Abstract
Hooked apex stolons and initial swelling stolons of potato plants were treated with 3 × 10–8 mol l–1 jasmonic acid (JA) to study the effect of this compound on histology, cell expansion and tissue differentiation. In hooked apex stolons, JA application increased the meristem thickness and reduced the length of the leaf primordia, whereas in initial swelling stolons narrowing of the apical region, absence of leaf primordia and swelling of the subapical meristem were evident. Early vascular tissue differentiation was observed in response to JA treatment, especially of xylem elements from regions proximal to the tunic. Protoxylem elements, such as tracheal elements, were present with thin primary cell walls. The cell area was measured in two zones: zone I, central mother cells situated immediately under the tunic; and zone II, rib meristem cells. JA caused a four‐ and six‐fold increase in cell area in both zones in hooked apex stolons and initial swelling stolons, respectively. Thus, tuber formation is concluded to occur as a consequence of increased cell expansion, a reduction in the length of leaf primordia, enlargement of meristems, and early vascular tissue differentiation.
Key words: Solanum tuberosum L., potato, stolons, jasmonic acid, cell expansion, tissue differentiation
INTRODUCTION
A broad spectrum of plant processes is regulated by jasmonates (JAs), among them seed germination and root growth, flower and fruit development, senescence and tuber formation (for reviews, see Sembdner and Parthier, 1993; Creelman and Mullet, 1997; Wasternack and Hause, 2002).
There are two aspects of potato tuber formation: (a) the morphological development of a tuber; and (b) the biochemical changes resulting in starch synthesis and storage (Xu et al., 1998). The morphogenetic steps consist of stolon elongation and tuber initiation (Booth, 1963; Vreugdenhil and Struik, 1989). During the early stages of tuber formation the growth habit of stolons is altered, with subapical radial growth taking place instead of elongation growth. The first indication of in vivo tuber formation is a thickening of the eighth internode counted from the apex (Xu et al., 1998). The earliest changes associated with tuber formation are a marked increase in both radial expansion and number of mitotic cells (Vreugdenhil et al., 1999) in the subapical stolon region, concomitant with the deposition of starch and protein (Ewing and Struik, 1992; Xu et al., 1998).
Transformation of the stolon into a tuber implies changes in cell division, direction and microtubule orientation, and also in cell expansion (Koda, 1997). Cell division and expansion are followed by a massive deposition of starch and of storage proteins, such as patatin, processes coordinated by the expression of genes involved in the synthesis of these substances (Prat et al., 1990; Visser et al., 1994).
Jasmonic acid (JA) and its methyl ester (MeJA) are able to induce cell expansion of medullary tissue in potato tuber discs cultured in vitro (Takahashi et al., 1994) and in the corpus cells of tuber buds (Castro et al., 1999). Once JA has induced cell expansion of a potato tuber, reorientation of the cortical microtubules occurs (Shibaoka, 1991). The same effect was observed for cultured potato cells treated with MeJA (Matsuki et al., 1992) and tobacco BY‐2 cells treated with JA and MeJA (Abe et al., 1990).
Despite the fact that JA causes cell expansion in mature tuber discs (Takahashi et al., 1994) and in tuber buds (Castro et al., 1999), there is no report, as yet, into the effect of exogenous JA on the early stages of tuberization, especially in apical hooked stolons. Therefore, the effect of exogenously applied JA on the histology, cell expansion and differentiation of hooked apex stolons and initial swelling stolons (stages correspondent to the early ontogenetic steps of tuber formation) were studied.
MATERIALS AND METHODS
Plant material
Potato tubers of Solanum tuberosum L. ‘Spunta’ were planted on soil in a glasshouse under controlled conditions: 15 h photoperiod, 30/12 °C day/night. Plants were harvested at stolon appearance between 8 and 10 weeks after planting, and hooked apex stolons (first stage) and initial swelling stolons (second stage) were collected.
Jasmonic acid treatment
Plant material corresponding to the first and second stages, excised 1 cm from the tip, was washed in distilled water and placed in 25 ml Erlenmeyer flasks containing 3 × 10–8 mol l–1 JA. For controls, only water was used. Flasks were maintained in darkness at 25 °C for 3 d.
Histological studies
Material was fixed in FAA (ethanol : water : formaldehyde : acetic acid, 50 : 35 : 10 : 5, v/v), dehydrated in graded series of ethanol and ethanol‐xylol, and embedded in histowax (D’Ambrogio de Argüeso, 1986). Longitudinal microtome sections (10 µm) were obtained and stained with a safranine‐fast green combination (Dizeo de Strittmatter, 1979). Sections were examined using a Zeiss Axiophot microscope.
To study cell expansion, cell area was from determined from serial sections using an image analysis system (VIDAS 25; Kontron Elektronik GmbH, Ecking, Germany). Two zones were delimited for the analysis: zone I, corresponding to the central mother cells situated immediately under the tunic; and zone II, corresponding to the rib meristem cells. The values obtained (in number of pixels) were multiplied by the size of the objective lens used.
Five repetitions were made; each repetition corresponded to the average of ten subsamples of the experimental unit. The recorded variables corresponded to the cell area in each of the delimited zones of the two stages. For each of the zones, cell areas were analysed using ANOVA. A posteriori comparison of means was made using the LSD Fisher Test.
RESULTS
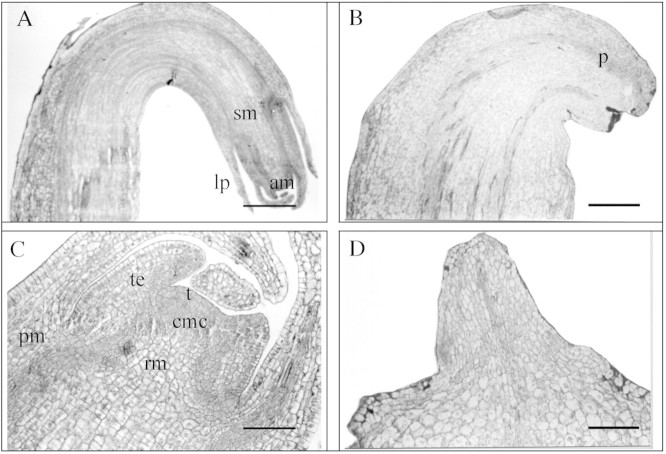
The typical morphology of a control hooked apex stolon (first stage) and an initial swelling stolon (second stage), showing the apical and subapical meristem, leaf primordia and vascular tissues, is presented in Fig. 1A and C. As seen in Fig. 1C, the apical meristem consists of two regions: the tunica—the outermost two layers of cells; and the corpus. Three main zones can be distinguished in the corpus: (a) the zone of central mother cells, situated below the tunica; (b) the rib meristem; and (c) the peripheral meristem.
Fig. 1. Photomicrographs of apical and subapical meristem of the first (A and B) and second tuberization stage (C and D) treated with jasmonic acid (B and D), or water (control) (A and C). am, Apical meristem; cmc, central mother cells; lp, leaf primordium; pm, peripheral meristem; p, procambium; rm, rib meristem; sm, subapical meristem; t, tunica; te, tracheal elements. Bars = 250 µm (A and B), 50 µm (C) and 100 µm (D).
Treating stolons of the first stage with JA led to an increase in thickness of the apical and subapical meristem, and caused leaf primordia to cease growth and remain as rudimentary primordia (Fig. 1B).
Remarkable changes in the morphology of the apical meristem were effected by JA in the initial swelling stolons (second stage). Narrowing of the apical region, an absence of leaf primordia and swelling of the subapical meristem were evident. In addition, early vascular tissue differentiation was observed in the regions proximal to the tunic. Following JA treatment, procambial strips stained more intensely than neighbouring tissues in the first stage (Fig. 1B), differentiating into xylem elements in the second stage. Protoxylem elements, such as tracheal elements, with thin primary cell walls and ring‐shaped secondary wall thickenings were observed (Fig. 1D).
At the cellular level, treatment with JA led to a marked expansion of the cells in both zones of the two stages analysed (Fig. 2B and D) compared with control cells (Fig. 2A and C). The expanded cells lost their polyhedral form (Fig. 2B and D).
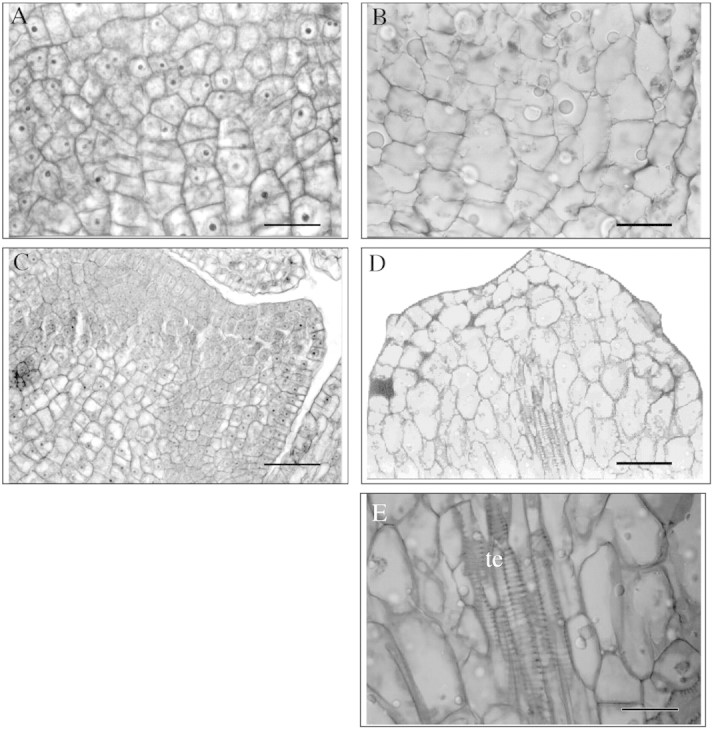
Fig. 2. Photomicrographs of zone I showing the effect of jasmonic acid on cell expansion and differentiation. First tuberization stage (A and B) and second tuberization stage (C and D). Control (A and C). Treated with jasmonic acid (B, D and E). te, Tracheal elements. Bars = 10 µm (A, B and E) and 25 µm (C and D).
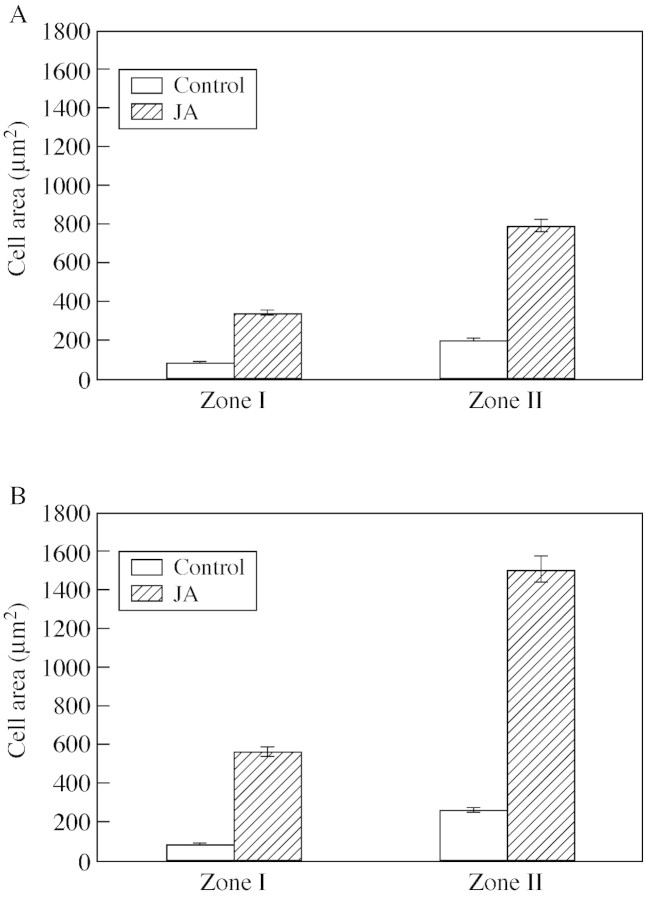
The increase in cell expansion caused by JA was statistically significant (P < 0·05). JA treatment caused a four‐fold increase in cell area in the first stage (Fig. 3A), and a six‐fold increase in the second stage (Fig. 3B) for both zones analysed. Likewise, an early formation of starch granules in response to JA was observed in zone II of the first stage and in zone I of the second stage. The parenchymatic cells that specialize in starch accumulation were associated with the conducting elements in the first and second (Fig. 2D and E) stage.
Fig. 3. Effect of jasmonic acid on cell area in zone I and II of the first (A) and second (B) tuberization stages (n = 5, P < 0·05). Bars represent ± s.e.
DISCUSSION
It is well known that the tuberization process is characterized by pronounced anatomical, hormonal and biochemical changes (Ewing and Struik, 1992; Jackson, 1999; Vreugdenhil et al., 1999; Fernie and Willmitzer, 2001). Previous studies have established the involvement of JAs in the growth and induction of tuber formation in potato (Koda et al., 1991, 1992; Koda, 1997; Castro et al., 1999). Recently, Abdala et al. (2002) reported that levels of endogenous JA increased in stolons of Solanum tuberosum L. ‘Spunta’ during the transition from stolons to tubers. Moreover, A. Cenzano, A. Vigliocco, O. Miersch, G. Abdala (unpubl. res.), studying the level of different jasmonates in stolons divided into the apical region and stolon, found high levels of JA and 12‐OH‐JA (tuberonic acid) in the apical regions, with levels decreasing through the ontogenic stages of tuberization. Levels of JA and tuberonic acid remained constant in stolons.
In the present work, application of JA to the early ontogenetic stages of tuberization led to modifications in the meristematic regions of the first and second stage. The thickness of the hooked apex stolons (first stage) and of initial swelling stolons (second stage) increased, accompanied by changes in leaf primordia. These results indicate that application of exogenous JA accelerated tuberization. The formation of tubers was thus accompanied by a shift in the growth axis from elongation growth towards lateral growth. Hence, exogenous JA may play a role similar to that of the endogenous compound in tuber formation.
It is known that JA and related compounds are synthesized in leaves (Sembdner and Parthier, 1993; Creelman and Mullet, 1997; Mueller, 1997) and roots (Abdala et al., 2003), and that JA is metabolized to tuberonic acid glucoside (TAG) and transported to all parts of the plant. When the concentration of TAG reaches a sufficiently high level, the subapical meristem of the stolon begins to swell (Yoshihara et al., 1996). Related to this, JA caused a considerable increase in the level of sucrose in potato cells before the initiation of cell expansion (Takahashi et al., 1995).
Previous studies have demonstrated that cell division and cell enlargement contribute to potato tuber formation. The timing and location of cell division and cell enlargement were found to differ in various regions of the developing stolon and tuber. Transverse cell divisions occurred first in the apical region of the stolon. When the stolon tips started to swell transverse cell division in the apex stopped. Meanwhile, from the basal part of the subapical region upwards, cells enlarged and then divided longitudinally (Xu et al., 1998). This agrees with the suggestion of Sanz et al. (1996) that cell enlargement precedes cell division during the initiation of radial tuber growth.
The data presented in this paper clearly show that JA produced a dramatic increase in cell area, but we were not able to observe cell division in response to JA treatment. However, we cannot ignore the possibility that JA had some effect on this process.
Following the increase in cell area in response to JA, large amounts of starch accumulated in the tip of the stolon. In relation to these events, increased cell division and expansion in the developing tuber is followed rapidly by a massive deposition of starch and storage of proteins (Visser et al., 1994; Viola et al., 2001).
To our knowledge, the present study is the first to report the involvement of JA in vascular tissue differentiation. In the longitudinal sections of stolons corresponding to the second stage and treated with JA, the presence of xylem elements was clearly observed. However, we were not able to detect phloem tissue despite the fact that differentiation of this tissue normally occurs prior to differentiation of xylem. Hence, the early differentiation of vascular tissues in the apical region may be directly related to water and ion movement through the stolons. Thus, we can assume that the role of JA in tuber formation is in the enlargement of meristems, the increase in cell expansion, the reduction of leaf primordia length and the early vascular tissue differentiation, thus facilitating the movement of substances to the stolon tip.
ACKNOWLEDGEMENTS
We thank the language consultant, Professor Iliana A. Martínez, for his help. This work was supported by a grant from SECYT‐UNRC to G.A., and fellowships from CONICET to A.C. and A.V.
Supplementary Material
Received: 13 November 2002; Returned for revision: 15 January 2003; Accepted: 5 March 2003
References
- AbdalaG, Castro G, Miersch O, Pearce D.2002. Changes in jasmonates and gibberellins during development of potato plant (Solanum tuberosum L.). Plant Growth Regulation 36: 121–126. [Google Scholar]
- AbdalaG, Miersch O, Kramell R, Vigliocco A, Agostini E, Forchetti G, Alemano S.2003. Jasmonate and octadecanoid occurrence in tomato hairy roots. Endogenous level changes in response to NaCl. Plant Growth Regulation 40: 21–27. [Google Scholar]
- AbeM, Shibaoka H, Yamane H, Takahashi N.1990. Cell cycle‐dependent disruption of microtubules by methyl jasmonates in tobacco BY‐2‐cells. Protoplasma 156: 1–8. [Google Scholar]
- BoothA.1963. The role of growth substances in the development of stolons. In: JD Ivins, FL Milthorpe, eds. The growth of the potato London: Butterworth, 99–113. [Google Scholar]
- CastroG, Kraus T, Abdala G.1999. Endogenous jasmonic acid and radial cell expansion in buds of potato tubers. Journal of Plant Physiology 155: 706–710. [Google Scholar]
- CreelmanRA, Mullet JE.1997. Biosynthesis and actions of jasmonates in plants. Annual Review of Plant Physiology and Plant Molecular Biology 48: 355–381. [DOI] [PubMed] [Google Scholar]
- D’Ambrogio de ArgüesoA.1986.Manual de técnicas en histología vegetal Buenos Aires: Hemisferio Sur, 5–12. [Google Scholar]
- Dizeo de StrittmatterC.1979. Modificación de una coloración safranina‐fast green. Boletín de la Sociedad Argentina de Botánica 18: 121–122. [Google Scholar]
- EwingEE, Struik PC.1992. Tuber formation in potato: induction, initiation and growth. Horticultural Reviews 14: 89–198. [Google Scholar]
- FernieAR, Willmitzer L.2001. Molecular and biochemical triggers of potato tuber development. Plant Physiology 127: 1459–1465. [PMC free article] [PubMed] [Google Scholar]
- JacksonSD.1999. Multiple signaling pathways control tuber induction in potato. Plant Physiology 119: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KodaY.1997. Possible involvement of jasmonates in various morphogenic events. Physiologia Plantarum 100: 639–646. [Google Scholar]
- KodaY, Kikuta Y, Kitahara T, Nishi T, Mori K.1992. Comparisons of various biological activities of stereoisomers of methyl jasmonate. Phytochemistry 31: 1111–1114. [Google Scholar]
- KodaY, Kikuta Y, Tazaki H, Tsujino Y, Sakamura S, Yoshihara T.1991. Potato tuber‐inducing activities of jasmonic acid and related compounds. Phytochemistry 30: 1435–1438. [Google Scholar]
- MatsukiT, Tazaki H, Fujimori T, Hogetsu T.1992. The influences of jasmonic acid methyl ester on microtubules in potato cells and formation of potato tubers. Bioscience Biotechnology and Biochemistry 56: 1329–1330. [Google Scholar]
- MuellerMJ.1997. Enzymes involved in jasmonic acid biosynthesis. Physiologia Plantarum 100: 653–663. [Google Scholar]
- PratS, Frommer WB, Höfgen R, Keil M, Kossmann J, Köster‐Töpfer M, Liu XJ, Müller B, Peña‐Cortés M, Rocha‐Sosa Met al.1990. Gene expression during tuber development in potato plants. FEBS Letters 286: 334–338. [DOI] [PubMed] [Google Scholar]
- SanzMJ, Mingo‐Castel A, van Lammeren AAM, Vreugdenhil D.1996. Changes in the microtubular cytoskeleton precede in vitro tuber formation in potato. Protoplasma 191: 46–54. [Google Scholar]
- SembdnerG, Parthier B.1993. The biochemistry and the physiological and molecular actions of Jasmonates. Annual Reviews of Plant Physiology and Plant Molecular Biology 44: 569–589. [Google Scholar]
- ShibaokaH.1991. Microtubules and the regulation of cell morphogenesis by plant hormones. In: CW Lloyd, ed. The cytoskeletal basis of plant growth and form London: Academic Press, 159–168. [Google Scholar]
- TakahashiK, Fujino K, Kikuta Y, Koda Y.1994. Expansion of potato cells in response to jasmonic acid. Plant Science 100: 3–8. [Google Scholar]
- TakahashiK, Fujino K, Kikuta Y, Koda Y.1995. Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Science 111: 11–18. [Google Scholar]
- ViolaR, Roberts AG, Haupt S, Gazzani S, Hancock RD, Marmiroli N, Machray GC, Oparka KJ.2001. Tuberization in potato involves a switch from apoplastic to symplastic phloem unloading. The Plant Cell 13: 385–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VisserRGF, Vreugdenhil D, Hendriks T, Jacobsen EJ.1994. Gene expression and carbohydrate content during stolon to tuber transition in potatoes (Solanum tuberosum). Physiologia Plantarum 90: 285–292. [Google Scholar]
- VreugdenhilD, Struik PC.1989. An integrated view of the hormonal regulation of tuber formation in potato (Solanum tuberosum). Physiologia Plantarum 75: 525–31. [Google Scholar]
- VreugdenhilD, Xu X, Jung JS, van Lammeren AAM, Ewing EE.1999. Initial anatomical changes associated with tuber formation on single‐node potato (Solanum tuberosum L.) cuttings: a re‐evaluation. Annals of Botany 84: 675–680. [Google Scholar]
- WasternackC, Hause B.2002. Jasmonates and octadecanoids – signals in plant stress response and development. In: Moldave K, ed. Progress in nucleic acid research and molecular biology New York: Academic Press, 165–221. [DOI] [PubMed] [Google Scholar]
- XuX, Vreugdenhil D, van Lammeren AAM.1998. Cell division and cell enlargement during potato tuber formation. Journal of Experimental Botany 320: 573–582. [Google Scholar]
- YoshiharaT, Amanuma M, Tsutsumi T, Okumura Y, Matsuura H, Ichihara A.1996. Metabolism and transport of [2‐14C] (±) jasmonic acid in the potato plant. Plant Cell Physiology 37: 586–590. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.