agro 400 lamps) and the night temperature was maintained at a minimum of 15 °C by electric heating.
The leaves of plants that were maintained in the shade for the duration of the experiment are referred to as shade‐leaves, while those that developed fully in the shade and were then transferred to full sunlight are referred to as transferred‐leaves. The leaves of the transferred plants that expanded and developed in full sunlight are referred to as sun‐leaves. Sun‐leaves were only sampled from plants grown in full sunlight for 21 and 28 d. Leaf samples were harvested and measurements of variable/maximal fluorescence (Fv/Fm) were made between 1100 and 1300 h to minimize diurnal fluctuations. Irradiance curves were generated 28 d after the transfer of plants to full sunlight.
Pigment quantification
Chlorophylls and carotenoids were extracted in dimethysulfoxide (DMSO). Discs (1 cm2) were weighed and placed in 5 ml DMSO for 48 h, at room temperature. Chlorophyll and carotenoid pigments were measured using a Jasco V‐550 dual‐beam spectrophotometer and determined, on a fresh weight basis, following the formulae of Chapelle et al. (1992).
Chlorophyll fluorescence measurements
Chlorophyll fluorescence was measured using a Pulse Amplitude Modulation (PAM‐2000) portable chlorophyll fluorometer (Walz, Effeltrich, Germany). Six plants from each treatment were brought into the laboratory and adapted to the dark, at ambient temperature for 30 min. To quantify the efficiency of photon capture by open PSII reaction centres, leaf surfaces were exposed to a saturating pulse of light for determination of the ratio of Fv : Fm (Butler and Kitajima, 1975).
The light response curves of ETR, qP and NPQ were also determined. Minimal fluorescence Fo and Fm were determined for dark‐adapted leaves. The leaves were then acclimated at 100 µmol m–2 s–1 for 10 min before being exposed to gradual increases in PPFD provided by an external Halogen lamp (2050‐H). At each light intensity the leaves were allowed to acclimate for 6·5 min and then given a saturating pulse of light. Following the saturating pulse, the actinic light was turned off and minimal fluorescence in the light (Fo′) measured after a 5·5 s exposure to far‐red light. The quantum yield of PSII was derived as per Gentry et al. (1989): ΔF/Fm′ = (Fm′ – Ft)/Fm′, where Fm′ is maximal fluorescence in the light, and Ft is fluorescence at time t. ETR was determined from yield × PAR × 0·5 × 0·84, qP from (Fm′ – Ft)/(Fm′ – FO′) and NPQ from (Fm – Fm′)/Fm′.
Hydrogen peroxide determination
Frozen leaf tissue was ground in 50 mm ice‐cold potassium phosphate buffer (3 ml g–1 f. wt), containing 200 mm perchloric acid and the catalase inhibitor hydroxylamine (1 mm), and centrifuged at 10 000 g for 15 min at 4 °C. The perchloric acid was removed and hydrogen peroxide levels determined using the spectrophotometric method described by Cui et al. (1999).
Lipid extraction and peroxide analysis
Leaf tissue was boiled in isopropanol and lipids were extracted, as described by Navari‐Izzo et al. (1991). Lipid hydroperoxides were determined using the method of Droillard et al. (1987). Briefly, 2 ml lipid extract was added to a solution of 5 ml ethanol, 0·2 ml 1 m HCl and 0·1 ml 35 mm ferrous ammonium sulfate. One millilitre of 2·6 m ammonium thiocyanate was added and the absorbance was read at 480 nm. A calibration curve with t‐butyl hydroperoxide was used for quantification.
Oxidative damage to proteins
Frozen leaf tissue was ground in 50 mm ice‐cold potassium phosphate buffer (pH 7·0) (3 ml g–1 f. wt), containing 1 mm EDTA and 2·5 µg ml–1 phenylmethylsulfonyl fluoride and leupeptin, and centrifuged at 10 000 g for 15 min at 4 °C. Contaminating nucleic acids were removed by treatment with streptomycin sulfate, and the oxidative damage to proteins was estimated as the protein carbonyl content, as determined by reaction with 2,4‐dinitrophenylhydrazine (Reznick and Packer, 1994).
Glutathione extraction and determination.
Tissue was homogenized in ice‐cold 5 % (w/v) sulfosalicylic acid (5 ml g–1 f. wt), centrifuged at 10 000 g for 15 min at 4 °C, and the supernatant was used for total and GSSG determinations by the 5,5′‐dithio‐bis‐(2‐nitrobenzoic acid)/GSSG reductase recycling procedure (Anderson et al., 1992). GSSG was determined after removal of GSH by 2‐vinylpyridine derivatizations, according to Anderson (1985). The contents of total glutathione and GSSG were calculated as per Sgherri et al. (1994), and GSH was determined by subtraction of GSSG (as GSH equivalents) from the total glutathione content.
Ascorbate extraction and determination
Tissue was homogenized in 5 % (w/v) metaphosphoric acid (10 ml g–1 f. wt) and the homogenate centrifuged at 10 000 g for 15 min at 4 °C. Total and reduced ASA in the supernatant were determined using the method of Hodges et al. (1996). Briefly, total ascorbate was determined in a 1 ml reaction mixture containing 200 µl supernatant, 60 mm K‐Na phosphate (pH 7·4), 3 mm EDTA and 1 mm dithiothreitol (DTT). After 10 min at 25 °C 100 µl of 40 mm N‐ethylmaleimide was added. To determine reduced ascorbate the reaction mixture was as above, except 200 µl H2O replaced the DTT and N‐ethylmaleimide. Colour was developed in both sets of reaction mixtures by the addition of 400 µl of 0·61 m trichloroacetic acid, 400 µl of 0·8 m orthophosphoric acid, 400 µl of 0·26 m α,α′‐dipyridyl in 70 % ethanol and 200 µl of 0·19 M FeCl3, and after 1 h at 40 °C, the absorbance was measured at 525 nm. Oxidized ascorbate (DHA) was calculated by subtracting reduced ascorbate from total levels.
Extraction and assay of antioxidant enzymes
Tissue was ground to a fine powder in liquid nitrogen and total proteins were extracted by homogenizing the powdered tissue in extraction buffer (3 ml g–1 f. wt). The extraction buffer contained 100 mm potassium phosphate (pH 7·0), 40 mm KCl, 10 % (w/v) glycerol, 0·25 % (w/v) Triton X‐100 and 2 % (w/v) Polyclar AT (Serva Chemicals Ltd, Heidelberg, Germany). Ascorbate (1 mm) was included in the extraction buffer when extracts were to be used for assaying APOX. Homogenates were centrifuged at 10 000 g for 20 min at 4 °C. The supernatants obtained were divided into aliquots and stored at –80 °C. Protein concentrations were determined according to the method of Pederson (1977), using bovine serum albumin as a standard.
APOX (EC 1.11.1.11) was assayed by following the decrease in A290 as ascorbate disappeared (Rao et al., 1996). The reaction mixture (1 ml) contained 100 mm potassium phosphate (pH 7·0), 0·5 mm ascorbate, 0·2 mm H2O2 and up to 50 µl extract. Activity was calculated using the extinction coefficient 2·8 mm–1 cm–1.
Dehydroascorbate reductase (DHAR) (EC 1.8.5.1) was assayed by monitoring the change in absorbance at 265 nm, as described by Miyake and Asada (1992), and monodehydroascorbate reductase (MDHAR) (EC 1.6.5.4) by measuring the decrease in absorbance at 340 nm, as described by Foyer et al. (1989). For DHAR the reaction mixture (1 ml) contained 50 mm HEPES/KOH (pH 7·0), 2·5 mm GSH 0·2 mm DHA, 0·1 mm EDTA and up to 50 µl of enzyme extract. DHAR activity was calculated using an extinction coefficient of 7·0 mm–1 cm–1. For MDHAR the reaction mixture (1 ml) contained 0·4 units ascorbate oxidase, 100 mm HEPES/KOH (pH 7·6), 2·5 mm ascorbate, 25 µm NADPH and up to 100 µl enzyme extract.
GR (EC 1.6.4.2) activity was determined following the procedure of Sgherri et al. (1994), by measuring the decrease in absorbance at 340 nm. The reaction mixture (1 ml) contained 0·2 m potassium phosphate (pH 7·5), 0·2 mm Na2 EDTA, 1·5 mm MgCl2, 0·25 mm GSSG, 25 µm NADPH and up to 50 µl of enzyme extract. The reaction was initiated by the addition of NADPH, and corrections for GSSG‐independent NADPH oxidation were not necessary. Activity was calculated using the extinction coefficient 6·2 mm–1 cm–1.
Superoxide dismutase (EC 1.15.1.1) was assayed by the inhibition of xanthine oxidase‐dependent reduction of nitroblue tetrazolium (McCord and Fridovich, 1969). The reaction mixture (1 ml) contained 50 mm potassium phosphate (pH 7·8), 0·5 mm nitroblue tetrazolium, 0·5 mm EDTA, 0·1 mm xanthine and 0·05 units xanthine oxidase. One unit of SOD is defined as the amount of enzyme that inhibits, by 50 %, the control rate (0·025 units of absorbance at 560 nm min–1).
Catalase (EC 1.11.1.6) was assayed by following the decrease in A240 as H2O2 was consumed (Rao et al., 1996). The reaction mixture (1 ml) contained 50 mm potassium phosphate (pH 7·0), 37·5 mm H2O2 and up to 50 µl enzyme. Activity was calculated using the extinction coefficient 39·4 mm–1 cm–1.
All enzyme assays were conducted at 25 °C using a Jasco 550 spectrophotometer fitted with a MFC‐132 temperature control cell. The conditions for all assays were chosen so that the rate of reaction was constant for the entire experimental period and proportional to the amount of enzyme added.
Extraction and determination of G3PDH and chloroplast FBPase
Tissue was ground to a fine powder in liquid nitrogen and total proteins were extracted by homogenizing the powdered tissue in extraction buffer (3 ml g–1 f. wt). For G3PDH (EC 1.2.1.13) the extraction buffer contained 100 mm potassium phosphate (pH 8·0), 0·1 mm EDTA and 4 % (w/v) Polyclar AT, and for FBPase (EC 1.11.1.9), 100 mm potassium phosphate (pH 8·0), 1·0 mm EDTA, 10 mm MgCl2 and 4 % (w/v) Polyclar AT. Enzymes were assayed using the procedures of Harten and Eickmeier (1986) for G3PDH and Hurry et al. (1995) for FBPase. For G3PDH the reaction mixture (1 ml) contained 100 mm Tris–HCl (pH 8·0), 5 mm MgCl2, 2 mm ATP, 1 mm 3‐phosphoglyceric acid, 0·06 units of 3‐phosphoglyceric phosphokinase from bakers’ yeast, 0·14 mm NADPH and up to 50 µl enzyme extract. For chloroplast FBPase the reaction mixture (1 ml) contained 100 mm Tris–HCl (pH 8·0), 0·5 mm Na2EDTA, 10 mm MgCl2, 0·6 mm fructose‐1,6‐bisphosphate, 0·6 units glucose‐6‐phosphate dehydrogenase, 1·2 units glucose phosphate isomerase, both from bakers’ yeast, 0·3 mm NADPH and up to 100 µl enzyme extract. For both enzymes the decrease in A340 was measured, and the enzyme activity was calculated using the extinction coefficient 6·2 mm–1 cm–1. Total activity was assayed on aliquots of enzyme extract incubated for 20 min with 20 mm DTT.
Statistical analysis
The significance of differences between mean values was determined by Duncan’s multiple range test (SPSS 10 for Mac computers).
RESULTS
Effect of full sunlight on chlorophyll pigments
The chlorophyll and carotenoid contents of pre‐existing and new leaves of plants transferred from shade to full sunlight were compared with those of shaded control plants, 28 d after transfer. The pigment composition of the leaves of shaded plants (shade‐leaves) was typical of a shade‐demanding species, with a high Chl (a + b) content, a low Chl a : Chl b ratio and a high Chl (a + b) : carotenoid ratio (Table 1). In contrast, new leaves developing on plants transferred to full sunlight (sun‐leaves) had lower Chl (a + b) contents, a higher Chl a : Chl b ratio and a lower Chl (a + b) : carotenoid ratio (Table 1). Transferred‐leaves had a significantly lower Chl (a + b) content and Chl a : Chl b ratio than shade‐leaves, but no significant difference in the Chl (a + b) : carotenoid ratio (Table 1). No significant difference in pigment composition was found when the pre‐existing and new leaves of shaded plants were compared (data not presented).
Table 1.
Chlorophyll and carotenoid contents of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun)
| Chl a : Chl b ratio | Chl a + b (mg g–1 f. wt) | Carotenoids (mg g–1 f. wt) | Chl (a + b) : carotenoids ratio | |
| Shade | 2·41 ± 0·13a | 1·55 ± 0·21b | 0·41 ± 0·07b | 3·78 ± 0·34b |
| Transferred | 2·79 ± 0·21b | 0·99 ± 0·19a | 0·28 ± 0·03a | 3·54 ± 0·29b |
| Sun | 3·11 ± 0·25b | 1·16 ± 0·14a | 0·43 ± 0·09c | 2·70 ± 0·31a |
Mean values ± s.e. (n = 6).
Letters indicate values that differ significantly at P < 0·05.
Chlorophyll a fluorescence
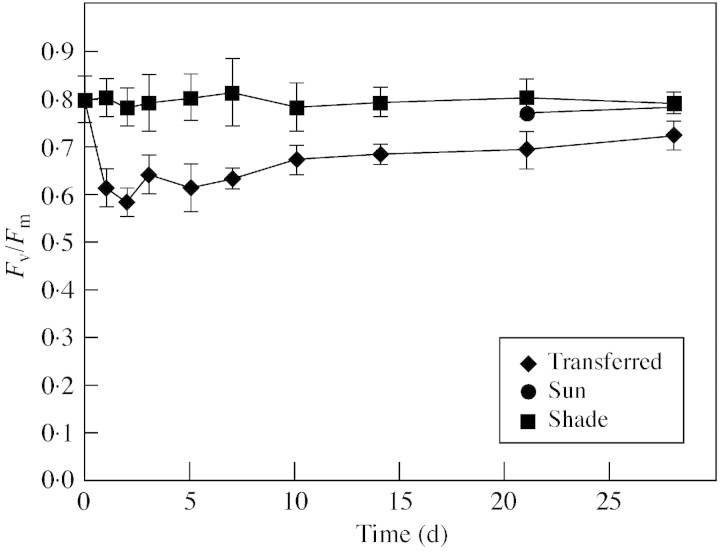
Transfer to full sunlight rapidly caused a significant decline in Fv/Fm values (Fig. 1). The Fv/Fm values of transferred‐leaves failed to recover completely, remaining significantly less than those of shade‐ and sun‐leaves. The Fv/Fm values of sun‐leaves were not significantly different to those of shade‐leaves (Fig. 1).
Fig. 1. The Fv : Fm ratios of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 6).
The light response curves for ETR, qP and NPQ showed significant differences between the three leaf types (Fig. 2). ETR was irradiance‐saturated for shade‐leaves at 148 µmol m–2 s–1, transferred‐leaves at 248 µmol m–2 s–1 and for sun‐leaves at 405 µmol m–2 s–1 (Fig. 2A). The ETRs of sun‐leaves were higher than those of shade‐leaves at irradiances greater than 148 µmol m–2 s–1, increasing to a maximum 75 % higher at irradiances of 405 µmol m–2 s–1 or greater. The ETRs of transferred‐leaves were also higher than those of shade‐leaves at irradiances greater than 148 µmol m–2 s–1, but only reached a maximum 25 % higher than those of shade‐leaves at irradiances of 248 µmol m–2 s–1 or greater.
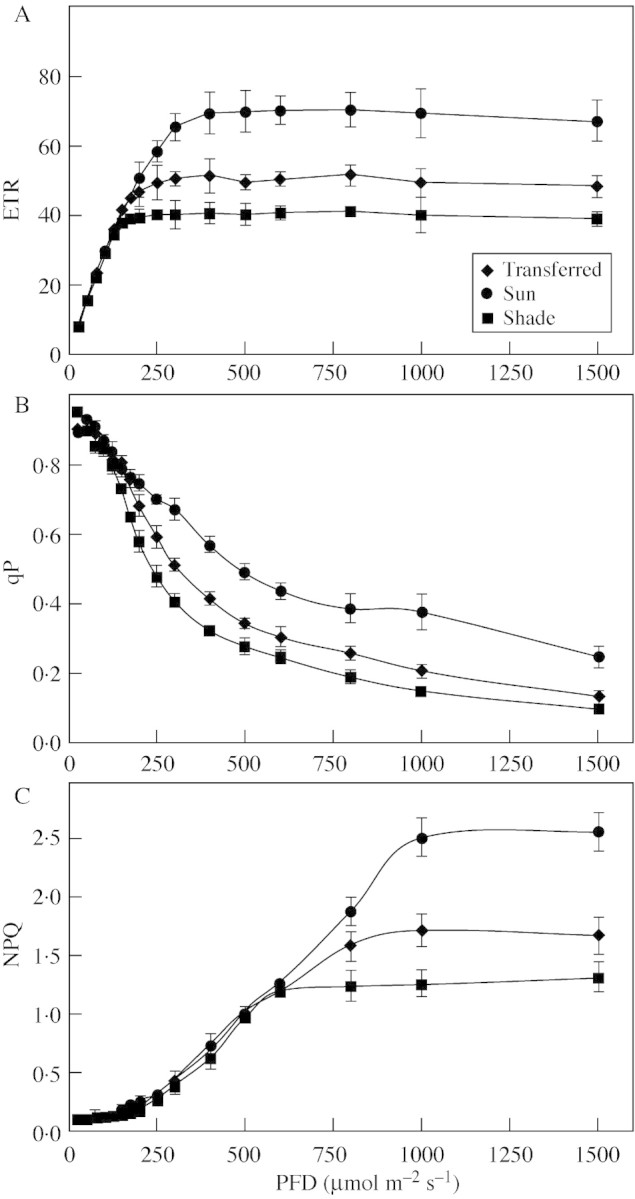
Fig. 2. Light response curves for ETR (A), qP (B) and NPQ (C) of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 6).
Typically, qP decreased in shade‐, sun‐ and transferred‐leaves as irradiance was increased (Fig. 2B). At low irradiances (up to 100 µmol m–2 s–1) there was little difference in qP values between the shade‐, transferred‐ and sun‐leaves, but above 148 µmol m–2 s–1 qP values of transferred‐ and sun‐leaves were significantly higher than those of the shade‐leaves. At irradiances above 200 µmol m–2 s–1 qP values of sun‐leaves exceeded those of transferred‐leaves.
The dependence of NPQ on irradiance is useful for estimating the relative increase in the non‐radiative dissipation of absorbed excitation energy. Higher NPQ values were observed for sun‐ and transferred‐leaves than shade‐leaves, although the maximum NPQ value of transferred‐leaves was still significantly lower than that of sun‐leaves (Fig. 2C). Leaves from all sources showed irradiance‐saturation of NPQ; for shade‐leaves this occurred at 597 µmol m–2 s–1 and for transferred‐ and sun‐leaves at 1004 µmol m–2 s–1.
Effect of full sunlight on H2O2 levels, lipid hydroperoxides and protein oxidation
Transfer to full sunlight rapidly caused a significant increase in the levels of leaf H2O2 (Fig. 3A). Twenty‐four hours following transfer, levels of H2O2 had increased by nearly 300 %, remaining at high levels for 5 d and then declining to levels twice those found in shade‐leaves (Fig. 3A). Levels of H2O2 in sun‐leaves were not significantly different from those found in shade‐leaves (Fig. 3A).
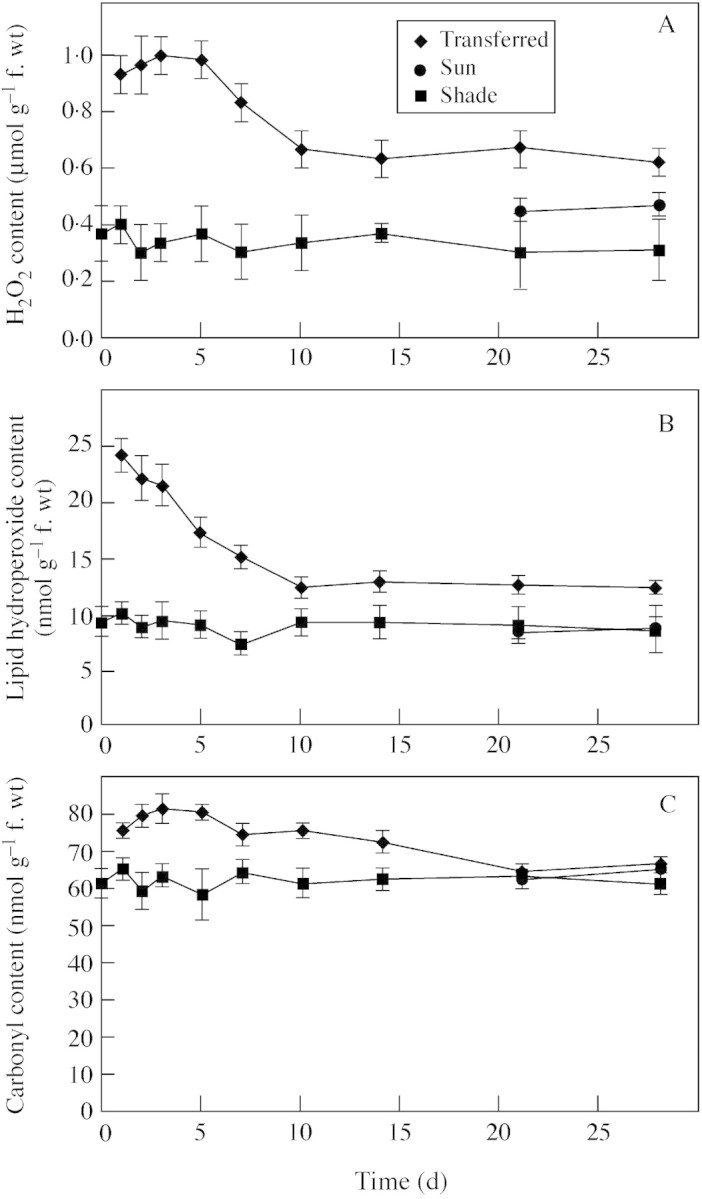
Fig. 3. The influence of growth conditions on the levels of H2O2 (A), lipid hydroperoxides (B), and carbonyl content (C) of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 5).
As observed for H2O2, levels of lipid hyperoxides increased rapidly when plants were transferred to full sunlight (Fig. 3B). Levels remained high for 7 d following transfer and then declined to levels approx. 30 % greater than those of shade‐leaves. The levels of lipid hyperoxides in sun‐leaves were not significantly different from those found in shade‐leaves (Fig. 3B).
The carbonyl content of leaves increased slightly following transfer to full sunlight, with levels remaining significantly elevated for 14 d (Fig. 3C). The carbonyl content of sun‐leaves was not significantly different from that of shade‐leaves (Fig. 3C).
Effect of full sunlight on metabolites
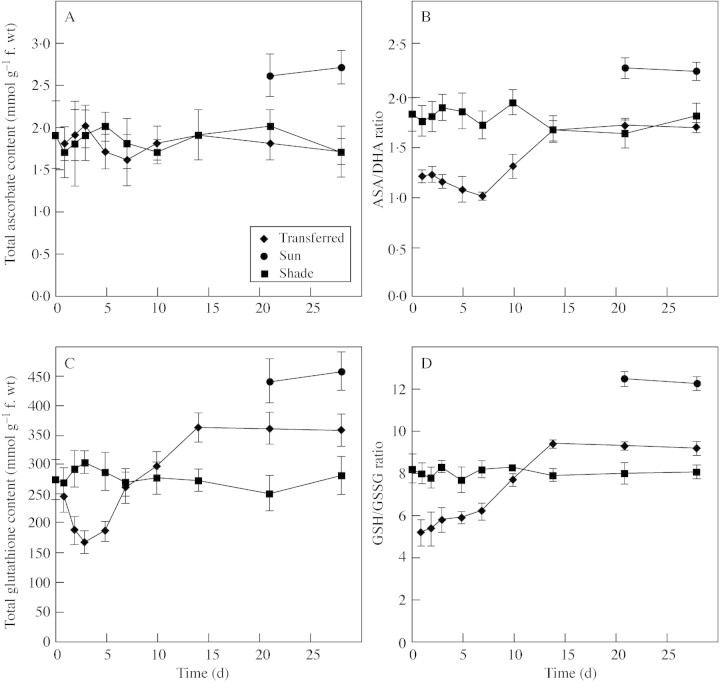
No significant differences in the total ascorbate pool were observed when transferred‐ and shade‐leaves were compared (Fig. 4A). However, the total ascorbate pool of sun‐leaves was 50 % greater than that of both shade‐ and transferred‐leaves (Fig. 4A). Although no differences in total ascorbate were observed, in transferred‐leaves the ASA : DHA ratio declined following transfer to full sunlight, and by day 7 was only 60 % that of shade‐leaves (Fig. 4B). This decline reflects a decrease in reduced abscorbate (ASA) and an increase in oxidized ascorbate (DHA). Sun‐leaves contained both larger pools of ascorbate and higher ASA : DHA ratios than shade‐leaves, indicating a greater capacity for AOS scavenging (Fig. 4A and B). Unlike the ascorbate pool, the total glutathione pool in transferred‐leaves declined rapidly upon exposure to full sunlight, dropping to almost 50 % that of shade‐leaves (Fig. 4C). Glutathione levels then increased, reaching levels about 30 % greater than those found in shade‐leaves after 10 d and remained at this level (Fig. 4C). Changes in the GSH : GSSG ratio paralleled the changes observed for total gluthathione, initially decreasing and then increasing to a value greater than that of shade‐leaves after 14 d (Fig. 4D). As seen with ascorbate, sun‐leaves contained both a larger pool of glutathione and a higher GSH : GSSG ratio than shade leaves (Fig. 4C and D).
Fig. 4. The influence of growth conditions on total ascorbate (A), the reduced (ASA) : oxidized (DHA) ascorbate ratio (B), total glutathione (C) and the reduced (GSH) : oxidized (GSSG) glutathione ratio (D) of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 5).
Effect of full sunlight on antioxidative enzymes
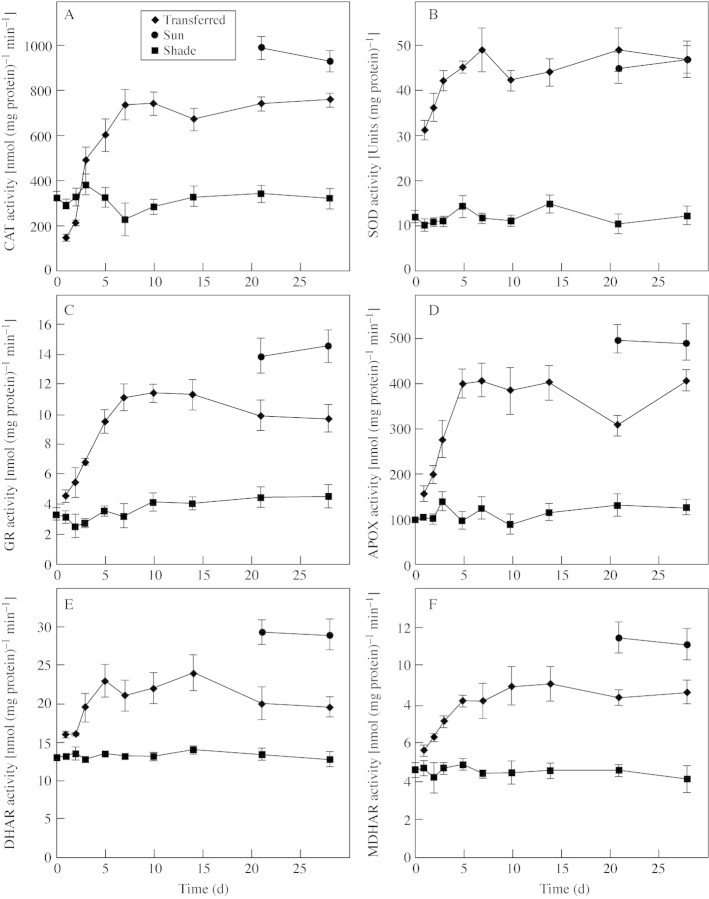
With the exception of catalase all of the antioxidant enzymes assayed showed increased activity 24 h after transfer to full sunlight (Fig. 5A–F). SOD activity increased rapidly, reaching a peak of activity four times greater than that of shade‐leaves within 7 d, and remained at this level (Fig. 5B). SOD activity of sun‐leaves was four times greater than that of shade‐leaves and was not significantly different from that of transferred leaves at 21 and 28 d (Fig. 5B).
Fig. 5. The influence of growth conditions on the levels of CAT (A), SOD (B), GR (C), APOX (D), DHAR (E) and MDHAR (F), in B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 5).
GR, APOX, DHAR and MDHAR activities all showed a more gradual increase than SOD, peaking 5–10 d following transfer to full sunlight (Fig. 5C–F). After 28 d, activities had stabilized at approx. three, three, two and three times those found in shade‐leaves, for GR, APOX, DHAR and MDHAR, respectively. In sun‐leaves GR, APOX, DHAR and MDHAR activities were higher than those found in transferred‐leaves after 28 d in full sunlight (Fig. 5C–F).
CAT activity initially declined upon transfer to full sunlight, dropping by 50 % within 24 h, before increasing to levels almost three times those found in shade‐leaves (Fig. 5A). CAT activity in sun‐leaves was higher than in transferred‐leaves exposed to full sunlight for 28 d (Fig. 5A).
Effect of full sunlight on G3PDH and chloroplast FBPase activities
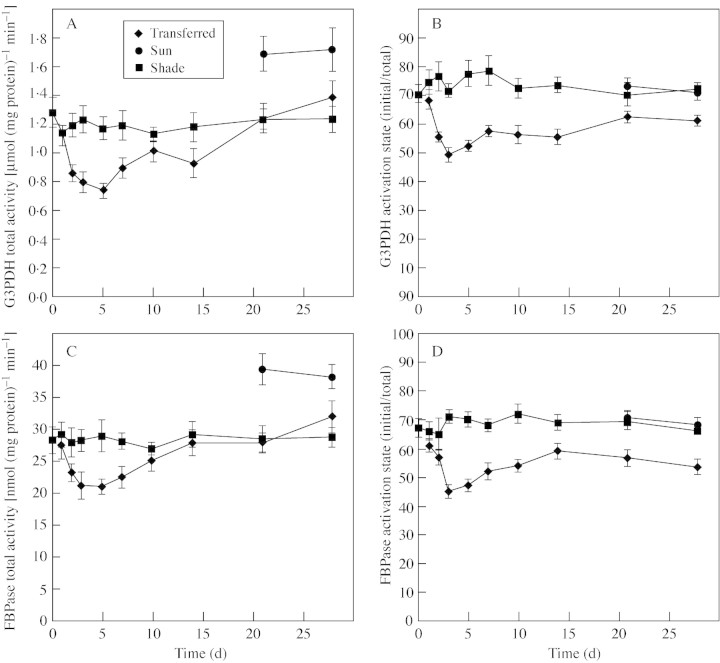
Both G3PDH and FBPase activities in transferred‐leaves showed the same trends, with declines in both total activity and activation state upon transfer to full sunlight (Fig. 6). However, after 28 d in full sunlight the total activities of these enzymes had recovered to similar levels found in the shade, but the activation states of both enzymes remained lower than in shaded plants (Fig. 6). In sun‐leaves, while the total activities of G3PDH and FBPase were almost 30 % higher than in shade‐leaves, the activations states were similar (Fig. 6).
Fig. 6. The influence of growth conditions on the total avtivity of G3PDH (A), the activation state of G3PDH (B), the total activity of FBPase (C), and the activation state of FBPase (D) of B. × erythrophylla leaves that developed in the shade (Shade), developed in the shade and were then transferred to full sunlight (Transferred), or developed in full sunlight (Sun). Mean values ± s.e. (n = 5).
DISCUSSION
Many studies have shown that photo‐inhibition occurs when plants are exposed to a PPFD higher than that required for the rate of CO2 fixation and higher than that to which they have been acclimated (Asada, 2000). When compared with plants grown in the shade, those grown under higher PPFD usually have a decreased capacity to absorb incident radiation and an increased ability to dissipate excess excitation energy. Begonia × erythrophylla plants grown in the shade had Fv/Fm values of approx. 0·80, close to the 0·83 value typical for non‐photo‐inhibited vascular plants (Björkman and Demmig, 1987). However, upon transfer to full sunlight a decrease in Fv/Fm was observed, indicating a strong inhibition of photosynthetic efficiency. Fv/Fm remained depressed for over 1 week and even after 28 d had not fully recovered. This rapid decrease in Fv/Fm suggests a significant transient loss of PSII function followed by a slow and possibly incomplete recovery.
The limitations of pre‐existing leaves transferred to full sunlight were also evident when the irradiance response curves for ETP and qP were compared with those of leaves that developed in full sunlight. At irradiances above 200 µmol m–2 s–1 the ETR and qP of sun‐leaves greatly exceeded those of transferred‐leaves, clearly indicating that transferred‐leaves, unlike sun‐leaves, were not fully adapted to utilize higher PPFD. As acclimation to high light levels involves a complex array of complementary and interdependent structural and functional alterations that enable efficient carbon gain in a new environment (Naidu and Delucia, 1997), it is logical to assume that full acclimation to high light levels can only be achieved in developing begonia leaves that have significant developmental plasticity and not in fully developed leaves.
A major contributor to the protection of the photosynthetic apparatus in plants growing in an environment with excess excitation energy is an increased ability to dissipate this energy via non‐photochemical quenching mechanisms such as the xanthophyll cycle, which is one of a variety of protection and repair mechanisms, including AOS scavenging systems, that help chloroplasts avoid damage due to excess PPFD (Gilles and Vidaver, 1990; Adamska, 1997; Logan et al., 1998). According to Demmig‐Adams (1990), the irradiance resulting in an increase in 1 – qP to a value above 0·6 is excess irradiance. Therefore the begonia plants grown in the shade (155 µmol m–2 s–1) were grown under a low to moderate excitation pressure (1 – qP < 0·3), while those under excess excitation pressure grown in the sun were 1 – qP > 0·6. NPQ is thought to be a good indicator of the concentration of dissipating complexes (Gilmore et al., 1995), and hence the ability of a plant to dissipate light energy in excess of that required for CO2 assimilation. Compared with leaves that developed in full sunlight, those of shaded plants and the pre‐existing leaves of plants transferred to full sunlight had lower NPQ values at higher light levels than sun‐leaves. This would probably result in greater damage to the photosynthetic apparatus and the generation of higher concentrations of AOS when shade‐leaves are exposed to high PPFD. This could lead rapidly to photo‐bleaching and hence the loss of photosynthetic pigments seen in this study. In the long term, the loss of photosynthetic pigments could be viewed as a protection mechanism as it would decrease the capacity of the leaf to absorb incident radiation and hence reduce the amount of excess excitation energy that has to be dissipated by NPQ.
The results presented here show that H2O2 is rapidly produced following transfer of B. × erythrophylla plants from shade to full sunlight. The amount of H2O2 produced was within the range 0·1–1 µmol g–1 f. wt and is similar to levels found previously in plant cells (Veljovic‐Jovanovic et al., 2003). Relatively low levels of H2O2 have been shown to cause the de‐activation of key enzymes required for photosynthetic carbon reduction, such as chloroplastic FBPase and G3PDH (Charles and Halliwell, 1981; Takeda et al., 1995). Therefore, it is important that photosynthetic cells have only low levels of hydrogen peroxide and that it is scavenged efficiently. When B. × erythrophylla plants grown in the shade were transferred to full sunlight, ASA and GSH levels decreased rapidly as did the size of the total glutathione pool. In addition to the general oxidation of proteins, rapid decreases in both the initial and total activities of chloroplastic FBPase and G3PDH were observed. GSH is less stable than GSSG under conditions of oxidative stress because AOS can rapidly oxidize GSH to GSSG, but the reduction of GSSG to GSH requires GR and NADPH. High H2O2 levels, combined with low GSH : GSSG and ASA : DHA ratios and a reduction in the size of the glutathione pool would leave the essential sulfhydryl groups of FBPase and G3PDH vulnerable to oxidative damage and could account for the reduced activities observed in this study.
Although the initial activities of FBPase and G3PDH recovered partially as transferred‐leaves began to acclimate to higher irradiances, and the glutathione levels and ASA : DHA and GSH : GSSG ratios increased, the activation states of both enzymes remained lower than before transfer to full sunlight and lower than those of sun‐leaves. This suggests that even though the activities of antioxidant enzymes increased progressively in response to the oxidative stress that occurred following the transfer of leaves to full sunlight, this increased AOS scavenging capacity was still not sufficient to allow the activation states of FPBase and G3PDH to recover to levels found in shade‐ and sun‐leaves. This reduction in enzyme activity would result in pre‐existing leaves having a lower capacity to fix carbon in full sunlight than newly formed leaves or leaves of plants grown in the shade, and hence having reduced photosynthetic efficiency. Interestingly, in addition to elevated levels of antioxidant enzymes, the pools of both ascorbate and glutathione in sun‐leaves were greater than those in shade‐leaves and transferred‐leaves, even after 28 d in full sunlight. Therefore it appears that under full sunlight, an increase in the size of both the ascorbate and glutathione pools, and not just an increase in the ability to regenerate ASA and GSH, could be important in keeping AOS at low levels and controlling the cellular redox status.
In addition to inhibiting the activity of key enzymes, cellular H2O2 can react with O2– in the presence of Fe2+, to form highly reactive hydroxyl radicals (OH·) that can trigger the autocatalytic process of lipid peroxidation and cause severe membrane damage (Halliwell and Gutteridge, 1989). The increase in lipid hydroperoxides observed upon transfer of plants to full sunlight is a clear indicator that membrane damage occurs in the leaves of B. × erythrophylla plants transferred from shade to full sunlight. Using isolated thylakoids, Jakob and Heber (1996) demonstrated that hydroxyl radicals can also inactivate both PSI and PSII, although PSI is considered to be more vulnerable to hydroxyl radical inactivation as the Fe‐S centres in the vicinity of P700 provide an environment ideal for hydroxyl radical formation (Sonoike, 1998). Inactivation of PSII and/or PSI would reduce the ability of pre‐existing leaves to utilize higher irradiances, and hence result in a further reduction in photosynthetic efficiency upon transfer to full sunlight. The combined effects on photosynthetic efficiency of oxidative damage to the photosystems and key enzymes required for carbon acquisition would result in pre‐existing leaves presenting a greater maintenance load on the plant and hence a temporary reduction in growth efficiency while pre‐existing leaves are replaced with new, high‐light acclimated, leaves.
Previous studies have indicated that increases in both the levels of cellular antioxidants and the activities of enzymes involved in antioxidant metabolism generally accompany exposure to high light levels (Foyer et al., 1997). Logan et al. (1998) suggested that in Cucurbita and Vinca plants exposed to increasing light intensities, photo‐oxidative stress is linked to photo‐inhibition and that protection against photo‐oxidative stress is important for the acclimation of plants to high light levels. The change in the size of the glutathione pool, the redox status of the glutathione and ascorbate pools and the activities of enzymes involved in the scavenging of AOS, following transfer of B. × erythrophylla plants to full sunlight, are indicative of oxidative stress. In particular, the changes in the ratios of reduced to oxidized antioxidants represent a change in the redox state of cells indicating a more strongly oxidizing environment for the first 7–10 d following transfer to full sunlight. This shift in redox status is followed by a progressive activation of the antioxidant systems that help protect cells against AOS, and so could be important in regulating antioxidant metabolism (Foyer and Noctor, 2000; Noctor et al., 2000). These findings suggest that in B. × erythrophylla, as in Cucurbita and Vinca plants, oxidative stress and high‐light stress are linked.
It has been suggested that enhancements in the activities of activated oxygen scavenging enzymes during high‐light stress occur mostly in the chloroplasts, because in photosynthetic tissues the ascorbate–gluthathione cycle is localized principally in the chloroplasts, which are the major sites of AOS production in leaves (Foyer et al., 1997). The rapid increase in SOD activity and the gradual increase in the activities of APOX, GR, DHAR and MDHAR under full sunlight indicate that this is true in B. × erythrophylla. However, the activity of antioxidant enzymes in other cell compartments may also be important for scavenging AOS. There is increasing evidence indicating the importance of the cytosolic isoforms of antioxidative enzymes, and those associated with the mitochondria and peroxisomes, in plants grown under various environmental stress conditions (Alscher et al., 1997; Karpinski et al., 1997; Logan et al., 1998).
Of the enzymes assayed in this study only CAT showed a decrease in activity. This decrease was transient and occurred immediately upon transfer to full sunlight. This response is not surprising, as it has been reported that both the synthesis and degradation of CAT are light sensitive (Hertwig et al., 1992). The decline in CAT activity combined with the rapid oxidation of both the ascorbate and glutathione pools, and the rapid increase in SOD activity that would have resulted in the rapid conversion of O2– to H2O2, could alone have resulted in the rapid increase in cellular H2O2 observed following transfer to full sunlight. However, a reduction in CAT could also lead to reduced breakdown of the H2O2 formed as a result of photorespiration and mitochondrial electron transport. Hence H2O2 from different sources could contribute to the increased levels of cellular H2O2 observed in B. × erythrophylla. Karpinski et al. (1997) demonstrated that exposure of arabidopsis plants to light stress not only triggers AOS production in the chloroplast, but also in the cytosol. They suggested that induction of AOS scavenging mechanisms in the cytosol provides an important secondary defence against AOS that occurs before the chloroplast AOS scavenging systems are saturated and that cytosolic APOXs play an important role in this process. Such a mechanism could be important in B. × erythrophylla as the transient decline in CAT activity following transfer to full sunlight could result in a rapid build up of cytosolic H2O2.
The results of this study show that shade‐acclimated leaves of B. × erythrophylla have a limited ability for photosynthetic acclimation following transfer to full sunlight, as demonstrated by lower NPQ values than sun‐leaves, and an inability to completely recover Fv/Fm and the activation states of FBPase and G3PDH, elevated levels of AOS and oxidative damage compared with sun‐ and shade‐leaves. However, pre‐existing leaves do have the ability to rapidly up‐regulate their capacity to scavenge AOS by increasing levels of SOD, CAT and the enzymes of the ascorbate–glutathione cycle, following transfer to full sunlight. Increased GR activity would help maintain the pool of glutathione in the reduced state, allowing GSH to be used by DHAR to reduce DHA to ASA (Noctor et al., 1998). In addition, elevated levels of MDHAR would allow more MDHA to be regenerated directly back to ASA providing enough NAD(P)H is available. ASA can then be used as a reductant by APOX to catalyse the reduction of H2O2 to H2O. Elevated APOX in combination with elevated CAT would lower H2O2 levels, which would in turn lead to a reduction in lipid peroxidation and reduced damage to the photosystems under full sunlight.
Of particular interest is what triggers the increased activities of these enzymes. As increased H2O2 levels, oxidative damage and a shift in redox to a more oxidized cellular environment occur within 24 h of transfer to full sunlight, redox signalling could be important for the up‐regulation of antioxidant enzyme activities. There is evidence that both the ASA : DHA ratio and the glutathione redox state may function as cellular regulatory signals (Foyer et al., 1997; Noctor et al., 2000). How the activity of the scavenging enzymes is up‐regulated in response to cellular signals is also unknown and will require further investigation. Mobilization of inactive enzyme pools, adaptive changes in the catalytic properties of the enzymes induced by the cellular environment and/or the transcription of usually silent genes are all possibilities.
In conclusion, AOS metabolism is clearly important for B. × erythrophylla plants developing in full sunlight. Although leaves that develop in full sunlight have reduced pigment concentrations and a greater capacity for NPQ than those of plants grown in the shade, they still develop significantly larger ascorbate and glutathione pools, and have greater activities of CAT, SOD, GR, APOX, DHAR and MDHAR. In addition, shade‐leaves of B. × erythrophylla possess a limited capacity for acclimation to high light levels, with an enhanced capacity for scavenging AOS appearing to play an important role.
Supplementary Material
Received: 21 August 2002; Returned for revision: 12 December 2002; Accepted: 10 February 2003 Published electronically: 3 April 2003
References
- AdamskaI.1997. ELIPs – light‐induced stress proteins. Physiologia Plantarum 100: 794–805. [Google Scholar]
- AlscherRG, Donahue JL, Cramer CL.1997. Reactive oxygen species and antioxidants: relationships in green cells. Physiologia Plantarum 100: 224–233. [Google Scholar]
- AndersonME.1985. Determination of glutathione and glutathione disulfide in biological samples. Methods in Enzymology 113: 548–555. [DOI] [PubMed] [Google Scholar]
- AndersonJV, Chevone BI, Hess JL.1992. Seasonal‐variation in the antioxidant system of eastern white‐pine needles – evidence for thermal‐dependence. Plant Physiology 98: 501–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AsadaK.1994. Production and action of active oxygen in photosynthetic tissue. In: Foyer CH, Mullineaux PM, eds. Causes of photooxidative stress and amelioration of defense systems in plants Boca Raton, FL: CRC Press, 77–104. [Google Scholar]
- AsadaK.2000. The water‐water cycle as alternative photon and electron sinks. Philosophical Transactions of the Royal Society of London Series B Biological Sciences 355: 1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BjörkmanO, Demmig B.1987. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 170: 489–504. [DOI] [PubMed] [Google Scholar]
- ButlerWL, Kitajima M.1975. Fluorescence quenching in photosystem II of chloroplasts. Biochimica et Biophysica Acta 376: 116–125. [DOI] [PubMed] [Google Scholar]
- ChappelleEW, Kim MS, McMurtrey III JE.1992. Ratio analysis of reflectance spectra (RARS): an algorithim for the remote estimation of the concentrations of chlorophyll a, chlorophyll b, and carotenoids in soybean leaves. Remote Sensing and Environment 39: 239–247. [Google Scholar]
- CharlesSA, Halliwell B.1981. Light activation of fructose biophosphatase in isolated spinach chloroplasts and deactivation by hydrogen peroxide. Planta 151: 242–246. [DOI] [PubMed] [Google Scholar]
- CuiKR, Xing GS, Liu XM, Xing GM, Wang YF.1999. Effect of hydrogen peroxide on somatic embryogenesis of Lycium arbarum L. Plant Science 146: 9–16. [Google Scholar]
- Demmig‐AdamsB.1990. Carotenoids and photoprotection in plants: a role for the xanthophyll zeaxanthin. Biochimica et Biophysica Acta 1020: 1–24. [Google Scholar]
- Demmig‐AdamsB, Adams III WW.1992. Photoprotection and other responses of plants to high light stress. Annual Review of Plant Physiology and Plant Molecular Biology 43: 599–626. [Google Scholar]
- DroillardMJ, Paulin A, Massot JC.1987. Free radical production, catalase and superoxide dismutase activities and membrane integrity during senescence of petals of cut carnations (Dianthus caryo). Physiologia Plantarum 71: 197–202. [Google Scholar]
- FoyerCH, Noctor G.2000. Oxygen processing in photosynthesis: regulation and signalling. New Phytologist 146: 359–388. [Google Scholar]
- FoyerCH, Dujardyn M, Lemoine Y.1989. Responses of photosynthesis and the xanthophyll and ascorbate‐glutathione cycle to changes in irradiances, photoinhibtion and recovery. Plant Physiology and Biochemistry 27: 751–760. [Google Scholar]
- FoyerCH, Lelandais M, Kunert KJ.1994. Photooxidative stress in plants. Physiologia Plantarum 92: 696–717. [Google Scholar]
- FoyerCH, Lopez‐Delgado H, Dat JF, Scott IM.1997. Hydrogen peroxide‐ and glutathione‐associated mechanisms of acclimatory stress tolerance and signalling. Physiologia Plantarum 100: 241–254. [Google Scholar]
- GentyB, Briantais JM, Baker NR.1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92. [Google Scholar]
- GilmoreAM, Hazlett TL, Govindjee.1995. Xanthophyll cycle‐dependent quenching of photosystem II chlorophyll a fluorescence: formation of a quenching complex with a short fluorescence lifetime. Proceedings of the National Academy of Sciences of the USA 92: 2273–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GillesSL, Vidaver W.1990. Resistance to photodamage in evergreen conifers. Physiologia Plantarum 80: 148–153. [Google Scholar]
- GillhamJ, Dodge A.1987. Chloroplast superoxide and hydrogen peroxide scavenging systems from pea leaves. Seasonal variation. Plant Science 50: 105–109. [Google Scholar]
- GraceSC, Logan BA.1996. Acclimation of foliar antioxidant systems to growth irradiance in three broad leaf evergreen species. Plant Physiology 112: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GreerDH, Jeffares D.1998. Temperature‐dependence of carbon acquisition and demand in relation to shoot growth of kiwifruit (Actinidia deliciosa) vines grown in controlled environments. Australian Journal of Plant Physiology 25: 843–850. [DOI] [PubMed] [Google Scholar]
- HartenJB, Eickmeier WG.1986. Enzyme dynamics of the resurrection plant Selaginella lepidophylla (Hook and Grev.) Spring during rehydration. Plant Physiology 82: 61–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HalliwellG, Gutteridge JMC.1989.Free radicals in biology and medicine, 2nd edn. Oxford: Clarendon Press. [Google Scholar]
- HausladenA, Alscher RG.1993. Glutathione. In: Alscher RG, Hess JL, eds. Antioxidants in higher plants Boca Raton, FL: CRC Press, 1–30. [Google Scholar]
- HertwigB, Streb P, Feierabend J.1992. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiology 100: 1547–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HodgesDM, Andrews CJ, Johnson DA, Hamilton RI.1996. Antioxidant compound responses to chilling stress in differentially sensitive inbred maize lines. Physiologia Plantarum 98: 685–692. [Google Scholar]
- HurryVM, Keerberg O, Parnik T, Gardestrom P, Oquist G.1995. Cold‐hardening results in increased activity of enzymes involved in carbon metabolism in leaves of winter rye (Secale cereale L.). Planta 195: 554–562. [Google Scholar]
- JakobB, Heber U.1996. Photoproduction and detoxification of hydroyl radicals in chloroplasts and leaves and relation to photoinactivation of photosystems I and II. Plant and Cell Physiology 37: 629–635. [Google Scholar]
- KarpinskiS, Escobar C, Karpinska B, Creissen G, Mullineaux PM.1997. Photosynthetic electron transport regulates the expression of cytosolic ascorbate peroxidase genes in Arabidopsis during excess light stress. Plant Cell 9: 627–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaingW, Greer D, Sun O, Beets P, Lowe A, Payn T.2000. Physiological impacts of Mg deficiency in Pinus radiata: growth and photosynthesis. New Phytologist 146: 47–57. [Google Scholar]
- LeeDW, Bone RA, Taris SL, Storch D.1990. Correlates of leaf optical properties in tropical forest sun and extreme shade. American Journal of Botany 77: 370–380. [Google Scholar]
- LoganBA, Demmig‐Adams B, Adams WW, Grace SC.1998. Antioxidants and xanthophyll cycle‐dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDs in the field. Journal of Experimental Botany 49: 1869–1879. [Google Scholar]
- McCordJM, Fridovich I.1969. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry 244: 6049–6055. [PubMed] [Google Scholar]
- MishraNP, Fatma T, Singhal GS.1995. Development of antioxidative defense system of wheat seedlings in response to high light. Physiologia Plantarum 95: 77–82. [Google Scholar]
- MiyakeC, Asada K.1992. Thylakoid‐bound ascorbate peroxidase in spinach‐chloroplasts and photoreduction of its primary oxidation‐product monodehydroascorbate radicals in thylakoids. Plant and Cell Physiology 33: 541–553. [Google Scholar]
- MysterJ.1999. The effects of temperature alternations, irradiance level, photoperiod, and day extension light quality on morphogenesis, growth, and flowering of Begonia × hiemalis fotsch Garten bauwissenschaft 64: 206–213. [Google Scholar]
- NaiduSL, DeLucia EH.1997. Growth, allocation and water relations of shade‐grown Quercus rubra L. saplings exposed to a late‐season canopy gap. Annals of Botany 80: 335–344. [Google Scholar]
- Navari‐IzzoF, Quartacci MF, Izzo R.1991. Free fatty acids, neutral and polar lipids in Hordeum vulgare exposed to long‐term fumigation with SO2 Physiologia Plantarum 81: 467–472. [Google Scholar]
- NoctorG, Foyer CH.1998. Ascorbate and glutathione: keeping active oxygen under control. Annual Review of Plant Physiology and Plant Molecular Biology 49: 249–279. [DOI] [PubMed] [Google Scholar]
- NoctorG, Veljovic‐Jovanovic S, Foyer CH.2000. Peroxide processing in photosynthesis: antioxidant coupling and redox signalling. Philosophical Transactions of the Royal Society of London Series B Biological Sciences 355: 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NowakJ, Fjeld T.1993. Light and ethylene effects on assimilate distribution, acid invertase activity and keeping quality of begonia. Plant Growth Regulation 13: 47–53. [Google Scholar]
- PedersonGL.1977. A simplification of the protein assay method of Lowry et al which is more generally applicable. Analytical Biochemistry 83: 346–356. [DOI] [PubMed] [Google Scholar]
- RaoMV, Paliyath C, Ormrod DP.1996. Ultraviolet‐B‐ and ozone‐induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana Plant Physiology 110: 125–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ReznickAZ, Packer L.1994. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods in Enzymology 233: 357–363. [DOI] [PubMed] [Google Scholar]
- RudnickiRM, Fjeld T, Moe R.1993. Effect of light quality on ethylene formation in leaf and petal disks of Begonia × Hiemalis‐Fotsch Cv Schwabenland Red. Plant Growth Regulation 13: 281–286. [Google Scholar]
- SgherriCLM, Loggini B, Puliga S, Navariizzo F.1994. Antioxidant system in Sporobolus stapfianus: changes in response to desiccation and rehydration. Phytochemistry 35: 561–565. [Google Scholar]
- SmirnoffH.1995. Antioxidant systems and plant response to the environment. In: Smirnoff H, ed. Environment and plant meta bolism Oxford, UK: BIOS Scientific Publishers, 217–244. [Google Scholar]
- SonoikeK.1998. Various aspects of inhibition of photosynthesis under light/chilling stress: ‘Photoinhibition at chilling temperatures’ versus ‘Chilling damage in the light’. Plant Research 111: 121–129. [Google Scholar]
- TakedaT, Yokota A, Shigeoka S.1995. Resistance of photosynthesis to hydrogen peroxide in algae. Plant and Cell Physiology 36: 1089–1095. [Google Scholar]
- Veljovic‐JovanovicS, Noctor G, Foyer CH.2002. Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiology and Biochemistry 40: 501–507. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.