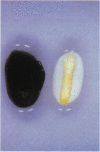
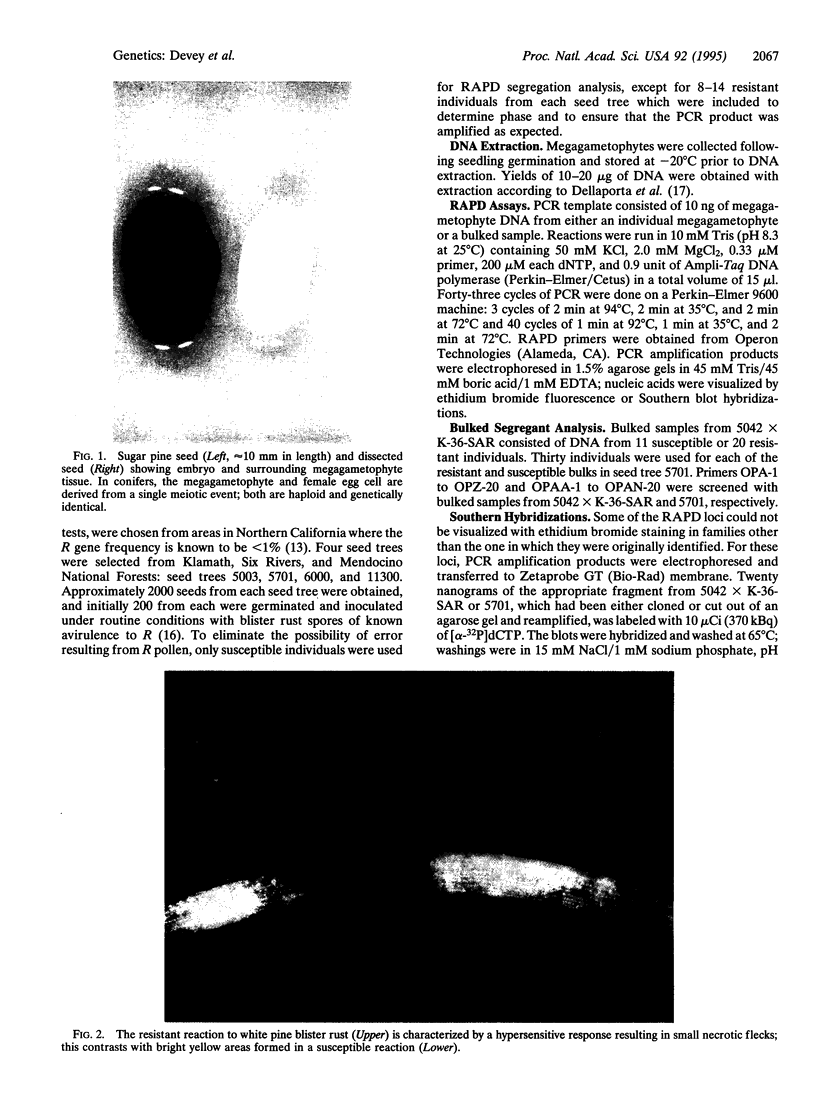
Abstract
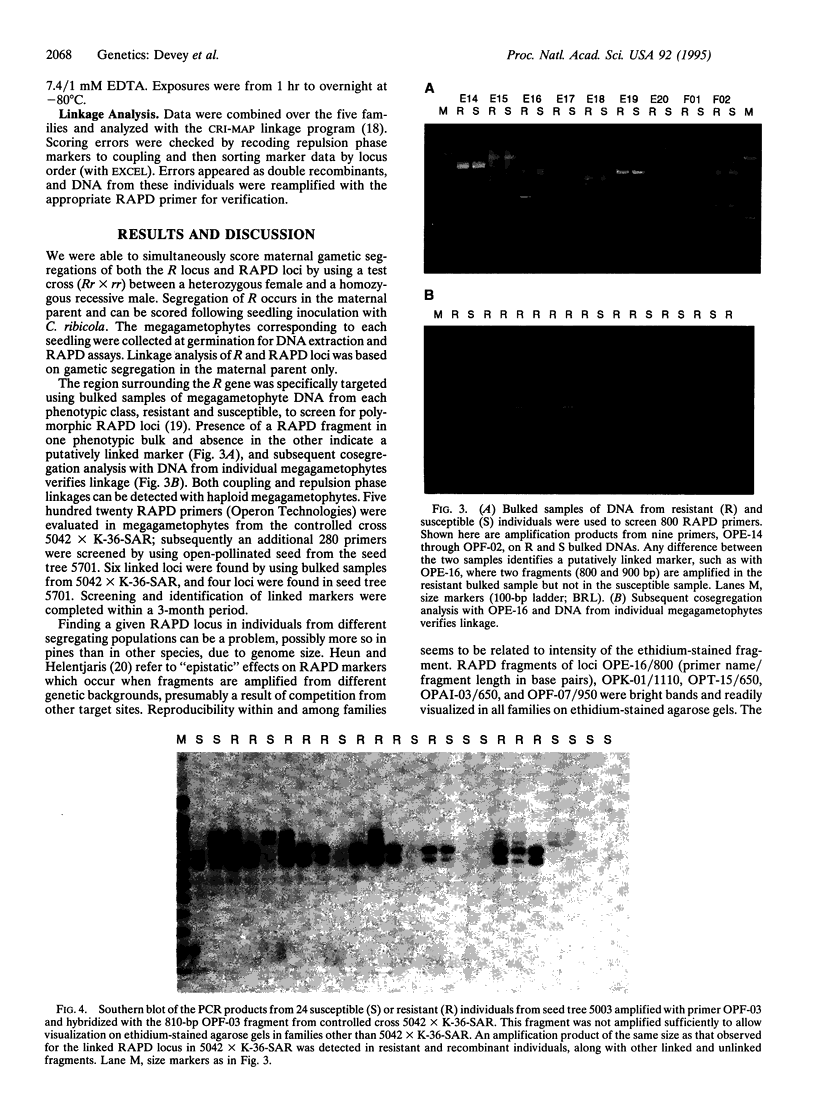
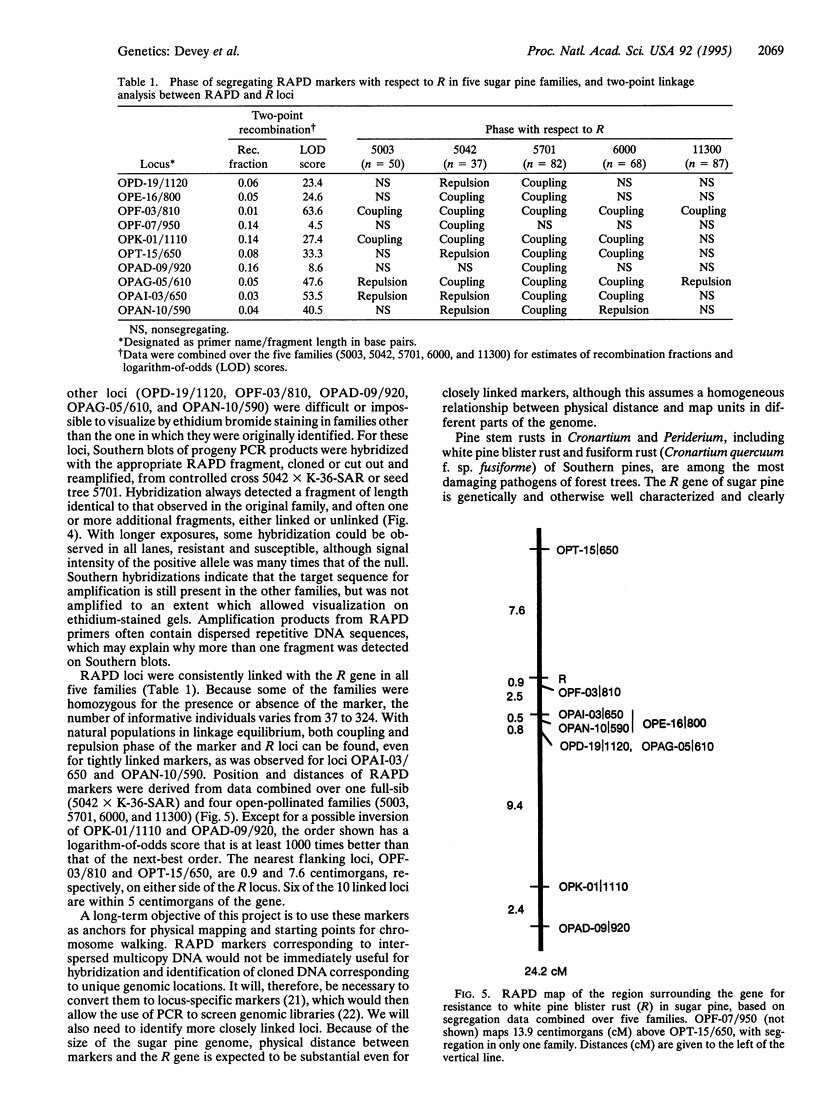
We have genetically mapped a gene for resistance to white pine blister rust (Cronartium ribicola Fisch.) in sugar pine (Pinus lambertiana Dougl.) by using an approach which relies on three factors: (i) the ability to assay for genetic markers in the haploid stage of the host's life cycle, using megagametophyte seed tissue; (ii) a simple and clearly defined pathosystem; and (iii) the use of random amplified polymorphic DNA (RAPD) markers that can be quickly and efficiently evaluated. Resistance to white pine blister rust in sugar pine is known to be controlled by a single dominant gene (R). Maternal segregation of R and dominant RAPD markers were scored simultaneously following collection of megagametophytes for DNA assays and seedling inoculation with C. ribicola. Bulked samples of haploid megagametophyte DNA from resistant and susceptible offspring of segregating full-sib and half-sib families were used to evaluate 800 random decanucleotide primers. Ten loci linked with the gene for resistance to white pine blister rust were identified and segregation data were obtained from five families. Six of the linked markers were within 5 centimorgans of the gene, and one marker was 0.9 centimorgan from R. These and other markers derived by this approach may provide starting points for map-based cloning of this important gene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983 Jun 3;220(4601):1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Green E. D., Olson M. V. Systematic screening of yeast artificial-chromosome libraries by use of the polymerase chain reaction. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1213–1217. doi: 10.1073/pnas.87.3.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johal G. S., Briggs S. P. Reductase activity encoded by the HM1 disease resistance gene in maize. Science. 1992 Nov 6;258(5084):985–987. doi: 10.1126/science.1359642. [DOI] [PubMed] [Google Scholar]
- Kinloch B. B., Jr, Parks G. K., Fowler C. W. White pine blister rust: simply inherited resistance in sugar pine. Science. 1970 Jan 9;167(3915):193–195. doi: 10.1126/science.167.3915.193. [DOI] [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993 Nov 26;262(5138):1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Michelmore R. W., Paran I., Kesseli R. V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9828–9832. doi: 10.1073/pnas.88.21.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsieram L. K., Glaubitz J. C., Kiss G., Carlson J. E. Single tree genetic linkage mapping in conifers using haploid DNA from megagametophytes. Biotechnology (N Y) 1992 Jun;10(6):686–690. doi: 10.1038/nbt0692-686. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Kubelik A. R., Livak K. J., Rafalski J. A., Tingey S. V. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 1990 Nov 25;18(22):6531–6535. doi: 10.1093/nar/18.22.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]