Abstract
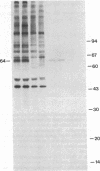
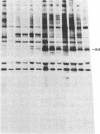
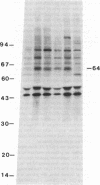
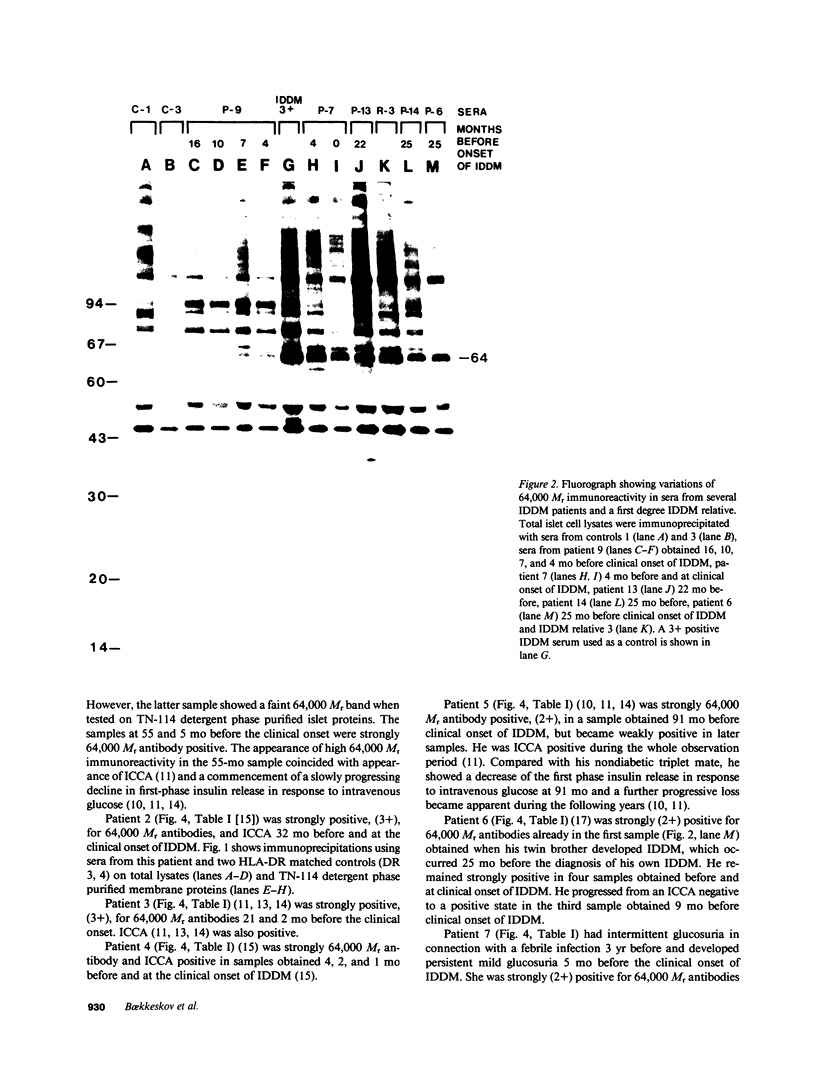
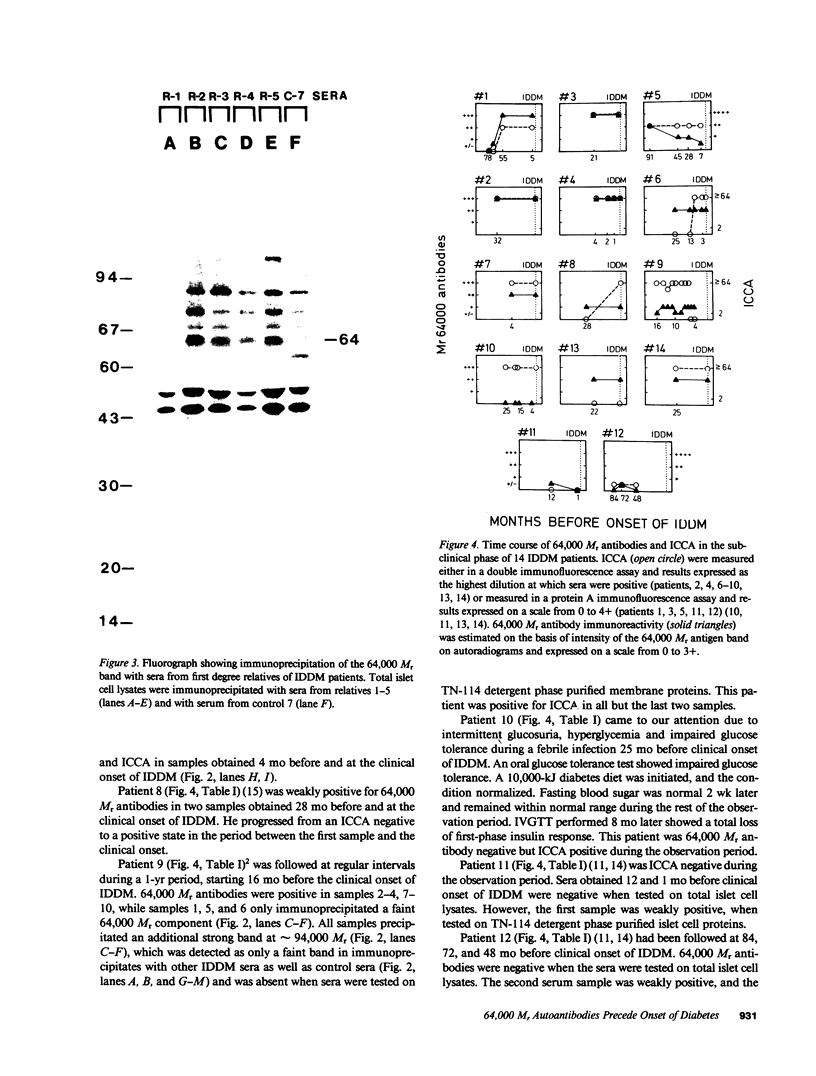
Antibodies in sera from newly diagnosed insulin-dependent diabetes mellitus (IDDM) patients are directed to a human islet cell protein of relative molecular mass (Mr) 64,000. Since IDDM seems to develop after a prodromal period of beta-cell autoimmunity, this study has examined whether 64,000 Mr antibodies could be detected in 14 individuals who subsequently developed IDDM and five first degree relatives who have indications of altered beta-cell function. Sera were screened by immunoprecipitation on total detergent lysates of human islets and positive sera retested on membrane protein preparations. Antibodies to the 64,000 Mr membrane protein were consistently detected in 11/14 IDDM patients, and in all 5 first degree relatives. 10 IDDM patients were already positive in the first samples, obtained 4-91 mo before the clinical onset of IDDM, whereas 1 patient progressed to a high 64,000 Mr immunoreactivity, at a time where a commencement of a decline in beta-cell function was detected. 64,000 Mr antibodies were detected before islet cell cytoplasmic antibodies (ICCA) in two patients. In the control groups of 21 healthy individuals, 36 patients with diseases of the thyroid and 5 SLE patients, the 64,000 Mr antibodies were detected in only one individual, who was a healthy sibling to an IDDM patient. These results suggest that antibodies against the Mr 64,000 human islet protein are an early marker of beta-cell autoimmunity and may be useful to predict a later development of IDDM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baekkeskov S., Dyrberg T., Lernmark A. Autoantibodies to a 64-kilodalton islet cell protein precede the onset of spontaneous diabetes in the BB rat. Science. 1984 Jun 22;224(4655):1348–1350. doi: 10.1126/science.6374896. [DOI] [PubMed] [Google Scholar]
- Baekkeskov S., Nielsen J. H., Marner B., Bilde T., Ludvigsson J., Lernmark A. Autoantibodies in newly diagnosed diabetic children immunoprecipitate human pancreatic islet cell proteins. Nature. 1982 Jul 8;298(5870):167–169. doi: 10.1038/298167a0. [DOI] [PubMed] [Google Scholar]
- Bonnevie-Nielsen V., Steffes M. W., Lernmark A. A major loss in islet mass and B-cell function precedes hyperglycemia in mice given multiple low doses of streptozotocin. Diabetes. 1981 May;30(5):424–429. doi: 10.2337/diab.30.5.424. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bruining G. J., Molenaar J. L., de Jongh B. M., Aarsen R. S., Visser H. K. Prediction of type 1 diabetes mellitus--a report on three cases. Eur J Pediatr. 1985 Jan;143(3):175–178. doi: 10.1007/BF00442131. [DOI] [PubMed] [Google Scholar]
- Feutren G., Papoz L., Assan R., Vialettes B., Karsenty G., Vexiau P., Du Rostu H., Rodier M., Sirmai J., Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986 Jul 19;2(8499):119–124. doi: 10.1016/s0140-6736(86)91943-4. [DOI] [PubMed] [Google Scholar]
- Ganda O. P., Srikanta S., Brink S. J., Morris M. A., Gleason R. E., Soeldner J. S., Eisenbarth G. S. Differential sensitivity to beta-cell secretagogues in "early," type I diabetes mellitus. Diabetes. 1984 Jun;33(6):516–521. doi: 10.2337/diab.33.6.516. [DOI] [PubMed] [Google Scholar]
- Gepts W., Lecompte P. M. The pancreatic islets in diabetes. Am J Med. 1981 Jan;70(1):105–115. doi: 10.1016/0002-9343(81)90417-4. [DOI] [PubMed] [Google Scholar]
- Gorsuch A. N., Spencer K. M., Lister J., Wolf E., Bottazzo G. F., Cudworth A. G. Can future type I diabetes be predicted? A study in families of affected children. Diabetes. 1982 Oct;31(10):862–866. doi: 10.2337/diab.31.10.862. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logothetopoulos J., Valiquette N., Madura E., Cvet D. The onset and progression of pancreatic insulitis in the overt, spontaneously diabetic, young adult BB rat studied by pancreatic biopsy. Diabetes. 1984 Jan;33(1):33–36. doi: 10.2337/diab.33.1.33. [DOI] [PubMed] [Google Scholar]
- Madsen O. D., Olsson M. L., Bille G., Sundkvist G., Lernmark A., Dahlqvist G., Ludvigsson J. A two-colour immunofluorescence test with a monoclonal human proinsulin antibody improves the assay for islet cell antibodies. Diabetologia. 1986 Feb;29(2):115–118. doi: 10.1007/BF00456121. [DOI] [PubMed] [Google Scholar]
- Nerup J., Lernmark A. Autoimmunity in insulin-dependent diabetes mellitus. Am J Med. 1981 Jan;70(1):135–141. doi: 10.1016/0002-9343(81)90420-4. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Eisenbarth G. S., Soeldner J. S. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med. 1983 Feb 10;308(6):322–325. doi: 10.1056/NEJM198302103080607. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Gleason R. E., Jackson R. A., Soeldner J. S., Eisenbarth G. S. Pre-type I diabetes. Linear loss of beta cell response to intravenous glucose. Diabetes. 1984 Aug;33(8):717–720. doi: 10.2337/diab.33.8.717. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Jackson R. A., Brink S. J., Fleischnick E., Yunis E., Alper C., Soeldner J. S., Eisenbarth G. S. Pre-type 1 (insulin-dependent) diabetes: common endocrinological course despite immunological and immunogenetic heterogeneity. Diabetologia. 1984 Jul;27 (Suppl):146–148. doi: 10.1007/BF00275674. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Jackson R. A., Gleason R. E., Kaldany A., Garovoy M. R., Milford E. L., Carpenter C. B., Soeldner J. S., Eisenbarth G. S. Type I diabetes mellitus in monozygotic twins: chronic progressive beta cell dysfunction. Ann Intern Med. 1983 Sep;99(3):320–326. doi: 10.7326/0003-4819-99-3-320. [DOI] [PubMed] [Google Scholar]
- Srikanta S., Rabizadeh A., Omar M. A., Eisenbarth G. S. Assay for islet cell antibodies. Protein A--monoclonal antibody method. Diabetes. 1985 Mar;34(3):300–305. doi: 10.2337/diab.34.3.300. [DOI] [PubMed] [Google Scholar]
- Svenningsen A., Dyrberg T., Markholst H., Binder C., Lernmark A. Insulin release and pancreatic insulin is reduced in young prediabetic BB rats. Acta Endocrinol (Copenh) 1986 Jul;112(3):367–371. doi: 10.1530/acta.0.1120367. [DOI] [PubMed] [Google Scholar]