Abstract
Aberrant expression of gonadotropin-releasing hormone receptor (GnRHR) has been reported in human adrenal tissues including aldosterone-producing adenoma (APA). However, the details of its expression and functional role in adrenals are still not clear. In this study, quantitative RT-PCR analysis revealed the mean level of GnRHR mRNA was significantly higher in APAs than in human normal adrenal (NA) (P=0.004). GnRHR protein expression was detected in human NA and neoplastic adrenal tissues. In H295R cells transfected with GnRHR, treatment with GnRH resulted in a concentration-dependent increase in CYP11B2 reporter activity. Chronic activation of GnRHR with GnRH (100 nM), in a cell line with doxycycline-inducible GnRHR (H295R-TR/GnRHR), increased CYP11B2 expression and aldosterone production. These agonistic effects were inhibited by blockers for the calcium signaling pathway, KN93 and calmidazolium. These results suggest GnRH, through heterotopic expression of its receptor, may be a potential regulator of CYP11B2 expression levels in some cases of APA.
Keywords: Gonadotropin-releasing hormone receptor (GnRHR), adrenal aldosterone-producing adenoma (APA), CYP11B2, aldosterone
INTRODUCTION
Aldosterone-producing adenoma (APA) represents the major cause of hyperaldosteronism. Growing evidence suggests that many adrenal tumors have aberrant expression of G-protein coupled receptors that lead to inappropriate growth and steroid hormone production (Ye et al., 2007). The gonadotropin-releasing hormone receptor (GnRHR), also known as the luteinizing hormone-releasing hormone receptor (LHRHR), is a member of the G protein-coupled receptor (GPCR) family. GPCR is known to play roles in many biological processes, including cell proliferation and metabolism (Chakraborty, 2001). Recently, it was demonstrated that GnRHR mRNA is highly expressed in some cases of APA (Ye et al., 2007; Zwermann et al., 2009). Ziegler et al. reported GnRHR expression in human normal adrenal gland, adrenocortical adenoma and the SW-13 adrenocortical tumor cell-line (Ziegler et al., 2009).
A number of studies involving gonadotropin-releasing hormone (GnRH)-mediated signal transduction indicate that GnRHR activates both adenylate cyclase and phospholipase C via G-protein coupled receptors (Arora et al., 1998; Liu et al., 2002). However, the role of GnRHR in the regulation of adrenal function is still not clear. Herein, we examined the expression of GnRHR protein in human normal and neoplastic adrenal tissues. In addition, we performed quantitative RT-PCR (qPCR) analysis in order to confirm the statistical significance of the mRNA levels transcribed. In addition, we developed an adrenocortical cell line with doxycycline (doxy)-inducible GnRHR to examine the functional role of GnRHR in regulation of CYP11B2 expression and aldosterone production.
MATERIALS AND METHODS
Subjects and tissues
APA, cortisol-producing adenoma (CPA), non-functioning adenoma lacking clinical hormonal abnormalities (NFA), adrenocortical carcinoma (ACC) and normal adrenal gland (NA) resected with renal carcinoma were retrieved from the surgical pathology files of Tohoku University Hospital (Sendai, Japan). For immunohistochemistry, the specimens were fixed in 10% formalin for 24–48 h at room temperature and embedded in paraffin wax. Western or immunoblotting analysis was performed using lysates from frozen tissue specimens. In qPCR analysis, NA and APA were obtained through the Department of Medicine at UT Southwestern, (Dallas, TX), the Cooperative Human Tissue Network (Philadelphia, PA) and Clontech (Palo Alto, CA). Adrenal samples were acquired from autopsy (NA) performed within 6 h after death where the cause of death was unrelated to adrenal functions. Adenoma samples were obtained at the time of surgery. Specimens for RNA isolation were snap-frozen and stored at −80 °C until use. Research protocols for this study were approved by the ethics committee at Tohoku University School of Medicine (Sendai, Japan), the Institutional Review Boards of the University of Texas Southwestern Medical Center (Dallas, TX, USA) and Georgia Health Sciences University (Augusta, GA).
RNA extraction and reverse transcription reaction
Total RNA from NAs (n=16) and APAs (n=20) was extracted using TRIzol Reagent (50–100mg/ml) (Life Technologies, Carlsbad, CA). The purity and integrity of the RNA were assessed by the Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and its quantity was determined by the NanoDrop spectrophotometers (ND1000, NanoDrop Technologies, Wilmington, DE). Total RNA of 2 μg was reverse transcribed using the High Capacity cDNA Archive Kit (Life Technologies, CA) according to the manufacturer’s recommendations and incubated at 25° C for 10 min and 37° C for 2 h. The synthesized cDNA was subjected to 1:10 dilution and stored at −20° C.
qPCR analysis
The primer and probe set for human CYP11B2 was designed using Primer Express 3.0 (Life Technologies, CA) and purchased from IDT (Integrated DNA Technologies Inc., Coralville IA, USA) as published previously (Saner-Amigh et al. 2006). Taqman gene expression assays used for GnRHR RNA detection was commercially obtained from Life Technologies (Ye et al. 2007). For the GnRHR reaction, the 20μl total volume consisted of TaqMan Fast Universal PCR Master Mix (2×) (Life Technologies) 10μl, 900nM of each primer, 400nM of the probe and 5μl of each first-strand cDNA sample. CYP11B2 reaction mix consisted of 10μl TaqMan PCR Master Mix (2×) (Life Technologies), 100nM of primer/probe mix and 5μl of each first-strand cDNA sample (20ng). Each reaction included 10μl of TaqMan PCR Master Mix (2×) (Life Technologies), 100nM probe and 50nM each primer (Ye et al., 2007). Quantitative normalization of cDNA in each tissue-derived specimen was performed using expression of18S rRNA (tissue) or cyclophilin (PPIA) (for transduced cells) as an endogenous internal controls. 18s mRNA was detected and quantified using TaqMan Ribosomal RNA Control Reagents (Vic Probe) (Life Technologies) (Ye et al., 2007). PPIA was detected and quantified using commercially available TaqMan gene expression assay (FAM Probe) (Life Technologies) (Monticone et al., 2012). Negative controls contained water instead of first-strand cDNA. The generated Ct value of each gene was normalized by its respected Ct value of 18S rRNA or PPIA (ΔCt). Each gene was subsequently normalized using the average ΔCt value of the normal adult adrenal or basal group (ΔΔCt). The final fold expression changes were calculated using the equation 2−ΔΔCt (Livak & Schmittgen, 2001).
Western blotting analysis
Human adrenal specimens were homogenized in a fivefold volume of the triple detergent lysis buffer, containing 50 mmol/L Tris–HCl (pH 8.0), 150 mmol/L NaCl, 0.02% sodium azide, 0.1% sodium dodecyl sulfate (SDS), 100 μg/ml phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 μg/ml Nonidet P40, 1 μg/ml leupeptin, 1 μg/ml pepstatin A, 1 μg/ml antipain and 0.5% sodium deoxycholate. The homogenates were centrifuged at 15,000 × g for 10 min, and the supernatant was subjected to western blot analysis. The protein extracts (15μg/lane) were electrophoresed on SDS polyacrylamide gels (10%) and then transferred to Immobilon-P transfer membrane (EMD Millipore Corporation, Billerica, MA). The membrane was incubated for 1 h with the antibody against GnRHR (sc-8682, Santa Cruz Biotechnology Inc.) (1:500). The membrane was washed several times with TBST-20 buffer and then incubated with horseradish peroxidase (HRP)-coupled secondary antibodies (1:20,000; sc-2004, Santa Cruz Biotechnology Inc.) for 30 min. Immune complexes were visualized using an Immobilon western chemiluminescent HRP substrate (Millipore Corporation). Expression of β-actin was utilized as an internal control using anti-β-actin monoclonal antibody (1:20,000; sc-81178, Santa Cruz Biotechnology Inc.) and its secondary antibody (goat anti-mouse IgG-HRP, 1:20,000; sc-2005, Santa Cruz Biotechnology Inc.).
For quantification of protein expression, the relative immunointensity of the bands at 38 kDa for GnRHR (NCBI Reference Sequence: NP_000406.2) or at 42 kDa for β-actin were determined using NIH Image software.
Immunohistochemistry
Immunohistochemical analysis was performed employing the streptavidin-biotin amplification method using a Histofine Kit (Nichirei, Tokyo, Japan) and goat polyclonal antibody for GnRHR (sc-8682) (Santa Cruz Biotechnology, Inc.). Antigen retrieval was performed by heating the slides in an autoclave at 120 C for 5 min in citric acid buffer (2 mM citric acid and 9 mM trisodium citrate dehydrate, pH 6.0). The dilution of GnRHR antibody used in this study was 1:300. The antigen-antibody complex was visualized with 3,3′-diaminobenzidine solution [1 mM 3,3′-diaminobenzidine, 50 mM Tris-HCl buffer (pH 7.6), and 0.006% H2O2] and counterstained with hematoxylin. Normal goat IgG was also used in place of the primary antibodies as a negative control.
Cell culture
H295R human adrenocortical tumor cells were cultured in Dulbecco’s modified Eagles/Ham’s F12 medium (Life Technologies, Carlsbad, CA) supplemented with 10% cosmic calf serum (Hyclone, Logan, UT) and antibiotics. The adrenocortical H295R-TR cells expressing the tet repressor were transduced with the doxy-inducible GnRHR lentivirus (Ye et al, 2009). Cells were cultured as previously described (Ye et al 2009). Cells were subcultured and treated with agonists Ang II (10 nM) (Sigma-Aldrich, St. Louis, MO) or GnRH alone or with inhibitors KN93 (1 μM) (EMD Millipore Corporation) calmidazolium (0.3 μM) (Sigma-Aldrich). Doxy (0.25 μg/ml) was present throughout the experiment.
Plasmids
The subcloned chimeric construct containing the human CYP11B2 5′-flanking region from −1521 to +2 relative to the transcription start site fused upstream of the luciferase cDNA (pGL3-Basic, Promega, Madison, WI) was used for the transient transfection study (Bassett et al., 2000). The expression construct of human GnRHR, encoding the full-length human GnRHR, was purchased from the UMR cDNA Resource Center (Rolla, MO). The human GnRHR was subcloned into the pLenti-TO-V5/DEST vector system (Life Technologies) to generate a lentivector expressing doxy-inducble GnRHR. Lentiviruses were prepared in HEK293T cells as previously described (Nogueira et al., 2009).
Transient Transfection assays of CYP11B2-luciferase reporter plasmid and GnRHR expression plasmid
For transfection experiments, cells were subcultured onto 12 well dishes at a density of 400,000 cells per well. Transfections were carried out, for 6 hours, using the transfection reagent Transfast (Promega) according to manufacturer’s directions. To normalize luciferase activity, cells were co-transfected with 0.05μg/well of β-galactosidase plasmid (Promega). Following recovery of the cells, for 20–24 hours, they were treated with increasing concentrations of human GnRH (Sigma Aldrich) (3nM, 30nM, 300nM and 3,000nM) for 6 hours prior to being lysed and assayed for activity using the Luciferase assay system (Promega).
Effect of GnRHR activation on endogenous CYP11B2 and aldosterone production in H295R-TR/GnRHR cells
Cells were plated at a density of 50,000 cells per well in a 48 well dish in growth medium containing 2% NuSerum and 1% ITS and incubated at 37° C for 48 h (6 replicates). Eighteen hours prior to starting the experiments, the cells were changed to a low-serum experimental medium (DMEM/F12 supplemented with 0.1% NuSerum and antibiotics). The cells were subsequently treated in the same low-serum experimental medium with indicated agonists and/or calmodulin inhibitor (calmidazolium) or calcium (Ca2+) calmodulin kinases inhibitor, KN93. Cells stimulated with 10 nM Ang II served as a positive control, and non-treated cells were considered as the basal (control). At the end of 4 days of incubation, media was collected for aldosterone measurement, protein was isolated from one well per treatment, and RNA was isolated from the remainder of the replicates. cDNA was generated from the RNA and analyzed for gene expression using qPCR. Each bar represents the fold increase ± S.D. over basal (no treatment, control) by the 2−ΔΔCt method, using human PPIA for normalization. Aldosterone measurements were determined as described earlier (Ye et al., 2009), using the aldosterone Coat-A-Count RIA kit from Siemens (New York, NY), following the manufacturer’s guidelines. Aldosterone levels were normalized to protein.
Statistical analysis
For qPCR results, the data obtained were analyzed and compared with control values (mean of normal adrenal samples) using the Mann–Whitney Rank Sum test with the SigmaStat 3.0 software package (SPSS, Chicago, IL, USA). Results of other in vitro experiments were analyzed by ANOVA followed by post hoc Tukey test. The results were considered significantly different when the P value was ≤ 0.05.
RESULTS
GnRHR mRNA expression in APA VS NA
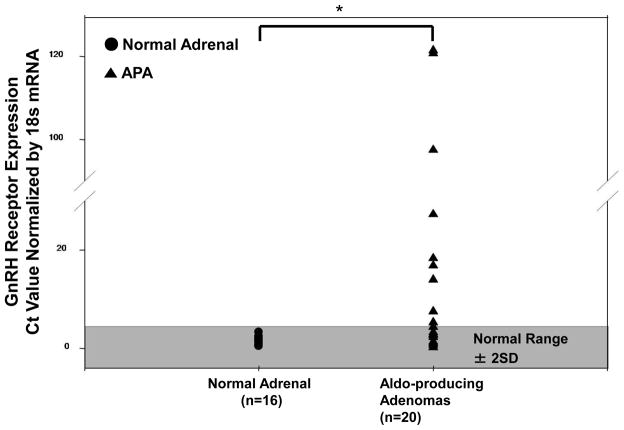
The results of qPCR analysis demonstrated that GnRHR mRNA expression in all of the 16 NAs (100%) were within the normal range (defined as ± 2SD from the mean of the normal adrenals) but only 9 of 20 APA samples (45%) within the normal range (Figure 1). Two APA samples had more than a 120-fold elevation in GnRHR expression over the normal controls (Figure 1). The mean level of GnRHR mRNA was significantly higher in APAs than in NAs (p=0.004) (Figure 1).
Figure 1.
Relative GnRHR expression as measured by real-time RT-PCR, in 16 normal adrenals and 20 aldosterone producing adenomas. A vertical point scatterplot comparing GnRH receptor expression in normal adrenals vs aldosterone-producing adenomas. All 16 normal adrenals (100%) fell within the normal range (defined as ±2 SD from the mean of the normal adrenals) as opposed to only 9 out of 20 APA samples (45%). The difference in the mean values between the two groups is statistically significant (*P < 0.05).
GnRHR protein expression in adrenocortical tissues
GnRHR protein expression in human adrenal tissues was studied using Western blot analysis (Figure 2A). The human adrenal tissue extracts, including APA, CPA, NFA, ACC and NA, demonstrated two bands with a size of about 38 kDa. Since the presumed molecular weight of human GnRHR is 37,600 (NCBI Reference Sequence: NP_000406.2), either of these two bands presumably represents GnRHR protein and the other may be a GnRHR protein with some modification, such as glycosylation. Semi-quantitative analysis of the bands corresponding to GnRHR and β-actin did demonstrate that relative expression levels of GnRHR protein in 4APAs were 1.2-fold higher than those in 4 NAs, but not statistically significant (P=0.20) (Figure 2B).
Figure 2.
(A) Western blotting analysis of GnRHR and β-actin in aldosterone-producing adenoma (APA), cortisol-producing adenoma (CPA), non-functioning adenoma (NFA), adrenocortical carcinoma (ACC) and normal adrenal gland (NA). Western blot analysis was repeated twice and similar results were obtained. (B) Relative expression levels of GnRHR protein in tissues of APA (n=4) and NA (n=4). The relative immunointensity of the band representing GnRHR protein was normalized with that of β-actin protein. The ratio of each normalized value to that of NA was demonstrated as the relative expression levels of GnRHR. Data are shown as mean + SEM.
As predicted from the Western results, immunoreactivities for GnRHR were sparsely detected in all the adrenal cases including NA (in the zona glomerulosa (ZG) and fasciculata (ZF)), APA, CPA, NFA and ACC (Figure 3). The immunopositivities were found in the cell membrane as well as the cytoplasm, compatible with a previous report (Albiger et al, 2011; Xing et al., 2009). Immunohistochemical analysis revealed that GnRHR positive tumor cells were sparsely distributed in APA, and the mean ratio of the positive cells was approximately 10% in the whole tumor area of the cases examined (data not shown).
Figure 3.
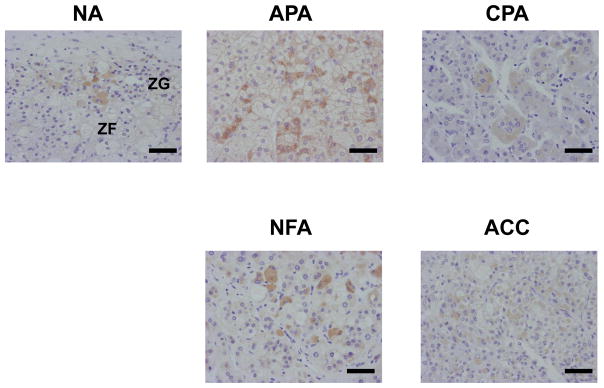
Immunohistochemistry for GnRHR in the cortex of normal adrenal gland (NA), aldosterone-producing adenoma (APA), cortisol-producing adenoma (CPA), non-functioning adenoma (NFA) and adrenocortical carcinoma (ACC). We performed the immunohistochemical analysis at least in five cases. In the representative figures, the immunopositive cells were sparsely detectable in the zona glomerulosa (ZG) and fasciculate (ZF) of NA, and these tumors. The percentage of GnRHR-positive cells in the representative cases were approximately 5% in NA, 10% in APA, 5% in CPA, 10% in NFA and 5% in ACC, respectively. Bar, 100 μm.
Concentration-dependent effects of GnRH on CYP11B2 reporter gene activity and in H295R cells
In H295R cells co-transfected with CYP11B2 and GnRHR, treatment with GnRH significantly increased CYP11B2 promoter activity starting at 30nM compared to basal levels (approximately 4-fold) (P<0.05) (Figure 4). As a positive control, we also confirmed that angiotensin II (Ang II) (100nM) stimulated CYP11B2 reporter activity (approximately 8-fold) in this experimental model (data not shown).
Figure 4.
Concentration-dependent effects of GnRH on CYP11B2 reporter gene activity in H295R cells cotransfected with GnRHR-containing vector. H295R cells were co-transfected with CYP11B2 luciferase reporter constructs at a concentration of 1 μg/well, along with GnRH receptor expression plasmid, at a concentration of 0.3 g/well. Cells were allowed to recover overnight at 37°C then treated with the indicated amounts of GnRH for 6 h and then lysed and assayed for luciferase activity. Data were normalized to cotransfected β-galactosidase. Data shown were expressed as fold induction over basal reporter plus GnRH receptor. Results represent the mean +/− SEM of data from at least three independent experiments, each performed in triplicate (* P < 0.05, vs. Basal).
Effect of GnRHR activation on endogenous CYP11B2 transcript levels and aldosterone production
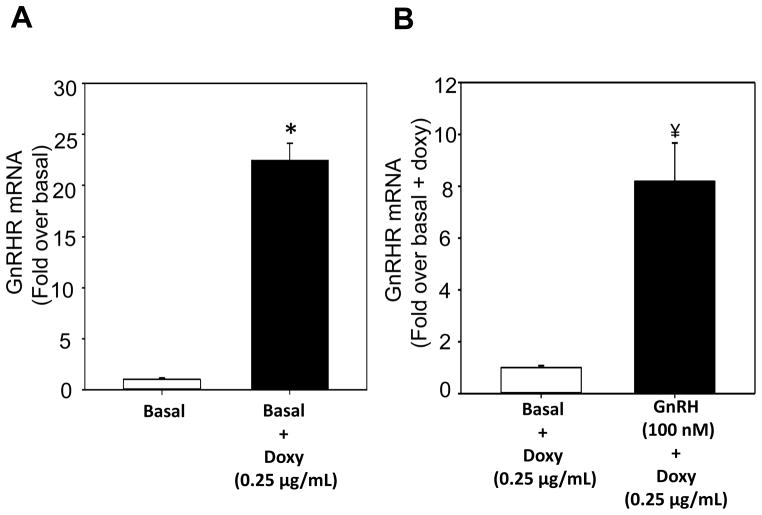
H295R-TR/GnRHR cells treated doxy elevated GnRHR mRNA by 23-fold (P<0.001) (Figure 5A). Treatment with GnRH (100 nM) demonstrated significant increase in GnRHR mRNA by 8-fold (P<0.001) (Figure 5B), CYP11B2 mRNA by over 100-fold (P<0.001) (Figure 6), and aldosterone production by 50-fold (P<0.001) (Figure 7). In addition, the treatment with CA2+ signaling inhibitors calmidazolium and KN93, inhibited the stimulatory effects of GnRH on CYP11B2 mRNA expression by 30% and 60 %, respectively. Similarly the stimulation in aldosterone production was inhibited by KN93 and calmidazolium by 95% and 40%, respectively. The positive control consisting of the cells treated with 10 nM Ang II for 4 days, demonstrated a 4-fold increase in aldosterone production.
Figure 5.
GnRHR expression in H295R-TR/GnRHR cells. (A) H295R-TR/GnRHR cells (6 replicates) were plated in growth media without (Basal) or with (Basal+Doxy) the presence of doxycycline for 48 h. Cells were treated with low serum (LS) media for 4 days. RNA was then isolated for cDNA generation and quantification of mRNA expression levels of GnRHR. (B) H295R-TR/GnRHR cells (6 replicates) were plated for 48 h. Media was then replaced with fresh LS GnRH (100 nM). After 4 days, RNA was isolated for cDNA generation and quantification of GnRHR mRNA expression levels. Media also contained 0.25μg/mL doxy, throughout this experiment. Bars represent the Mean ± S.D. of fold increase in mRNA levels versus basal and normalized to PPIA, (*P <0.001 vs. Basal, ¥ P <0.001, vs. Basal + Doxy).
Figure 6.
Effect of chronic GnRH treatment on CYP11B2 expression. H295R-TR/GnRHR cells (6 replicates) were pre-treated with 0.3 μM calmidazolium (calmodulin inhibitor) or 1 μM KN93 (calcium calmodulin kinases inhibitor). Media was then replaced with fresh LS containing inhibitors and agonists Ang II (10 nM) or GnRH (100 nM). After 4 days RNA was isolated for cDNA generation and quantification of mRNA expression levels of CYP11B2. Bars represent the Mean ± S.D. of fold increase in mRNA levels vs Basal and normalized to PPIA. Media also contained 0.25μg/mL throughout the experiment (*P <0.001 vs. Basal, §P <0.001, vs. respective agonist).
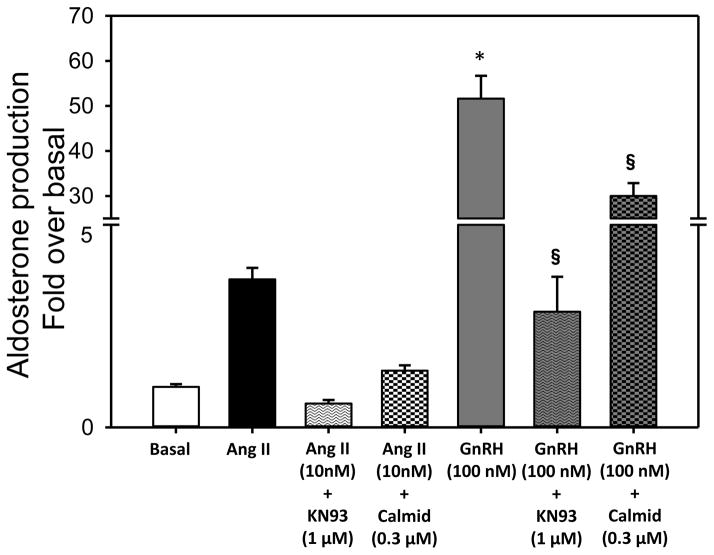
Figure 7.
Effect of chronic GnRHR activation on aldosterone production. H295R-TR/GnRHR cells (6 replicates) were initially pre-treated with 0.3 μM calmidazolium (calmodulin inhibitor) or 1 μM KN93 (calcium calmodulin kinases inhibitor). Media was then replaced with fresh LS containing inhibitors and agonists Ang II (10 nM) or GnRH (100 nM). After 4 days, media was collected for aldosterone assay, and protein lysates from each treatment group were collected for normalization of steroid estimation. Bars represent the Mean±S.D. of fold increase in aldosterone levels per mg protein (vs Basal). Media also contained 0.25μg/mL doxy, throughout the experiment (*P<0.001 vs. Basal, §P<0.001, vs. respective agonist).
DISCUSSION
GnRHR is abundantly expressed in pituitary gonadotropic cells, where it can trigger the synthesis and release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) activated by GnRH secreted from the hypothalamus (La Rosa et al., 2000). In addition, GnRHR has also been detected in many extra-pituitary tissues including some tumor cells (Chen et al., 1999; Hapgood et al., 2005; Kottler et al., 1997; Minaretzis et al., 1995; Peng et al., 1994; Yin et al., 1998; Yeung et al., 2005). Results of our present study demonstrated that GnRHR mRNA and protein were expressed in human normal and neoplastic adrenal tissues including APA, consistent with those in previous reports (Albiger et al., 2011; Ye et al., 2007; Ziegler et al., 2009; Zwermann et al., 2009). In the results of Western blotting analysis, GnRHR protein levels tended to be higher in APA than NA although statistically not significant possibly due to limited number of adrenal cases examined in this study. However, we could not deny the possibility that increased expression of GnRHR mRNA did not result in increased GnRHR protein expression in APA. It awaits further study for clarification in the future.
In human tissues, the ligand for GNRHR, GnRH, has two forms: GNRH1 and GNRH2 (Chen et al., 1998). It has been reported that GNRH1 is mainly expressed in the hypothalamus, and GNRH2 is expressed at significantly higher levels outside the brain, including the kidney, bone marrow and prostate (Albiger et al, 2011; White et al., 1998). On the other hand, we have previously reported that in human adult adrenal, predominant isoform of GNRH is GnRH1, and the mRNA level is approximately 8-fold lesser compared to human adult hypothalamus (Xing et al., 2009). Taken together, it is postulated that the aberrant GNRHR in human adrenal tissue may be controlled by the paracrine/autocrine action of GNRH1 expressed in the adrenal gland.
It was previously reported that three patients with APA showed increased serum aldosterone levels following GnRH stimulation, suggesting the expression of GnRHR in these adrenal tissues (Zwermann et al., 2009). Albiger and colleagues were the first to report a case of primary aldosteronism (PA) during pregnancy with response to GnRH and human chorionic gonadotropin (hCG) administration, which also suggested the roles of aberrant expression of non-adrenal GPCRs as one of the sporadic causes of PA (Albiger et al, 2011). They further demonstrated, in a series of non-pregnant PA patients, that aberrant GnRHR was expressed and several of these patients increased aldosterone secretion in response to GnRH administration (Albiger et al, 2011). Therefore, the ability of GnRHR to regulate intracellular signaling pathways known to activate aldosterone production made GnRHR as a potential candidate for regulator of APA steroidogenesis. Results of our present in vitro study also demonstrated that GnRH ligand elevated the promoter activity and expression level of CYP11B2 in H295R cells transfected with the GnRHR vector. In this study, a second adrenocortical cell line, H295R-TR/GnRHR with doxy-inducible GnRHR was also used to study the effect of GnRHR on endogenous CYP11B2 regulation in order to further confirm the results from reporter analyses in H295R cells. Chronic GnRH (100 nM) treatment increased CYP11B2 mRNA expression and aldosterone production An inhibition of this stimulatory effect by inhibitors calmidazolium and KN93 confirmed the involvement of Ca2+ signaling in CYP11B2 transcript stimulation through GnRHR activation. This stimulation in CYP11B2 expression and aldosterone production may be attributed in part to the observed increase in cell proliferation caused by GnRHR activation (data not shown). It may be speculated that APA with elevated GnRHR levels may exhibit increased cell proliferation, CYP11B2 expression and aldosterone production. Indeed, GnRH stimulation has shown to have anti-apoptotic effects in ovarian cancers (Gründker et al., 2000; Günthert et al., 2002). Furthermore, the increase in aldosterone production was also paralleled with decrease in the production of androstenedione by 50% without stimulation of cortisol production with 4 days GnRH treatment (data not shown), indicating a possible divergence of pathways towards mineralocorticoid production. It may therefore be speculated that chronic activation of GnRHR in adrenocortical cells triggers several different signaling cascades, including Ca2+ signaling pathways that increase aldosterone production and/or pathways that may increase cell proliferation. The importance of controlling adrenal cell intracellular Ca2+ has recently been shown to have clinical relevance. Somatic mutations of the KCNJ5 potassium channel have been reported to cause a loss of channel ion selectivity leading to sodium influx and increased adrenal cell Ca2+ via activation of voltage gated Ca2+ channels (Choi et al., 2011; Zennaro et al., 2011). In addition, somatic mutations of ATP1A1 and ATP2B3 in APA were also recently reported to result in increasing adrenal cell intracellular Ca2+ (Beuschlein et al., 2013). Therefore, it becomes pivotal to examine the association between GnRHR expression levels and these somatic mutations. However, in this study, the original APA materials used to determine GnRHR expression patterns were exhausted and could not be studied with regard to KCNJ5, ATP2B3 and ATP1A1 mutation status. It awaits further study for clarification in the future.
In summary, our current study demonstrated that human normal and neoplastic adrenal tissues expressed GnRHR. The increased GnRHR mRNA expression was also detected in APAs compared to normal adrenal cortex. We also demonstrated that GnRHR activation increased CYP11B2 promoter activity, CYP11B2 mRNA expression and aldosterone production. These findings all suggest novel and important roles of GnRHR in the regulation of adrenocortical functions.
References
- Albiger NM, Sartorato P, Mariniello B, Iacobone M, Finco I, Fassina A, Mantero F. A case of primary aldosteronism in pregnancy: do LH and GNRH receptors have a potential role in regulating aldosterone secretion? Eur J Endocrinol. 2011;164:405–12. doi: 10.1530/EJE-10-0879. [DOI] [PubMed] [Google Scholar]
- Arora KK, Krsmanovic LZ, Mores N, O’Farrell H, Catt KJ. Mediation of cyclic AMP signaling by the first intracellular loop of the gonadotropin-releasing hormone receptor. J Biol Chem. 1998;273:25581–25586. doi: 10.1074/jbc.273.40.25581. [DOI] [PubMed] [Google Scholar]
- Bassett MH, Zhang Y, White PC, Rainey WE. Regulation of human CYP11B2 and CYP11B1: comparing the role of the common CRE/Ad1 element. Endocr Res. 2000;26:941–951. doi: 10.3109/07435800009048620. [DOI] [PubMed] [Google Scholar]
- Beuschlein F, Boulkroun S, Osswald A, Wieland T, Nielsen HN, Lichtenauer UD, Penton D, Schack VR, Amar L, Fischer E, Walther A, Tauber P, Schwarzmayr T, Diener S, Graf E, Allolio B, Samson-Couterie B, Benecke A, Quinkler M, Fallo F, Plouin PF, Mantero F, Meitinger T, Mulatero P, Jeunemaitre X, Warth R, Vilsen B, Zennaro MC, Strom TM, Reincke M. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat Genet. 2013;45:440–444. doi: 10.1038/ng.2550. [DOI] [PubMed] [Google Scholar]
- Chakraborthy P. G-protein-mediated signaling and its control in macrophages and mammalian cells. Crit Rev Microbiol. 2001;27:1–8. doi: 10.1080/20014091096666. [DOI] [PubMed] [Google Scholar]
- Chen A, Yahalom D, Ben-Aroya N, Kaganovsky E, Okon E, Koch Y. A second isoform of gonadotropin-releasing hormone is present in the brain of human and rodents. FEBS Letters. 1998;435:199–203. doi: 10.1016/s0014-5793(98)01064-3. [DOI] [PubMed] [Google Scholar]
- Chen HF, Jeung EB, Stephenson M, Leung PC. Human peripheral blood mononuclear cells express gonadotropin-releasing hormone (GnRH), GnRH receptor, and interleukin-2 receptor gamma-chain messenger ribonucleic acids that are regulated by GnRH in vitro. J Clin Endocrinol Metab. 1999;84:743–750. doi: 10.1210/jcem.84.2.5440. [DOI] [PubMed] [Google Scholar]
- Choi M, Scholl UI, Yue P, Bjorklund P, Zhao B, Nelson-Williams C, Ji W, Cho Y, Patel A, Men CJ, Lolis E, Wisgerhof MV, Geller DS, Mane S, Hellman P, Westin G, Åkerström G, Wang W, Carling T, Lifton RP. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science. 2011;331:768–772. doi: 10.1126/science.1198785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gründker C, Schulz K, Günthert AR, Emons G. Luteinizing hormone-releasing hormone induces nuclear factor kappaB-activation and inhibits apoptosis in ovarian cancer cells. J Clin Endocrinol Metab. 2000;85:3815–3820. doi: 10.1210/jcem.85.10.6859. [DOI] [PubMed] [Google Scholar]
- Günthert AR, Gründker C, Hollmann K, Emons G. Luteinizing hormone-releasing hormone induces JunD-DNA binding and extends cell cycle in human ovarian cancer cells. Biochem Biophys Res Commun. 2002;294:11–15. doi: 10.1016/S0006-291X(02)00427-8. [DOI] [PubMed] [Google Scholar]
- Hapgood JP, Sadie H, van Biljon W, Ronacher K. Regulation of expression of mammalian gonadotrophin-releasing hormone receptor genes. J Neuroendocrinol. 2005;17:619–638. doi: 10.1111/j.1365-2826.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- Kottler ML, Starzec A, Carre MC, Lagarde JP, Martin A, Counis R. The genes for gonadotropin-releasing hormone and its receptor are expressed in human breast with fibrocystic disease and cancer. Int J Cancer. 1997;71:595–599. doi: 10.1002/(sici)1097-0215(19970516)71:4<595::aid-ijc14>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- La Rosa S, Celato N, Uccella S, Capella C. Detection of gonadotropin-releasing hormone receptor in normal human pituitary cells and pituitary adenomas using immunohistochemistry. Virchows Arch. 2000;437:264–269. doi: 10.1007/s004280000247. [DOI] [PubMed] [Google Scholar]
- Liu F, Usui I, Evans LG, Austin DA, Mellon PL, Olefsky JM, Webster NJ. Involvement of both G(q/11) and G(s) proteins in gonadotropin-releasing hormone receptor-mediated signaling in L beta T2 cells. J Biol Chem. 2002;277:32099–32108. doi: 10.1074/jbc.M203639200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−ΔΔ C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Minaretzis D, Jakubowski M, Mortola JF, Pavlou SN. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells. J Clin Endocrinol Metab. 1995;80:430–434. doi: 10.1210/jcem.80.2.7852501. [DOI] [PubMed] [Google Scholar]
- Monticone S, Hattangady NG, Nishimoto K, Mantero F, Rubin B, Cicala MV, Pezzani R, Auchus RJ, Ghayee HK, Shibata H, Kurihara I, Williams TA, Giri JG, Bollag RJ, Edwards MA, Isales CM, Rainey WE. Effect of KCNJ5 mutations on gene expression in aldosterone-producing adenomas and adrenocortical cells. J Clin Endocrinol Metab. 2012;97:1567–1572. doi: 10.1210/jc.2011-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira EF, Xing Y, Morris CA, Rainey WE. Role of angiotensin II-induced rapid response genes in the regulation of enzymes needed for aldosterone synthesis. J Mol Endocrinol. 2009;42:319–330. doi: 10.1677/JME-08-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, Fan NC, Ligier M, Vaananen J, Leung PC. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology. 1994;135:1740–1746. doi: 10.1210/endo.135.5.7956897. [DOI] [PubMed] [Google Scholar]
- Saner-Amigh K, Mayhew BA, Mantero F, Schiavi F, White PC, Rao CV, Rainey WE. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91:1136–1142. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proc Natl Acad Sci U S A. 1998;95:305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Nakamura Y, Rainey WE. G protein-coupled receptor expression in the adult and fetal adrenal glands. Mol Cell Endocrinol. 2009;300:43–50. doi: 10.1016/j.mce.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- Ye P, Nakamura Y, Lalli E, Rainey WE. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology. 2009;150:1303–1309. doi: 10.1210/en.2008-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung CM, An BS, Cheng CK, Chow BK, Leung PC. Expression and transcriptional regulation of the GnRH receptor gene in human neuronal cells. Mol Hum Reprod. 2005;11:837–842. doi: 10.1093/molehr/gah241. [DOI] [PubMed] [Google Scholar]
- Yin H, Cheng KW, Hwa HL, Peng C, Auersperg N, Leung PC. Expression of the messenger RNA for gonadotropin-releasing hormone and its receptor in human cancer cell lines. Life Sci. 1998;62:2015–2023. doi: 10.1016/s0024-3205(98)00173-8. [DOI] [PubMed] [Google Scholar]
- Zennaro MC, Jeunemaitre X. Mutations in KCNJ5 gene cause hyperaldosteronism. Circ Res. 2011;108:1417–1418. doi: 10.1161/RES.0b013e318224a359. [DOI] [PubMed] [Google Scholar]
- Ziegler CG, Brown JW, Schally AV, Erler A, Gebauer L, Treszl A, Young L, Fishman LM, Engel JB, Willenberg HS, Petersenn S, Eisenhofer G, Ehrhart-Bornstein M, Bornstein SR. Expression of neuropeptide hormone receptors in human adrenal tumors and cell lines: antiproliferative effects of peptide analogues. Proc Natl Acad Sci U S A. 2009;106:15879–15884. doi: 10.1073/pnas.0907843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwermann O, Suttmann Y, Bidlingmaier M, Beuschlein F, Reincke M. Screening for membrane hormone receptor expression in primary aldosteronism. Eur J Endocrinol. 2009;160:443–451. doi: 10.1530/EJE-08-0711. [DOI] [PubMed] [Google Scholar]