Abstract
Species within the genus Pseudowintera exhibit high rates of self‐sterility. Self‐sterility in the genus has been previously posited—but not confirmed—to be the result of late‐acting ovarian self‐incompatibility (OSI) functioning within nucellar tissue of the ovule to prevent self pollen tubes from entering the embryo sac. Structural and functional aspects of pollen–carpel interactions and early seed development following cross‐ and self‐pollination were investigated in P. axillaris to determine the site, timing and possible mechanisms of self‐sterility. No significant differences were observed between pollen tube growth, ovule penetration and double fertilization following cross‐ and self‐pollination. Pollen tubes exhibited phasic growth in an extracellular matrix composed of proteins and carbohydrates, as well as arabinogalactans/arabinogalactan proteins. A uniform failure in embryo sac development prior to division of the zygote was apparent within 15 d following double fertilization by self gametes. Results indicate that SI mechanisms in P. axillaris do not prevent double fertilization from occurring. Instead, mechanisms of self‐sterility affect post‐zygotic development of the embryo sac. Although self‐sterility may be attributed to inbreeding depression, given the post‐zygotic nature of failure in embryo sac development, the possibility of late‐acting OSI is discussed.
Key words: Embryo sac, late‐acting self‐incompatibility, ovarian self‐incompatibility, early‐acting inbreeding depression, pollination‐regulated development
INTRODUCTION
Early‐acting inbreeding depression and self‐incompatibility (SI) are the two main causes of reduced seed set following self‐pollination in flowering plants. Early‐acting inbreeding acts post‐zygotically and results in abortion of progeny homozygous for deleterious recessive alleles (Charlesworth and Charlesworth, 1987; Husband and Schemske, 1996). In comparison, self‐incompatibility is a genetically based self‐recognition system that reduces the frequency of self seed set through rejection of self pollen. Known SI systems function primarily pre‐zygotically with arrest of pollen function at the stigma or style (see Matton et al., 1994; Franklin et al., 1995; de Nettancourt, 1997), although it has been hypothesized that some species may exhibit post‐zygotic SI (see Seavey and Bawa, 1986; Gibbs and Bianchi, 1993, 1999; Sage et al., 1994; Gibbs et al., 1999).
The vessel‐less Winteraceae family of the subclass Magnoliidae has received considerable attention in recent decades as a result of the apparent primitiveness of many of its vegetative and floral features and its long fossil history (Bailey and Swamy, 1951; Sampson, 1980; Cronquist, 1981; Walker et al., 1983; Friis and Endress, 1990; Brenner and Bickoff, 1992; Suh et al., 1993). One genus within the family, Pseudowintera, exhibits high rates of self‐sterility (Norton, 1980; Godley and Smith, 1981; Lloyd and Wells, 1992). Qualitative studies on pollen tube growth in Pseudowintera have led to the conclusion that self‐rejection occurs in the ovary with genetic recognition/rejection of self pollen in ovules at the nucellus prior to embryo sac entry (Norton, 1980; Godley and Smith, 1981; Lloyd and Wells, 1992). Classification of self‐sterility as pre‐zygotic in ovules, particularly involving the nucellus, of Pseudowintera is still tentative. Characterization of self‐sterility in Pseudowintera requires quantitative analysis of pollen tube growth following cross‐ and self‐pollination and detailed structural studies to assess pollen–carpel interactions and early embryo/seed development. Although previous studies have provided some insights into the pollen tube pathway and pollen–carpel interactions as well as embryo/seed development (Bhandari, 1963; Sampson, 1963; Norton, 1980; Lloyd and Wells, 1992), quantitative comparisons of cross‐ and self‐pollen tube growth, as well as structural information on pollen‐carpel interactions and early seed development following cross‐ and self‐pollination are lacking for the genus. The purpose of this study is to provide such information for P. axillaris. The primary questions addressed were: (1) do cross and self pollen tubes exhibit differential qualitative and quantitative growth characteristics within the pistil; (2) do self pollen tubes effect double fertilization as successfully as cross pollen tubes; (3) are there differences in ovule/seed development following cross‐ vs. self‐pollination; and (4) if there are differences in ovule/seed development, when are they apparent and in what tissues are differences first manifested?
MATERIALS AND METHODS
Study organism
Experimental material of P. axillaris used in this study was located in the Akatarawa region at the southern end of Tararua Forest Park (40°58′S, 175°10′E), North Island, New Zealand. Six flowering trees were selected at random for cross‐ and self‐pollinations. Self‐pollinations were made on each of the six flowering trees, and cross‐pollinations were conducted on all six flowering trees between individual trees heterozygous at the isozyme locus PGI‐2 (determined by W. W. Cole, University of Toronto, Canada). Field and laboratory pollinations were conducted as described by Norton (1980) and Lloyd and Wells (1992). The shoot tip and leaf apices were removed from branches bearing 15–20 floral buds to allow easy application and removal of nylon mesh pollination bags. Pollinations were made on fully opened flowers with receptive stigmas that had not yet begun to shed pollen. Stigma receptivity was indicated by the presence of stigmatic secretions. Pollinations were performed from 0800 until 1100 h, at which time the secretions dried up. A freshly dehisced anther was removed using fine forceps and brushed on the stigmas of each flower.
Pollen–carpel interactions following cross‐ and self‐pollination
Structural and histochemical features of cross‐ and self‐ pollen tube growth and the transmitting tissues encountered by pollen tubes were characterized using light microscopy (LM) and scanning (SEM) and transmission (TEM) electron microscopy as described by Williams et al. (1993) and Sage and Williams (1995). SEM observations were made using a Philips 505 SEM at 2–5 kv (La B6 filament) following gold coating. TEM observations were made using a Philips 201 transmission electron microscope. Unpollinated flowers were sampled at anthesis and 25 h post‐anthesis (n = 9 at each time interval). Cross‐ and self‐pollinated flowers were harvested at 5‐h intervals for 25 h post‐pollination (n = 6–10 at each sample period).
To compare quantitative growth of cross and self pollen tubes, controlled hand‐pollinations were conducted in the laboratory as described by Lloyd and Wells (1992). Hand‐pollinated flowers were collected at 5‐h intervals for 25 h following pollination (n = 9 at each time interval) and prepared for fluorescence microscopy as described by Martin (1959). Parameters measured at each time interval included: (1) the number of germinated pollen grains; (2) the number of pollen tubes at the point of ovary entry; and (3) the number of ovules penetrated by pollen tubes. Mean pollen tube length at each time interval was determined as described by Cruzan (1986). ANOVA was performed to determine whether there were differences over time between (1) mean cross and self pollen tube length; (2) mean cross and self pollen tube density at the point of ovary entry; and (3) percentage ovule penetration by cross and self pollen tubes.
Double fertilization and early embryo/seed development following cross‐ and self‐pollination
Flowers from field pollinated trees were harvested 3 and 15 d after pollination (n = 9–16 for each time interval), fixed in formalin–acetic acid–ethanol (FAA), dehydrated in a tertiary butyl alcohol series, embedded in Paraplast wax, sectioned at 10 µm, and stained in Heidenhain’s iron‐alum haematoxylin with safranin and fast green as counterstains. Serial sections were scored for double fertilization as described by Sage et al. (1998). Double fertilization was indicated by: (1) the absence of unfused sperm nuclei within embryo sacs penetrated by a pollen tube; (2) the presence of a resting zygote; and (3) the presence of one or more endosperm nuclei. Embryo sac development and integument development were quantified using a Zeiss Axiophot microscope equipped with image analysis software (Northern Exposure; Empix Imaging, Ontario, Canada). Mean percentage double fertilization and embryo sac length and width following cross‐ and self‐pollination were contrasted using one‐way ANOVA.
RESULTS
Structural features of the pollen tube pathway
An exudate (Fig. 1A–C) that stained positive for protein, lipids and carbohydrates covered papillate stigmatic cells. Stigmatic papillae cells contained prominent vacuoles and dense peripheral cytoplasm with mitochondria and few Golgi (Fig. 1B and C). Endoplasmic reticulum was sparse, as were the basally situated plastids. Stigmatic papillae exhibited cell wall ingrowths (Fig. 1B). Inclusions similar in fine‐structure to some components of the extracellular exudate were localized within the cell wall (Fig. 1B and C). Although the winteraceous carpel is style‐less, papillate carpellary cleft cells and surrounding ground tissue cells between the stigma and placenta formed a core of cells resembling a solid style, hereafter referred to as sub‐stigmatic transmitting tissue. Exudate, similar in histochemistry and fine structure to that of the stigmatic papillate cells, was apparent between appressed papillate adaxial epidermal cells forming the carpel cleft (Fig. 1D). Branched plasmodesmata (secondary plasmodesmata; Lucas et al., 1993) were common between cleft cells (Fig. 1E). Cytoplasm of cells comprising sub‐stigmatic transmitting tissue contained prominent vacuoles and plastids, ribosomes (Fig. 1F and G) and short profiles of rough endoplasmic reticulum (RER). A granular substance filled the periplasmic space, and fibril strands were located within the vacuoles (Fig. 1F and G). Exudate was apparent in the extracellular matrix (Fig. 1G). This exudate was histochemically positive for proteins, carbohydrates, lipids and arabinogalactans/arabinogalactan proteins (AG/AGPs).
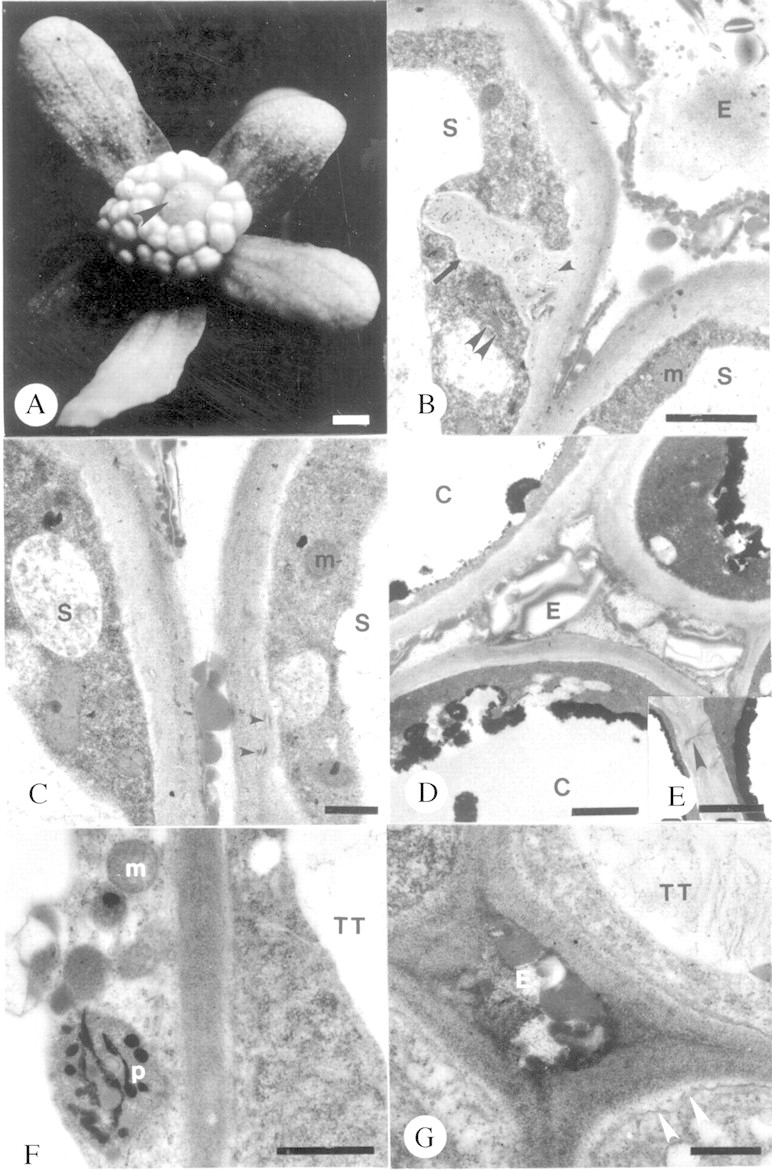
Fig. 1. Unpollinated stigma and sub‐stigmatic transmitting tissue of Pseudowintera axillaris at anthesis. A, Stigmatic exudate (arrowhead). Bar = 6·67 mm. B, Stigmatic papilla with transfer cell wall (arrow). Note cell wall inclusions (arrowhead) and exudate. Double arrowhead denotes Golgi body. Bar = 2 µm. C, Stigmatic papilla. Note cell wall inclusions (arrowheads). Bar = 0·05 µm. D, Exudate between marginal cleft cells. Bar = 2 µm. E, Secondary plasmodesmata (arrowhead) between marginal cleft cells. Bar = 1·5 µm. F and G, Fine structure of cells in solid core of sub‐stigmatic transmitting tissue. F, Bar = 1 µm. G, Note periplasmic exudate adjacent to the invaginated plasmalemma (arrowheads). Bar = 0·05 µm. C, Marginal cleft cell; E, exudate; m, mitochondrion; p, plastid; S, stigmatic papilla; TT, sub‐stigmatic transmitting tissue cells.
Placental transmitting cells contained protoplasts with prominent nuclei, numerous plastids, mitochondria (Fig. 2A and B) and ribosomes. RER was also present. Vesicles and electron‐dense droplets were apparent within the cell wall and periplasmic space adjacent to an invaginate plasmalemma (Fig. 2A and B). A heterogeneous exudate was apparent next to the placental epidermis (Fig. 2A and B) and within the micropyle of the bitegmic, anatropous ovules (Fig. 2C and D). This secretion stained positively for proteins, carbohydrates, lipids and AG/AGPs. Each pollinated ovule of P. axillaris contained a seven‐celled embryo sac at anthesis. Mean median embryo sac length was 103·19 ± 7·25 µm (n = 8) and mean median embryo sac width was 57·08 ± 6·58 µm (n = 8).
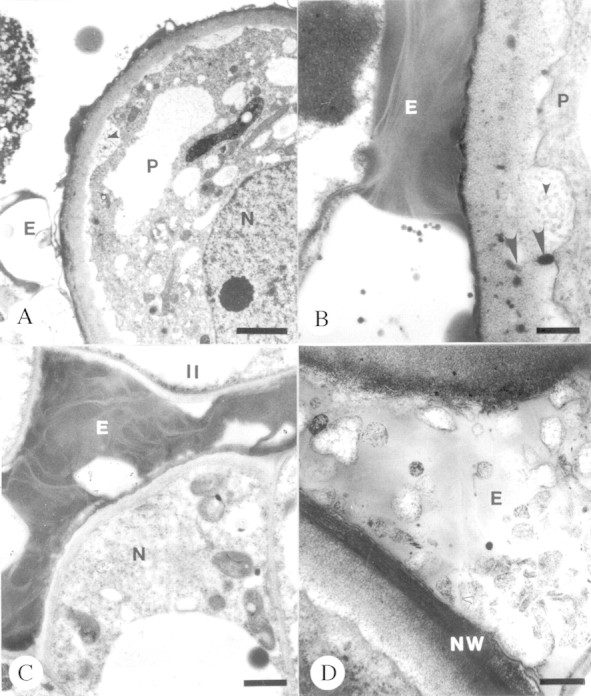
Fig. 2. Unpollinated transmitting tissue and associated exudate of placentae and micropyle of Pseudowintera axillaris at anthesis. A and B, Placental epidermal cells. Note vesiculate inclusions in periplasmic space (small arrowheads) and osmiophilic globules grading from periplasmic space into the cell wall (large arrowheads). Bars = 2 µm (A) and 0·05 µm (B). C and D, Micropylar exudate. Bars = 1 µm (C) and 0·03 µm (D). E, Exudate; II, inner integument; N, nucellus; NW, nucellar cell wall; P, placental epidermal cell.
Pollen–carpel interactions following cross‐ and self‐pollinations
No structural differences were observed in pollen tube growth following cross‐ and self‐pollination. The bicellular pollen grain within the coherent tetrad was covered with a proteinaceous coat (Fig. 3A) that formed an adhesion pad with the stigmatic papillae and intervening exudate (Fig. 3B). Pollen grains of tetrads germinated in the stigmatic crest secretion (Fig. 3C) and pollen tubes subsequently grew intercellularly between stigmatic papillae (Fig. 3D) and between cells of the sub‐stigmatic transmitting tissue (Fig. 3E). Upon entry into the ovary locule, tubes tracked along placental epithelial cells (Fig. 4A and B), entered the micropyle (Fig. 4B–E) and subsequently penetrated one synergid of the embryo sac (Fig. 4E). Pollen tubes developed a thin callosic wall during the first 10 h of growth. A ladder‐like array of callosic plugs was present by 15 h in sub‐stigmatic transmitting tissue. Callose plugs were never observed in tubes in the ovary during the initial 25 h of growth.
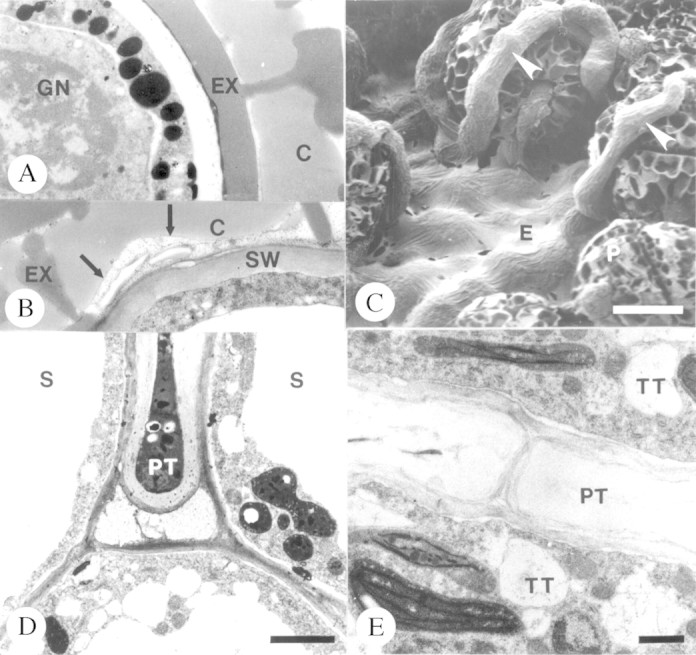
Fig. 3. Pollen tube growth on the stigma and in sub‐stigmatic transmitting tissue of Pseudowintera axillaris. A, Pollen coat of bicellular pollen grain. Bar = 1 µm. B, Adhesion zone between pollen grain and exudate of stigma papilla. Arrows denote the boundary. Bar = 3 µm. C, Germinating pollen tubes (arrowheads) on stigma. Bar = 10 µm. D, Pollen tube growing between stigmatic papillae towards sub‐stigmatic transmitting tissue. Bar = 2 µm. E, Pollen tube in solid sub‐stigmatic transmitting tissue. Bar = 1 µm. C, Pollen coat; E, exudate; EX, exine; GN, generative cell nucleus; P, pollen grain; PT, pollen tube; S, stigma papilla cells; SW, stigmatic papilla cell wall; TT, cells of sub‐stigmatic transmitting tissue.
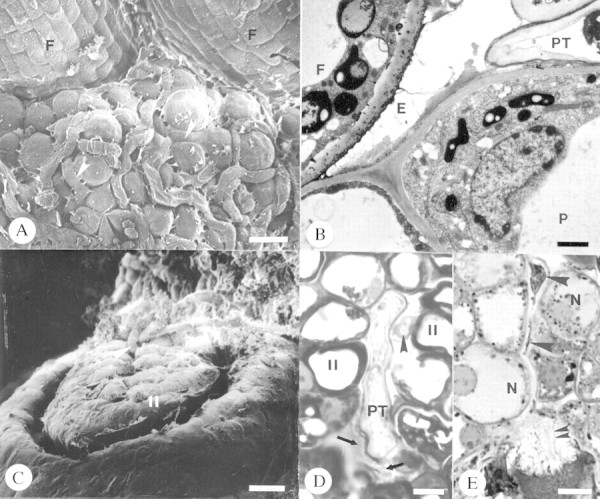
Fig. 4. Pollen tube growth in the ovary of Pseudowintera axillaris. A and B, Pollen tubes (arrowheads) growing between papillate epidermal cells of the placenta. Bars = 11 µm (A) and 2 µm (B). C, Arrowheads denote pollen tubes. Bar = 10 µm. D, Micropyle exudate (arrows). Arrowhead marks second pollen tube in micropyle. Bar = 2 µm. E, Intercellular growth of pollen tube in nucellus (large arrowheads) and penetrating synergid (double arrowheads). Bar = 8 µm. E, Exudate; F, funiculus; II, inner integument; N, nucellus; P, placenta; PT, pollen tube.
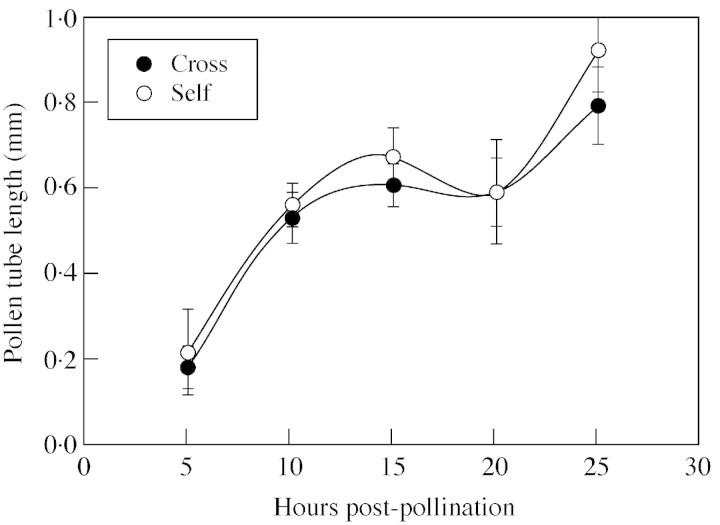
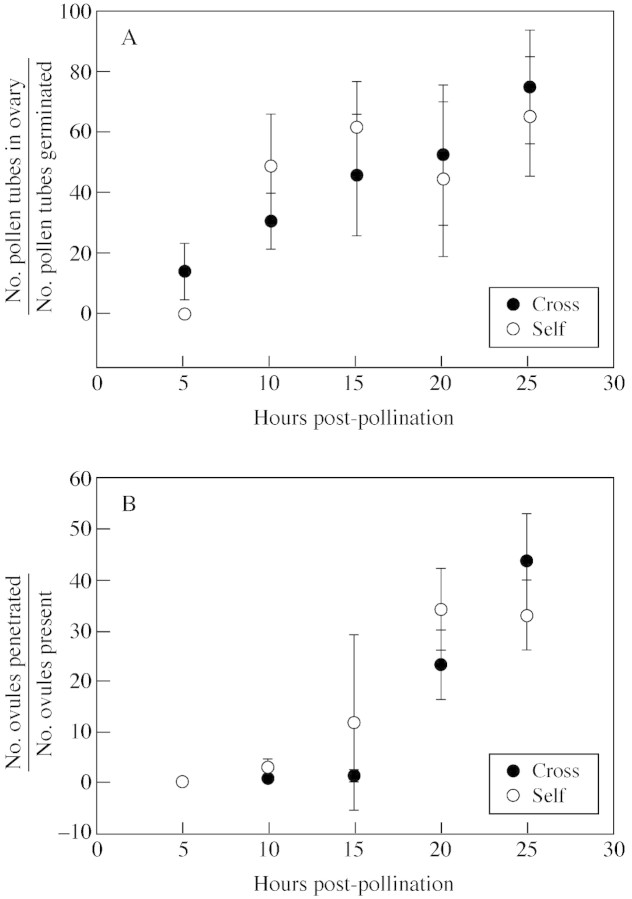
No significant differences were observed between cross and self pollen tube length during the 25 h of growth (d.f. = 4,122, F = 1·281, P = 0·285). Cross‐ and self‐pollen tubes exhibited biphasic growth (Fig. 5). Furthermore, no significant differences were observed in pollen tube number at the site of ovary entry (Fig. 6A; d.f. = 4,122, F = 3·223, P = 0·887) or the percentage of ovule penetration by cross and self pollen tubes during the 25 h of growth (Fig. 6B; d.f. = 4,122, F = 1·126, P = 0·347). All ovules appeared receptive at the time of pollen tube entry as indicated by the presence of a seven‐celled embryo sac and micropylar secretions.
Fig. 5. Changes in cross‐ and self‐pollen tube length over time for Pseudowitnera axillaris. Data are mean values ± s.d. See Results for details of the statistics.
Fig. 6. Graphs illustrating pollen tube density over time at the ovary entrance (A) and ovule penetration over time (B) following cross‐ and self‐pollination for P. axillaris. Data are mean values ± s.d. See Results for details of the statistics.
Double fertilization and early seed development following cross‐ and self‐pollination
There was no significant difference in the frequency at which double fertilization occurred following cross‐ and self‐pollination (cross‐pollination, 84·56 ± 3·04 %; self‐pollination, 80·00 ± 6·18 %; d.f. = 1,108, F = 0·439, P = 0·513). Double fertilization occurred within 3 d following pollination, as indicated by the presence of a zygote (Fig. 7A) and primary endosperm nucleus (Fig. 7B). The first primary endosperm division occurred by 15 d post‐pollination (Fig. 7C). Fifteen days after pollination, mean median embryo sac (ES) length was significantly greater following cross‐pollination (128·30 ± 3·08 µm) than following self‐pollination (116·54 ± 1·99 µm) (d.f. = 1,108, F = 11·01, P < 0·001). Similar observations were made for median embryo sac width (cross‐pollination, 71·667 ± 2·07 µm; self‐pollinationl, 63·24 ± 1·98 µm; d.f. = 1,108, F = 8·66, P = 0·004). No differences were noted in integument structure following either pollination treatment.
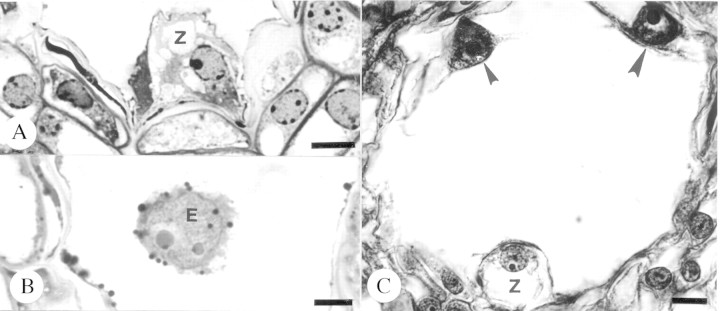
Fig. 7. Embryo sac contents after double fertilization following self‐pollination in Pseudowintera axillaris. A, Zygote, 3 d post‐pollination. Bar = 7·5 µm. B, Primary endosperm nucleus, 3 d post‐pollination. Bar = 7·5 µm. C, Zygote and endosperm nuclei (arrowheads), 15 d post‐pollination. Bar = 10 µm. E, Primary endosperm nucleus; Z, zygote.
DISCUSSION
It is apparent from structural observations of the present study that sterility mechanisms in P. axillaris are not acting to reduce self pollen tube growth prior to embryo sac entry at the nucellus, as was hypothesized by earlier workers (Norton, 1980; Lloyd and Wells, 1992). Rather, we note that double fertilization is similar following cross‐ and self‐pollination and there are no differences between cross and self pollen tube growth. Characterization of embryo/seed development indicates that self‐sterility in P. axillaris is manifested at the microscopic level by a significant uniform suppression in selfed embryo sac enlargement during the zygotic phase of embryogeny. We begin our discussion by placing the results of present study and of other studies on self‐sterility in the Winteraceae in the context of criteria proposed to distinguish a potential post‐zygotic SI from early‐acting inbreeding depression. Since P. axillaris fulfils some of the characteristics proposed for the presence of SI, we review mechanisms that may be functioning in self recognition and rejection, focusing on some of our structural–functional observations on the pollen tube pathway in P. axillaris. We emphasize that although self rejection results in post‐zygotic differences in embryo sac development, self recognition, as well as initial rejection, may be pre‐zygotic. We conclude by addressing the possible evolutionary significance of SI in P. axillaris and the Winteraceae given the primitive nature of floral features of the taxon and its long fossil history.
Inbreeding depression or SI?
The most widely held views regarding SI and early‐acting inbreeding depression contend that SI is a strictly pre‐zygotic phenomenon and that post‐zygotic self‐sterility is due to early‐acting inbreeding depression leading to embryonic abortion (Seavey and Bawa, 1986). However, it has been hypothesized that post‐zygotic SI may be present in flowering plants, and criteria to test the presence of post‐zygotic SI have been set forth (Charlesworth, 1985; Seavey and Bawa, 1986). In summary, these criteria are: (1) the timing of abortion. Uniform failure at a single stage of development may indicate SI mechanisms, whereas a continuum of failure throughout the life cycle of the new generation would be expected if early‐acting inbreeding depression was the primary cause of abortion. (2) The amount of variability in self seed set among individuals of a population. Variation in self seed set among individuals would be expected if inbreeding mechanisms are operating, whereas zero or near zero self seed set by most individuals in a genetically variable population suggests that SI is involved. (3) Dependence of abortion on the paternal vs. the progeny genotype. Evidence of cross‐incompatibility among siblings, or parent and offspring, with a limited number of cross‐ compatible groups indicative of segregating alleles would be evidence for SI with a genetic basis not dependent upon progeny genotype. (4) Response of embryos to rescue in tissue culture. Embryos carrying homozygous lethal genes would not survive, whereas those aborting as a result of incompatibility might have a chance of survival if the expression of incompatibility did not interfere with their autonomous physiology. The present study and others on self‐sterility in the Winteraceae address the first two criteria.
Self‐sterility in P. axillaris appears to occur uniformly at the zygotic stage of embryogeny in the absence of dif ferential rates of pollen tube growth, ovule penetration and double fertilization, thereby fulfilling the first criterion that differentiates SI from inbreeding depression. Similar observations have been documented for another winteraceous species, Drimys winteri (Sage et al., 1998). Failure of development during the zygotic phase of embryogeny in the absence of differential cross‐ and self‐pollen tube growth and/or ovule penetration and double fertilization rates has also been reported in other species from diverse groups of derived angiosperm families, including Gasteria verrucosa (Liliaceae; Sears, 1937), Asclepias spp. (Asclepiadaceae; Sparrow and Pearson, 1948; Sage and Williams, 1991), Rhododendron spp. (Ericaceae; Williams et al., 1984; Kaul et al., 1986), Chorisia spp. and Tabebuia spp. (Bombacaceae and Bignoniaceae; Gibbs and Bianchi, 1993, 1999), and Hymenaea stigonocarpa and Caesalpinia spp. (Leguminoseae; Lewis and Gibbs, 1999; Gibbs et al., 1999). Failure of development during the zygotic phase of embryogeny following selfing in numerous species provides increasing support for the presence of post‐zygotic SI using the first criterion.
Studies examining self seed set within populations of Pseudowintera and other genera in the Winteraceae address the second criterion and also provide support for the action of SI. Although limited in sample size, pollination studies on individuals within populations of P. axillaris, P. colorata and Drimys spp. have indicated that self‐pollination results in the absence of seed/fruit set [Norton, 1980; Godley and Smith, 1981; Thien, 1982; Lloyd and Wells, 1992; however, see Gottsberger et al. (1980) for D. brasiliensis]. The absence of seed/fruit production following selfing in populations of some species of the Winteraceae is in contrast to the wide range of seed set between individuals within populations of the self‐sterile Epilobium obcordatum (Onagraceae), a species once suspected of exhibiting late‐acting ovarian SI (OSI) but since demonstrated to experience early‐acting inbreeding depression (Seavey and Carter, 1994). And, while the rare palaeoendemic shrub Dedeckera eurekensis exhibits uniformly low seed set within populations following selfing, embryonic failure occurs at a variety of developmental stages indicative of genetically mediated embryonic abnormalities (Wiens et al., 1989). Furthermore, cross‐pollination in D. eurekensis does not result in significant increases in seed set over self‐pollination, suggesting the presence of high segregational genetic load. In contrast to pollination studies in D. eurekensis, cross‐pollination in P. axillaris and P. colorata results in significant increases in seed set over self‐pollination (Norton, 1980; Godley and Smith, 1981). Since self‐sterility in P. axillaris fulfils two of the criteria in support of SI, we cautiously speculate that self‐sterility in this species is due to SI rather than early‐acting inbreeding depression.
Mechanisms of OSI
A variety of mechanisms have been proposed for species exhibiting late‐acting OSI following double fertilization (Seavey and Bawa, 1986; Gibbs and Bianchi, 1993; Sage et al., 1994, 2000). Two major points focus on the timing of recognition with respect to rejection, and the sites of recognition and rejection. Although it is possible to posit that both self recognition and rejection function post‐zygotically in OSI, an alternative mechanism supports a pre‐zygotic recognition of self and is thus consistent with more traditional views on temporal aspects of SI. In contrast to commonly cited SI systems whereby recognition results in failure of pollen tube growth at the stigma or style (de Nettancourt, 1997), rejection reactions in some OSI systems are hypothesized to result in the modification of one or more post‐pollination stimulatory functions of pollen tube growth on carpel tissue development. Recognition and rejection may be temporally and spatially separated. The latter concept is supported by studies on Gasteria verrucosa (Sears, 1937), Theobroma cacao (Cope, 1962) and Asclepias exaltata (Sage and Williams, 1991). In all of these species, integument growth fails to proceed normally after self pollen tubes enter ovules. This led Sears (1937) to propose that SI reactions involved pollen tube–integument interactions. He suggested that interaction of compatible pollen tubes with integuments might be important for stimulation of normal seed development. Post‐pollination stimulation of carpel tissue development following cross‐pollination is well documented in flowering plants (Pimienta and Polito, 1983; Gilissen and Hoekstra, 1984; Singh et al., 1992; Sekhar and Heij, 1995; O’Neill, 1997; Pontieri and Sage, 1999). Significantly, with respect to post‐pollination signalling following selfing in an OSI species, Sage et al. (1999) reported that self‐sterility in Narcissus triandrus resulted from a pre‐zygotic failure in embryo sac development (Sage et al., 1999). Cross‐pollination in N. triandrus stimulated ovule development, and hence ovule receptivity. A similar induction was not observed following self‐pollination, even though self pollen tubes exhibited the same growth characteristics as cross pollen tubes. In N. triandrus, recognition and rejection are pre‐zygotic and rejection reactions are manifested in the ovary while pollen tubes are still in the style. Similar phenomena may also be functioning in Ipomopsis aggregata (Waser and Price, 1991).
Although the present study does not provide data for or against a pre‐zygotic self recognition affecting embryo sac development in P. axillaris, it does provide researchers with the structural framework for elucidation of the biology of a potential pre‐zygotic self recognition and rejection. Notably, we report the presence of extracellular matrix components that may function in recognition of self pollen. We show: (1) the presence of a pollen coat that forms an adhesive zone or foot with stigmatic secretions, and (2) the presence of transmitting tract secretions in the micropyle. Pollen coat in angiosperms contains compounds essential for adhesion, recognition, hydration and germination (Dickinson, 1995; Taylor and Hepler, 1997; Wolters‐Arts et al., 1998). Although the pollen coat has been demonstrated to contain the S‐gene products in Brassica (SSI with dry‐type stigmas; Schopfer et al., 1999), Willemse (1996) speculates that in the OSI species Gasteria verrucosa it may influence ovule receptivity, and hence play a role in self recognition. Micropylar secretions have been implicated in pollen tube guidance phenomena in flowering plants (Knox, 1984; Sanders and Lord, 1992; Hülskamp et al., 1995; Cheung, 1997; Lennon et al., 1998) and have been documented to occur in a number of phylogenetically older and younger taxa (for a review, see Pontieri and Sage, 1999). Significantly, with respect to OSI species, it has been suggested that micropylar secretions may contain S‐gene products (Sage et al., 1994). Confirmation of the role of pollen coat and micropylar secretions in temporal and spatial aspects of recognition and rejection of self in P. axillaris awaits further study.
In addition to the structural observations on components of the extracellular matrix of pollen grains and micropyle, we also note that pollen tubes of P. axillaris penetrate and grow in a solid transmitting tract en route to the ovary after stigmatic germination. The solid core of transmitting tissue encompasses ground tissue as well as marginal cleft cells that are post‐genitally fused, as indicated by the presence of branched (‘secondary’; Lucas et al., 1993) plasmodesmata. These results are significant since they suggest additional sites for pollen–carpel interactions that may be important in self vs. cross recognition. Similar growth patterns of pollen tubes in a solid transmitting tract en route to the ovary have been reported for another genus within the Winteraceae, Drimys winteri (Sage et al., 1998), the primitive angiosperm Saururus cernuus (Pontieri and Sage, 1997, 1999) and Nymphaea capensis (Orban and Bouharmount, 1995) of the Nymphaeaceae, a member of the basal ANITA clade (Qiu et al., 1999; Graham and Olmstead, 2000). Growth of pollen tubes following stigmatic germination within a solid transmitting tract in basal angiosperms may be important in the evolution of SI (see below) and other pollen–carpel signalling phenomena, and may have important implications for the reconstruction of the early evolution of the pollen tube pathway, a topic of long‐standing interest (Bailey and Swamy, 1951; Lloyd and Wells, 1992; Bell, 1995; Endress and Igersheim, 2000).
Evolutionary relationships of OSI and other forms of SI
Recent molecular data support the hypothesis that SI has evolved on multiple occasions throughout the history of flowering plant evolution (Matton et al., 1994; Franklin et al., 1995; de Nettancourt, 1997). However, the timing of evolution of SI with respect to angiosperm origins remains an unanswered question, as does the nature of primitive SI mechanisms and site‐specific origins of SI (Whitehouse, 1950; Bernhardt and Thien, 1987; Olmstead, 1989; Sage et al., 1994, 2000; Read et al., 1995; Weller et al., 1995; Weller and Sakai, 1999). Both stylar gametophytic SI (GSI) and stigmatic sporophytic SI (SSI) have been implicated as the first SI mechanisms to evolve (Zavada, 1984, 1990; Gibbs, 1986, 1991; Bernhardt and Thien, 1987). An intriguing hypothesis is that primitive SI mechanisms may have been some form of OSI (Kenrick et al., 1986; Barrett, 1988; Lloyd and Wells, 1992). Kenrick et al. (1986) hypothesized that SI mechanisms may have evolved and been operative on the adaxial surface of an open carpel in association with any structure which at the time was functionally important in pollen germination and transmission of the male gametophyte to the female gametophyte. Bell (1995) noted that SI reactions evolved in association with penetration of pollen tubes into ground tissue as well as with closure of the carpel.
The present study on P. axillaris and a recent study on self‐sterility in another winteraceous species, Drimys winteri (Sage et al., 1998), suggest the possible presence of OSI in a relictual flowering plant family. Given the primitive standing of Winteraceae, we support the ideas of Kenrick et al. (1986) and Barrett (1988) that some form of OSI may have been functional during the early stages of angiosperm evolution. However, we do not conclude that OSI was the only self recognition and rejection mechanism operating during the early evolution of angiosperms. Reports of a potential stigmatic SI in Austrobaileya scandens (Prakash and Alexander, 1984) and Trimenia moorei (T. L. Sage, pers. comm.), both woody members of the basal ANITA grade (Qiu et al., 1999; Graham and Olmstead, 2000), and stigmatic SI in the Saururaceae (Pontieri and Sage, 1997, 1999) indicate that angiosperms may have rapidly co‐opted many forms of SI. This scenario is consistent with multiple origins of SI and maintains the importance of SI as a player in the early success of angiosperms.
ACKNOWLEDGEMENTS
This research was funded by a grant from the Natural Sciences and Engineering Research Council of Canada to T.L.S. and a grant awarded to F.B.S. from Victoria University of Wellington. The authors thank Len Thien and Shirley Tucker for helpful comments on the manuscript, and Bill Cole for technical assistance. This manuscript is dedicated to the memory of Professor Hugh D. Gordon, Head of Botany 1947–1977, Victoria University of Wellington, New Zealand.
Supplementary Material
Received: 22 November 2002; Returned for revision: 16 December 2002; Accepted: 6 February 2003 Published electronically: 3 April 2003
References
- BaileyIW, Swamy BGL.1951. The conduplicate carpel of dicotyledons and its initial trends of specialization. American Journal of Botany 38: 373–379. [Google Scholar]
- BarrettSCH.1988. The evolution, maintenance, and loss of self‐incompatibility systems. In: Lovett Doust J, Lovett Doust L, eds. Plant reproductive ecology: patterns and strategies Oxford: Oxford University Press, 98–124. [Google Scholar]
- BellPR.1995. Incompatibility in flowering plants: adaptation of an ancient response. Plant Cell 7: 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BernhardtP, Thien LB.1987. Self‐isolation and insect pollination in the primitive angiosperms: a new evaluation of older hypotheses. Plant Systematics and Evolution 156: 159–176. [Google Scholar]
- BhandariNN.1963. Embryology of Pseudowintera colorata – a vesselless dicotyledon. Phytomorphology 13: 303–316. [Google Scholar]
- BrennerGJ, Bickoff IS.1992. Palynology and age of the Lower Cretaceous basal Kurnub Group from the coastal plain to the northern Negev of Israel. Palynology 16: 137–185. [Google Scholar]
- CharlesworthD.1985. Distribution of dioecy and self‐incompatibility in angiosperms. In: Greenwood PJ, Slatkin M, eds. Evolution: essays in honour of John Maynard Smith Cambridge: Cambridge University Press, 237–268. [Google Scholar]
- CharlesworthD, Charlesworth B.1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. [Google Scholar]
- CheungA.1997. The pollen tube growth pathway: its molecular and biochemical contributions and responses to pollination. Sexual Plant Reproduction 9: 330–336. [Google Scholar]
- CopeFW.1962. The mechanism of pollen incompatibility in Theobroma cacao L. Heredity 17: 157–182. [Google Scholar]
- CruzanMB.1986. Pollen tube distributions in Nicotiana glauca: evidence for density dependent growth. American Journal of Botany 73: 902–907. [Google Scholar]
- CronquistA.1981.An integrated system of classification of flowering plants. New York: Columbia University Press. [Google Scholar]
- de NettancourtD.1977.Incompatibility in angiosperms. Berlin: Springer‐Verlag. [Google Scholar]
- de NettancourtD.1997. Incompatibility in angiosperms. Sexual Plant Reproduction 10: 185–199. [Google Scholar]
- DickinsonH.1995. Dry stigmas, water and self‐incompatibility in Brassica Sexual Plant Reproduction 8: 1–10. [Google Scholar]
- EndressPK, Igersheim A.2000. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences 161: S211–S213. [DOI] [PubMed] [Google Scholar]
- FranklinFCH, Lawrence MJ, Franklin‐Tong VE.1995. Cell and molecular biology of self‐incompatibility in flowering plants. International Review of Cytology 158: 1–64. [Google Scholar]
- FriisEM, Endress PK.1990. Origin and evolution of angiosperm flowers. Advances in Botanical Research 17: 99–162. [Google Scholar]
- GibbsPE.1986. Do homomorphic and heteromorphic self‐incompatibility systems have the same sporophytic mechanisms? Plant Systematics and Evolution 154: 285–323. [Google Scholar]
- GibbsPE.1991. The ‘Zavada hypothesis’: a rebuttal rebutted. Taxon 40: 583–595. [Google Scholar]
- GibbsPE,Bianchi M.1993. Post‐pollination events in species of Chorisia (Bombacaceae) and Tabebuia (Bignoniaceae) with late‐acting self‐incompatibility. Botanica Acta 106: 64–71. [Google Scholar]
- GibbsPE,Bianchi M.1999. Does late‐acting self‐incompatibility (LSI) show family clustering? Two more species of Bignoniaceae with LSI: Dolichandra cynanchoides and Tabebuia nodosa Annals of Botany 84: 449–457. [Google Scholar]
- GibbsPE, Oliveira PE, Bianchi M.1999. Postzygotic control of selfing in Hymenaea stigonocarpa (Leguminosae‐Caesalpinioideae), a bat‐pollinated tree of the Brazilian cerrados. International Journal of Plant Science 160: 72–78. [Google Scholar]
- GilissenLJW, Hoekstra FA.1984. Pollination‐induced corolla wilting in Petunia hybrida Rapid transfer through the style of a wilting‐inducing substance. Plant Physiology 75: 496–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GodleyEJ, Smith DH.1981. Breeding systems in New Zealand plants. Pseudowintera colorata (Winteraceae). New Zealand Journal of Botany 19: 151–156. [Google Scholar]
- GottsbergerGI, Silberbauer‐Gottsberger I, Ehrendorfer F.1980. Reproductive biology in the primitive relic angiosperm Drimys brasiliensis (Winteraceae). Plant Systematics and Evolution 135: 11–39. [Google Scholar]
- GrahamS, Olmstead RG.2000. Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. American Journal of Botany 87: 1712–1730. [PubMed] [Google Scholar]
- HülskampMK, Schneitz K, Pruitt RE.1995. Genetic evidence for a long‐range activity that directs pollen tube guidance in Arabidopsis Plant Cell 7: 57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HusbandBC, Schemske DW.1996. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 50: 54–70. [DOI] [PubMed] [Google Scholar]
- KaulV, Rouse JL, Williams EG.1986. Early events in the embryo sac after intraspecific and interspecific pollinations in Rhododendron kawakamii and R retusum Canadian Journal of Botany 64: 282–291. [Google Scholar]
- KenrickJ, Kaul V, Williams EG.1986. Self‐incompatibility in Acacia retinoides: site of pollen‐tube arrest is the nucellus. Planta 169: 245–250. [DOI] [PubMed] [Google Scholar]
- KnoxRB.1984. Pollen–pistil interactions. Annual Review of Plant Physiology 17: 508–608. [Google Scholar]
- LennonK, Stephance A, Hepler PK, Lord EM.1998. The structure of the transmitting tissue of Arabidopsis thaliana (L.) and the path of pollen tube growth. Sexual Plant Reproduction 11: 49–59. [Google Scholar]
- LewisG, Gibbs P.1999. Reproductive biology of Caesalpinia calycina and C pluviosa (Leguminosae) of the caatinga of north‐eastern Brazil. Plant Systematics and Evolution 217: 43–53. [Google Scholar]
- LloydDG, Wells MS.1992. Reproductive biology of a primitive angiosperm, Pseudowintera colorata (Winteraceae), and the evolution of pollination systems in the Anthophyta. Plant Systematics and Evolution 181: 77–95. [Google Scholar]
- LucasWJ, Ding B, van der Schoot C.1993. Plasmodesmata and the supracellular nature of plants. Tansley Review No. 58. New Phytologist 125: 435–476. [DOI] [PubMed] [Google Scholar]
- MartinFW.1959. Staining and observing pollen tubes by means of fluorescence. Stain Technology 34: 436–437. [DOI] [PubMed] [Google Scholar]
- MattonDP, Nass N, Clarke AE, Newbigin E.1994. Self‐incompatibility: how plants avoid illegitimate offspring. Proceedings of the National Academy of Sciences of the USA 91: 1992–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NortonSA.1980.Reproductive ecology of Pseudowintera (Winteraceae). MSc Thesis, Victoria University of Wellington, New Zealand. [Google Scholar]
- OlmsteadRG.1989. The origin and function of self‐incompatibility in flowering plants. Sexual Plant Reproduction 2: 127–136. [Google Scholar]
- O’NeillS 1997. Pollination regulation of flower development. Annual Review of Plant Physiology and Plant Molecular Biology 48: 547–574. [DOI] [PubMed] [Google Scholar]
- OrbanI Bouharmount J.1995. Reproductive biology of Nymphaea capensis Thunb. var. zanzibarensis (Casp.) Verdc. (Nymphaeaceae). Botanical Journal of the Linnean Society 119: 35–43. [Google Scholar]
- PimientaE, Polito VS.1983. Embryo sac development in almond (Prunus dulcis (Mill.) D. A. Webb) as affected by cross‐, self‐ and non‐pollination. Annals of Botany 51: 469–479. [Google Scholar]
- PontieriV, Sage TL.1997. Characterization of pollen–carpel interactions following self‐ and cross‐pollination in the paleoherb family, Saururaceae. American Journal of Botany 84: 65. [Google Scholar]
- PontieriV, Sage TL.1999. Evidence for stigmatic self‐incompatibility, pollination‐induced ovule enlargement and transmitting tissue exudates in the palaeoherb, Saururus cernuus L. (Saururaceae). Annals of Botany 84: 507–519. [Google Scholar]
- PrakashN, Alexander III JH.1984. Self‐incompatiblity in Austrobaileya scandens In: Williams EG, Knox RB, eds. Pollination ’84 Melbourne: Melbourne, School of Botany: University of Melbourne, 214–216. [Google Scholar]
- QiuY‐L, Lee J, Bernasconi‐Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen Z, Savolainen V and Chase MW.1999. The earliest angiosperms: evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404–407. [DOI] [PubMed] [Google Scholar]
- ReadSM, Newbigin E, Clarke AE, McClure BA, Kao T.1995. Disputed ancestry: comments on the model for the origin of incompatibility in flowering plants. Plant Cell 7: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SageTL, Williams EG 1991. Self‐incompatibility in Asclepias Plant Cell Incompatibility Newsletter 23: 55–57. [Google Scholar]
- SageTL, Williams EG.1995. Structure, ultrastructure, and histochemistry of the pollen tube pathway in the milkweed Asclepias exaltata L. Sexual Plant Reproduction 8: 257–265. [Google Scholar]
- SageTL,Bertin RI, Williams EG.1994. Ovarian and other late‐acting self‐incompatibility systems. In: Williams EG, Knox RB, Clarke AE, eds. Genetic control of self‐incompatibility and reproductive development in flowering plants Dordrecht: Kluwer, 116–140. [Google Scholar]
- SageTL,Pontieri V, Christopher R 2000. Incompatibility and mate recognition in monocotyledons. In: Wilson KL, Morrison DA, eds. Monocots: systematics and evolution Melbourne: CSIRO, 270–276. [Google Scholar]
- SageTL, Strumas F, Cole B, Barrett SCH.1999. Differential ovule development following self‐ and cross‐pollination: the basis of self‐sterility in Narcissus triandrus (Amaryllidaceae). American Journal of Botany 86: 855–870. [PubMed] [Google Scholar]
- SageTL,Sampson FB, Bayliss P, Gordon MG, HeijEG.1998. Self‐sterility in the Winteraceae. In: Owens SJ, Rudell PJ, eds. Reproductive biology in systematics, conservation and economic botany Kew: Royal Botanic Garden, 317–328. [Google Scholar]
- SampsonFB.1963. The floral morphology of Pseudowintera, the New Zealand member of the vesselless Winteraceae. Phytomorphology 13: 403–423. [Google Scholar]
- SampsonFB.1980. Natural hybridism in Pseudowintera (Winteraceae). New Zealand Journal of Botany 18: 43–51. [Google Scholar]
- SandersLC, Lord EM.1992. A dynamic role for the stylar matrix in pollen tube extension. International Review of Cytology 140: 297–318. [Google Scholar]
- SchmittD, Perry, TO.1964. Self‐sterility in sweetgum. Forestry Science 10: 302–305. [Google Scholar]
- SchopferDR, Nasrallah ME, Nasrallah JB.1999. The male determinant of self‐incompatibility in Brassica Science 286: 1697–1700. [DOI] [PubMed] [Google Scholar]
- SearsER.1937. Self‐sterility in plants. Genetics 22: 130–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeaveySR, Bawa KS.1986. Late‐acting self‐incompatibility in angiosperms. Botanical Review 52: 195–218. [Google Scholar]
- SeaveySR, Carter SK.1994. Self‐sterility in Epilobium obcordatum (Onagraceae). American Journal of Botany 81: 331–338. [PubMed] [Google Scholar]
- SekharKNC, Heij EG.1995. Changes in proteins and peroxidases induced by compatible pollination in the ovary of Nicotiana tabacum L. Sexual Plant Reproduction 8: 369–374. [Google Scholar]
- SinghA, Evensen KB, Kao T.1992. Ethylene synthesis and floral senescence following compatible and incompatible pollinations in Petunia inflata Plant Physiology 99: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SparrowIK, Pearson NL.1948. Pollen compatibility in Asclepias syriaca Journal of Agricultural Research 77: 187–199. [Google Scholar]
- SuhY, Thien LB, Reeve HE, Zimmer EA.1993. Molecular evolution and phylogenetic implications of internal transcribed spacer sequences of ribosomal DNA in Winteraceae. American Journal of Botany 80: 1042–1055. [Google Scholar]
- TaylorLP, Hepler PK.1997. Pollen germination and tube growth. Annual Review of Plant Physiology and Plant Molecular Biology 48: 461–491. [DOI] [PubMed] [Google Scholar]
- ThienLB.1980. Patterns of pollination in the primitive angiosperms. Biotropica 12: 1–13. [Google Scholar]
- WalkerJW, Brenner GJ, Walker AG.1983. Winteraceous pollen in the lower Cretaceous of Israel: early evidence of a Magnolialean angiosperm family. Science 220: 1273–1275. [DOI] [PubMed] [Google Scholar]
- WaserNM, Price MV.1991. Reproductive costs of self pollination in Ipomopsis aggregata (Polemoniaceae): are ovules usurped? American Journal of Botany 78: 1036–1043. [Google Scholar]
- WellerSG, Sakai AK.1999. Using phylogenetic approaches for the analysis of plant breeding system evolution. Annual Review of Ecology and Systematics 30: 167–199. [Google Scholar]
- WellerSG, Donoghue MJ, Charlesworth D.1995. The evolution of self‐ incompatibility in flowering plants: a phylogenetic approach. In: Hoch PC, Stephenson AG, eds. Experimental and molecular approaches to plant biosystematics St Louis: Missouri Botanical Garden, 317–328. [Google Scholar]
- WhitehouseHLK.1950. Multiple‐allelomorph incompatibility of pollen and style in the evolution of angiosperms. Annals of Botany 14: 198–216. [Google Scholar]
- WiensD, Nickrent DL, Davern CI, Calvin DL, Vivrette NJ.1989. Developmental failure and loss of reproductive capacity in the rare paleoendemic shrub Dedeckera eurekensis Nature 338: 65–67. [Google Scholar]
- WillemseMTM.1996. Progamic phase and fertilization in Gasteria verrucosa (Mill.). H. Duval: pollination signals. Sexual Plant Reproduction 9: 348–352. [Google Scholar]
- WilliamsEG, Sage TL, Thien LB.1993. Functional syncarpy by intercalary growth of pollen tubes in a primitive apocarpous angiosperm, Illicium floridanum Ellis, Illiciaceae. American Journal of Botany 80: 137–142. [Google Scholar]
- WilliamsEG,Kaul V, Rouse JL, Knox RB.1984. Apparent self‐incompatibility in Rhododendron ellipticum, R. championae and R. amamiense: a post‐zygotic mechanism. Plant Cell Incompatibility Newsletter 16: 10–11. [Google Scholar]
- Wolters‐ArtsM, Lush WM, Mariani C.1998. Lipids are required for directional pollen‐tube growth. Nature 392: 818–821. [DOI] [PubMed] [Google Scholar]
- ZavadaJS.1984. The relation between pollen exine sculpturing and self‐incompatibility mechanisms. Plant Systematics and Evolution 147: 63–78. [Google Scholar]
- ZavadaJS.1990. Correlations between pollen exine sculpturing and angiosperm self‐incompatibility systems – a rebuttal. Taxon 39: 442–447. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.