Abstract
This review examines the electrochemical techniques used to study extracellular electron transfer in the electrochemically active biofilms that are used in microbial fuel cells and other bioelectrochemical systems. Electrochemically active biofilms are defined as biofilms that exchange electrons with conductive surfaces: electrodes. Following the electrochemical conventions, and recognizing that electrodes can be considered reactants in these bioelectrochemical processes, biofilms that deliver electrons to the biofilm electrode are called anodic, ie electrode-reducing, biofilms, while biofilms that accept electrons from the biofilm electrode are called cathodic, ie electrode-oxidizing, biofilms. How to grow these electrochemically active biofilms in bioelec-trochemical systems is discussed and also the critical choices made in the experimental setup that affect the experimental results. The reactor configurations used in bioelectrochemical systems research are also described and the authors demonstrate how to use selected voltammetric techniques to study extracellular electron transfer in bioelectrochemical systems. Finally, some critical concerns with the proposed electron transfer mechanisms in bioelectrochemical systems are addressed together with the prospects of bioelectrochemical systems as energy-converting and energy-harvesting devices.
Keywords: electrochemically active biofilms, electroactive biofilms, anode-respiring biofilms, bioelectrochemical systems, electron transfer, anode, cathode, microbial fuel cell, microbial electrolysis cell
Introduction
Microbial respiration is based on electron transfer from electron donors to electron acceptors, a series of reactions facilitated by a cascade of energetic substances; these are well-known reactions described in the literature (Stams et al. 2006; Gralnick and Newman 2007; Bird et al. 2011; Kraft et al. 2011). The donors and acceptors of electrons are typically dissolved substances; however, some microorganisms can use solid electron donors and/or solid electron acceptors, such as minerals and metals, in respiration (Nealson and Finkel 2011). The exact mechanisms of electron transfer between microorganisms and solid substances remain a matter of intensive debate in the literature (Reguera et al. 2005; Coursolle et al. 2010; El-Naggar et al. 2010; Malvankar et al. 2011; Snider et al. 2011). Two conflicting points of view are usually presented in this debate: (1) the recently proposed point of view, that electrons are transferred by conduction through extracellular materials or elongated appendages called nanowires (Reguera et al. 2005; El-Naggar et al. 2010; Malvankar et al. 2011), and (2) the more traditional point of view, that the electrons are transferred using redox mediators, also known as electron shuttles (Marsili et al. 2008a; Coursolle et al. 2010; Peng et al. 2010; Velasquez-Orta et al. 2010). Biofilms with microorganisms capable of electron transfer to and from solid electron acceptors have been used in microbial fuel cells (MFCs) to harvest energy from various environmental processes (Sharma and Kundu 2010). The biofilms grown on the electrodes of MFCs are called electrochemically active biofilms (EABs), which is a misnomer as all microorganisms are electroactive in the respiration process. EABs are also known under several other names in the literature dedicated to MFCs, such as electricigens, electrochemically active microbes, exoelectrogenic bacteria, and anode-respiring or anodophilic species (Marsili et al. 2010). However, because the hallmark of EABs is the ability to exchange electrons with solid surfaces such as electrodes, the authors believe that the term ‘electrochemically active biofilms’ refers to the most basic property of these biofilms and it is used in this review.
The use of EABs in MFCs is not new. For various reasons, these devices have attracted some attention in the literature recently. In particular, researchers have recognized their potential use as alternative sources of energy. The attention MFCs receive is fully justified although some expectations of their ability to deliver large amounts of energy combined simultaneously with high power seem overly optimistic. It has been demonstrated that MFCs can be used successfully as a source of energy to continuously power electronic devices that consume low levels of power or to intermittently power electronic devices requiring higher power (Shantaram et al. 2005; Donovan et al. 2008, 2011). Much of the interest in using MFCs stems from the idea of harvesting energy from wastewater treatment processes, which at present are wasteful processes where energy-rich streams are reclaimed without obtaining useful energy (Du et al. 2007). There has been an estimate presented at conferences referring to the amount of energy that could be harvested from all wastewater treated in the US if the entire chemical oxygen demand were converted to the equivalent number of electrons and used to power external devices. In the authors’ opinion, this calculation resembles the computations estimating how much gold could be extracted from seawater, and how rich an individual could get by doing so, if all the oceans in the world were to be treated. There is some truth in these calculations, of course, but they neglect the costs and the technical problems associated with harvesting energy from wastewater or extracting gold from seawater. MFCs are fascinating devices, and they no doubt will find practical applications. In the short term, however, it is difficult to see how they can meaningfully contribute to solving the impending energy crisis. On the other hand, using MFCs for special applications, to power battery-reliant systems that consume reasonably small amounts of energy, has been gaining interest, as can be seen in the exponential increase in publications on their applications (Hamelers et al. 2010). Just how practical these applications will become remains to be seen.
Collectively, MFCs and the newer biologically catalyzed electrochemical cells have come to be known as bioelectrochemical systems (BESs) (Rabaey et al. 2007; Arechederra and Minteer 2008; Ivanov et al. 2010; Manohar et al. 2010; Rosenbaum and Schroder 2010). As BES research becomes more sophisticated, it appears that BESs can provide new insights into the fundamental mechanisms of electron transfer between microorganisms and solid substances. This application can deliver interesting results sooner than the expectations of harvesting large amounts of energy from wastewater processes can be fulfilled. There is a lot of excitement about using BESs and understandably all expectations may not be fulfilled. For that reason a somewhat provocative phrase is used in the title of this review, referring to ‘facts and fiction.’ The authors’ immediate goal is to review the state of the art of BES research and review what is known and what needs to be known to characterize these devices as legitimate power sources or as bioelectrochemical reactors that behave in a predictable manner. However, they would also like to attract attention to something they find promising in a much shorter time frame: to use BESs as tools of discovery in studying the process of electron transfer in EABs. For example, high-throughput, efficient BESs could be used to select for new EABs (Torres et al. 2009; Call and Logan 2011). Many researchers could use this technology as a tool for understanding the biochemistry of these unique microorganisms. This aspect of using BESs may be much less glamorous than the promise of delivering power to the national grid, but at the same time the expectations set are more realistic than converting all or even a large part of the chemical oxygen demand in wastewater into electron equivalents and using it to power external devices. As BES researchers, the authors are somewhat concerned that focusing on these glamorous but, in their opinion, currently unrealistic expectations, may do damage to a legitimate and very interesting field of research. They hope that this paper can help identify and evaluate the strengths and the limitations of BESs for both generating energy and studying the mechanisms of electron transfer between microorganisms in biofilms and solid substances.
Electrochemically active biofilm preparation and reactor configurations
Although there are many techniques for quantifying EAB extracellular electron transfer mechanisms, the quality and interpretation of the results are highly dependent on the way the study is conducted. Factors which are often selected arbitrarily, such as (1) the biofilm electrode material, (2) how the EABs were grown on the biofilm electrodes, (3) the reactor configuration used to grow the EABs, and (4) the reactor configurations used to study the extracellular electron transfer processes have a critical impact on the resulting EAB and its ability to participate in extracellular electron transfer processes. Identifying the effects of each factor on EAB performance may serve as a basis for optimizing the systems toward maximizing the rate of energy conversion.
Electrode materials
The biofilm electrode material affects the measured current and open circuit potential (OCP) of electrodes with EABs grown on them, and the choice of electrode material is important for the standardization of reported values. Traditionally, cheaper graphite, carbon paper, carbon granule, carbon brush, or carbon felt electrodes are used in MFC practical applications (Wei et al. 2011; Zhou et al. 2011b). These carbon materials suffer from high background currents that can mask the electrochemical response of redox species at low concentrations. In the authors’ laboratory, glassy carbon electrodes are often used to observe electrochemical activity. One advantage of glassy carbon is that the background currents are practically zero in the potential ranges in which EABs are studied; another is that it is nonporous. Additionally, there is significant literature on electrochemistry utilizing glassy carbon electrodes, potentially opening up a vast amount of literature to EAB studies. The use of glassy carbon electrodes would provide more universal current values when studying fundamental electron transfer of EABs. For these reasons, the authors would recommend researchers to use, or at least test, their systems with glassy carbon electrodes. There are various glassy carbon types, and readers are referred to Kissinger and Heineman (1996) for a more thorough review. When glassy carbon electrodes are not compatible with an experiment, common substitutes include gold and indium tin oxide (ITO) electrodes (Richter et al. 2008). Gold offers the advantage of significant literature on self-assembled monolayers and the modification of surface functional groups (Gooding and Yang 2008; Eckermann et al. 2010; Mandler and Kraus-Ophir 2011). Thin gold films on a glass substratum have also been used in advanced spectroscopic techniques for direct electron transfer studies (Busalmen et al. 2010). ITO is used in spectroelectrochemical experiments where an optically transparent electrode is required (Jain et al. 2011; Liu et al. 2011). Users should be aware of the resistivity of ITO electrodes and their durability, since the conductive film is thin compared to glassy carbon (Liu et al. 2011). Platinum and other catalytic electrode materials could have unanticipated effects on an experiment and are best avoided. For the supporting electrode in a BES, which completes the electrochemical cell and functions as the electron source/sink for the electrons derived from the EAB, a cheaper carbon electrode with a larger surface area can be used.
Reactors and electrode configurations used to study electrochemically active biofilms
The positions of the biofilm electrode, supporting electrode, and reference electrode (RE) in a BES have direct effects on the measured current and should be accounted for beyond spatial geometric considerations (Kissinger and Heineman 1996; Logan et al. 2006; Manohar and Mansfeld 2009). For example, the simplest system to configure to study electron transfer in EABs could be an MFC (Figure 1A), although it is generally used to quantify power production in practical research (Logan et al. 2006; Clauwaert et al. 2008). The geometry or other experimental parameters of an MFC could be optimized to produce more power. However, an MFC cannot obtain information about the EABs on the individual electrodes since only cell potential can be measured. Without the individual electrode potentials, it is very difficult to determine the fundamental reason for an increase in power in an MFC. Thus, how EABs respond to variation in electrode potentials cannot be understood through electrochemical theory. The end result would be the inability to study electron transfer in EABs. The MFC reactor configuration can be enhanced by inserting a reference electrode to measure individual electrode potentials and characterize overpotentials and potential losses (Figure 1B) (Clauwaert et al. 2008; Rismani-Yazdi et al. 2008). The individual electrode potentials and different resistances to current flow could then be related to current from EABs. While a significant amount of information can be obtained with this reactor configuration, the biofilm electrode potential cannot be controlled. Ambiguity arises in the MFC reactor configuration when an experimental parameter is changed and both the potential and the current change.
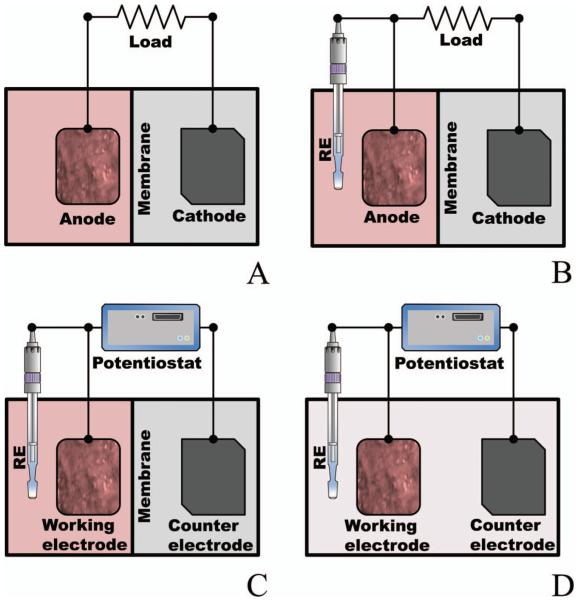
Figure 1.
EABs can be studied using four different configurations: (A) an MFC with an anode and a cathode; (B) an MFC with an anode, a cathode and a reference electrode (RE) used to monitor individual electrode potentials (against the RE); (C) an MFC with an anode and a cathode and an RE connected to a potentiostat; and (D) an electrochemical cell with a working electrode (WE) covered by biofilm and a counter electrode (CE) and RE immersed in the same solution. This is called a three-electrode bioreactor.
Therefore, a potentiostat is generally required to measure the current while fixing the biofilm electrode potential (Figure 1C or Figure 1D). This system is often called a three-electrode bioreactor and is used frequently to study biofilm voltammetry (Fricke et al. 2008; Marsili et al. 2008a; Bouhenni et al. 2010; Strycharz et al. 2011). When an experimental parameter such as the initial electron donor concentration is changed, the current can then be correlated without the effect of a varying electrode potential. Reactor configurations with (Figure 1C) and without (Figure 1D) an ion-selective membrane have distinct advantages. For membrane-less reactor configurations, the membrane potential loss is eliminated (Logan et al. 2006, 2008; Rozendal et al. 2008; Li et al. 2010). The disadvantage is that the supporting electrode reaction products are free to diffuse to the working electrode. This could potentially generate uncontrolled experimental parameters. In membrane-less MECs, the diffusion of hydrogen from the supporting electrode to the working electrode can be utilized by EABs to produce a current higher than that expected with the supplied electron donor (Lee et al. 2009a; Parameswaran et al. 2011). Regardless of the use of ion-selective membranes, potentiostatic systems provide more control over electron transfer than the MFC mode of operation (Figure 1A and B).
Current-limiting electrode
The current-limiting electrode is the electrode which cannot pass a higher current than the other electrode either because of its small size or because of limiting electrode reaction kinetics. If an electrode with an EAB limits the current of the BES, this practically means that the performance of the BES is limited by the EAB. Electron transfer in EABs can only be studied under this condition. The knowledge of which electrode limits the current is critical when BESs are studied. In the case of MFCs (Figure 1A), because both current and potential are variable when the resistance to the current flow is changed, it is important to confirm that the EAB under investigation is limiting the current. The simplest way to determine which electrode in an MFC setup is the current-limiting electrode is to monitor the individual electrode potentials using a RE (Figure 1B). When the resistor load is changed, the current-limiting electrode will undergo a significant change in electrode potential whereas the non-current-limiting electrode will not (Dewan et al. 2009). The current-limiting electrode concept also applies to potentiostatic systems (Figure 1C and D). The potentiostatic mode controls the biofilm electrode potential such that perturbations of the biofilm electrode potential cause a measurable change in the EAB under investigation. Thus, the current is controlled by the EAB. If, at any time, reactions at the supporting electrode affect the EAB under investigation, the controlled electrode cannot be called the limiting electrode and steps must be taken to ensure that the effect of the supporting electrode can be assumed to be negligible. This is especially important in BESs that place the biofilm electrode of interest and the supporting electrode in the same solution (Figure 1D). For fundamental electron transfer investigations, this concept cannot be ignored.
The preferred polarization potential for growing electrochemically active biofilms
The effect of the polarization potential (anode potential) has been studied for various BESs and EABs (Aelterman et al. 2008; Cheng et al. 2008; Lee et al. 2009b; Torres et al. 2009; Srikanth et al. 2010). There is no consensus on the exact magnitude of potential to apply; however, there is a clear understanding that applying a polarization potential more positive than the OCP of the biofilm electrode is sufficient to drive electrons from the EAB to the biofilm electrode. The concept of an optimal polarization potential is misleading, since the polarization potential can be limited by external factors such as the energy efficiency of the BES (Lee and Rittmann 2010). The preferred polarization potential when studying electron transfer from an EAB must be explored experimentally and be chosen from a range of polarization potentials from near OCP to a few hundred millivolts more positive than OCP. For example, if maximum current is desired, then a polarization potential that is in the current-limiting region for EABs (current independent from polarization potential) should be used (Lee and Rittmann 2010). The polarization potential could also be used to select for different types of EABs, with different abilities for extracellular electron transfer (Torres et al. 2009). However, for use in MFCs and other BESs, the polarization potential should be comparable to what is observed in the actual selection process.
Electrode acclimatization and growing electrochemically active biofilms
Electrode acclimatization refers to the processes that require time for EABs to populate an electrode surface and is often used for multi-species EABs (Kim et al. 2005; Finkelstein et al. 2006; Liu et al. 2008; Rodrigo et al. 2009; Wang et al. 2009). The purpose of acclimatization is to increase electrode performance by enhancing biofilm attachment and/or allowing the biofilm electrode to reach a steady-state OCP prior to use in a BES (Larrosa-Guerrero et al. 2010; Cheng et al. 2011; Kassongo and Togo 2011; Renslow et al. 2011a). The method of acclimatization affects the type of EAB grown on the biofilm electrode and can be focused on control of the current or of the biofilm electrode potential. Four acclimatization methods are common in the literature, viz. (1) Closed circuit: the biofilm electrode and the supporting electrode are short-circuited or connected across a resistor; (2) open circuit: the biofilm electrode is left disconnected; (3) controlled cell potential: a constant potential is applied between the biofilm electrode and the supporting electrode; and (4) controlled electrode potential: a constant polarization potential is placed between the biofilm electrode and the reference electrode.
Closed circuit and open circuit acclimatization are the simplest methods to configure. Closed circuit acclimatization allows the biofilm electrode to reach a steady-state cell potential, focusing on enhanced steady-state electron transfer. The choice of resistor controls the amount of current allowed to pass (Aelterman et al. 2008; Jadhav and Ghangrekar 2009; Zhang et al. 2011). Open circuit acclimatization allows the biofilm electrode potential to develop a steady-state OCP utilizing natural redox processes in the environment. Controlled cell potential and controlled electrode potential acclimatization requires powered external equipment to control cell potential and electrode potential irrespective of the natural redox processes in the environment. Controlled cell potential acclimatization controls steady-state electron transfer at a researcher-specified level. Controlled electrode potential acclimatization controls steady-state electron transfer irrespective of the supporting electrode. Both methods allow the user to expose the system to potentials not normally sustainable or possible. However, only controlled electrode potential acclimatization gives the researcher direct and consistent control of the biofilm electrode potential. To choose one method over the other, critical decisions must be made. First, does the researcher prefer to select for biofilm processes or natural redox processes that can take advantage of an applied potential or polarization potential? Does the researcher prefer to produce electrodes that reflect only the natural redox processes? When a current is passed through an electrode to or from its surroundings this will affect the state of redox processes around it. The choice of how electrodes are acclimatized affects the end result and should be reported clearly in published studies.
Growing EABs refers normally to pure cultures in the laboratory and can be achieved using two distinct acclimation methods. It should be noted that acclimatization refers to field experiments whereas acclimation refers to laboratory experiments. The first method is to grow EABs on an electrode in the presence of a soluble electron acceptor. This method is in line with open circuit acclimatization since no polarization potential is required for EAB growth. Once the EAB has reached a desired state (thickness, surface coverage, metabolic activity, OCP), the soluble electron acceptor can be removed and the EAB can be switched to respire on the biofilm electrode where a current is measured. The second method is to grow the EAB on an electrode which acts as the sole electron acceptor. This method is in line with closed circuit, controlled cell potential, and controlled electrode potential acclimatization where current produced reflects biofilm growth. Once the EAB generates a desired state (thickness, surface coverage, metabolic activity, current), the EAB can be used for further investigations. Both methods are able to produce laboratory-scale EABs; however, the EABs resulting from these two methods have different biofilm properties and electron transfer capabilities. Most likely, this is due to the acclimation of the EAB to each electron acceptor. Recently, Nevin et al. (2009) observed different 3D biofilm structures and electron transfer capabilities in Geobacter sulfurreducens depending on whether the EAB was grown on fumarate as a soluble electron acceptor or on an electrode as a solid state electron acceptor (see also Inoue et al. 2011). The authors have also observed structural differences between EABs grown using the two methods in their laboratory.
Electrochemical techniques for studying extracellular electron transfer of electrochemically active biofilms
When the correct reactor configuration has been chosen and the EAB has been successfully grown or acclimatized on an electrode, the next step is to study the electron transfer properties of the biofilm electrode using electrochemical techniques. There are many electrochemical techniques available; however, this review covers only those frequently used to study electron transfer processes in EAB literature. Note that there are many books on classical electrochemical techniques and describing these topics is not the purpose of this review (Oldham and Myland 1994; Kissinger and Heineman 1996; Bard and Faulkner 2001).
Long-term electrode polarization of electrochemically active biofilms
In a long-term electrode polarization experiment, a selected polarization potential is applied to an electrode with an EAB grown on it and the current is measured. In this way the total charge transferred in a batch system or the steady-state current produced by the EABs in a continuous system can be measured. A long-term electrode polarization experiment identifies sustainable current generation that can be systematically related to controlled parameters such as polarization potential. While the technique appears similar to controlled electrode potential acclimatization, in which EABs are grown on polarized electrodes, the intents of the two are distinct and should be distinguished. For example, Yi et al. (2009) allowed G. sulfurreducens DL1 to acclimatize on an electrode for 5 months and selected for a new strain, G. sulfurreducens KN400. They then compared the sustainable current productions of the two strains using long-term electrode polarization experiments. The key value of performing electrode polarization experiments is to observe the electrochemical response of an EAB to a change in a controlled experimental parameter or the effects of acclimatization methods.
When a significant current (above background and noise levels) is generated by an EAB such as that shown in Figure 2, the EAB is thought to exchange electrons with the biofilm electrode. To confirm that the current generated is related to the metabolism of the EAB or its metabolic by-products, certain long-term electrode polarization experiments can be performed. In batch experiments, the total consumption of the electron donor can be correlated to the total charge transferred. If the current trends towards zero when the electron donor concentration goes to zero, then the oxidation of the electron donor is the source of electrons and the coulombic efficiency can be calculated (Logan et al. 2006). For example, Bond and Lovley (2003) showed that current production by G. sulfurreducens biofilms is directly related to the consumption of acetate in batch mode. In continuous experiments, the electron donor feed concentration can be altered and subsequent steady-state current values measured. Replacing the bulk solution with fresh growth medium during electrode polarization experiments has been done to probe soluble extracellular electron transfer mechanisms. For example, Marsili et al. (2008a) replaced the spent solution in a Shewanella oneidensis MR-1 biofilm reactor to show that soluble redox mediators were responsible for the steady-state current generated. Bond and Lovley (2003) replaced the bulk solution of G. sulfurreducens biofilms with acetate-free growth medium to show acetate dependence. Other sophisticated experiments can be done to isolate controlled parameters in EAB experiments that affect extracellular electron transfer mechanisms. EABs can be genetically engineered to enhance/inhibit current generation. For example, Richter et al. (2008) observed that no current could be produced by a ΔpilA G. sulfurreducens mutant on gold electrodes. The pH can be adjusted to correlate proton transfer with current generation. Both Torres et al. (2008) and Patil et al. (2011) adjusted bulk pH and measured the current generation of their mixed species EABs.
Figure 2.
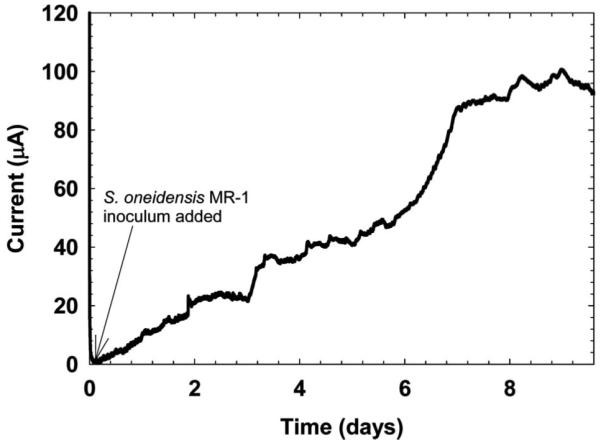
Current generation by S. oneidensis MR-1 biofilm on a graphite electrode under anaerobic conditions in the reactor configuration shown in Figure 1C. The current increased steadily over a period of 9 days. The polarization potential was 0 mVAg/AgCl.
Cyclic voltammetry
When a steady-state current or a current higher than the background is identified as being the result of EABs, cyclic voltammetry (CV) can be used to identify the biofilm electrode potential at which active redox couples related to the EAB are oxidized or reduced. CV is an electrochemical technique that applies a linear polarization potential scan from an initial polarization potential to a final polarization potential and measures the current. Because redox couples can only be reduced or oxidized at certain electrode potentials, CV can determine the biofilm electrode potential range in which extracellular electron transfer can occur in EABs (Fricke et al. 2008; Marsili et al. 2010). Under well-controlled conditions, CV can be used to determine whether EABs have the capability for electron transfer, whether freely diffusing species or surface-adsorbed species contribute to electron transfer, and whether EABs engage in catalytic activity towards specific substrates (Fricke et al. 2008; Richter et al. 2009; Katuri et al. 2010; Carmona-Martinez et al. 2011; Strycharz et al. 2011). CV studies, however, do not reflect the ability of EABs to produce long-term, sustainable current, which should be reserved for long-term electrode polarization experiments. Often CV is coupled to long-term electrode polarization experiments in which CV can explain how the active redox couples are affected by systematic changes in controlled parameters such as bulk pH (Patil et al. 2011).
There is an implicit assumption, however, that EABs growing on electrode surfaces can be described as a ‘well-controlled’ condition in which CV can be applied to study reaction mechanisms as in pure electrochemical systems. Beyond the reproducibility of the biofilm electrode surface, simply characterizing biofilm structure itself has historically been difficult (Yang et al. 2000, 2001; Renslow et al. 2011b). Furthermore, the result of biofilm heterogeneity is the local variation of not only diffusion coefficients, but local flow velocities as well (Beyenal et al. 1998; Beyenal and Lewandowski 2001, 2002; Renslow et al. 2010). The unknown mass transfer conditions suggest that not all cells in the EAB contribute equally to current production. The issue of mass transfer conditions being reproducible has historically been overcome through the use of rotating electrodes (Kissinger and Heineman 1996). However, no studies to date have used these types of mass-transfer-controlled systems for EABs. While the state of mass transfer in EABs cannot be ignored, the few studies that have attempted to resolve the reaction mechanisms in EABs are highly informative (Strycharz et al. 2011). However, there is still much room for improving the CV model of EABs.
Figure 3A shows an example CV of S. oneidensis MR-1 biofilm grown on glassy carbon taken without disturbing the biofilm (in situ). Figure 4B shows a CV of a G. sulfurreducens biofilm grown on glassy carbon as well. The peaks in EAB studies are typically broad with many individual peak responses overlapping; assigning peak responses to specific redox couples is difficult without more in-depth analysis (Rabaey et al. 2004; Fricke et al. 2008; Marsili et al. 2010; Strycharz et al. 2011). Often, the EAB can be placed in fresh growth medium or buffer solution, or removed altogether, and the resulting CV can be compared to that taken before the treatment (Marsili et al. 2008a; Babauta et al. 2011). Figure 3 shows example treatments in which the EAB was physically removed from the surface. A second CV was taken without the biofilm. The resulting CV shows that the behavior of the CV results is tied intimately to the EAB, although a significant fraction of electrochemically active species can be associated with the electrode surface. Often, mutants with specific gene deletions of the EAB being studied are used to strengthen CV analysis (Carmona-Martinez et al. 2011; Strycharz et al. 2011).
Figure 3.
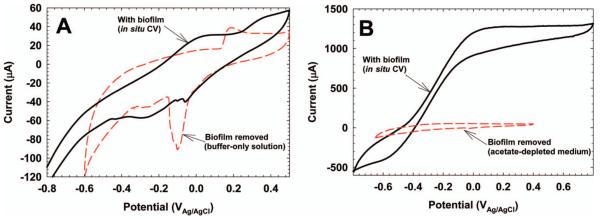
(A) In situ CV of S. oneidensis MR-1 (black trace). The biofilm was physically removed from the biofilm electrode and a second CV was performed (red dashed trace). A glassy carbon electrode (10 mm × 10 mm) was used. The scan rate was 10 mV s71. (B) In situ CV of G. sulfurreducens (black trace). The biofilm was physically removed from the biofilm electrode and a second CV was performed (red dashed trace). A glassy carbon electrode (25 mm × 25 mm) was used. The scan rate was 10 mV s71 for the first CV and 1 mV s71 for the second CV.
Figure 4.
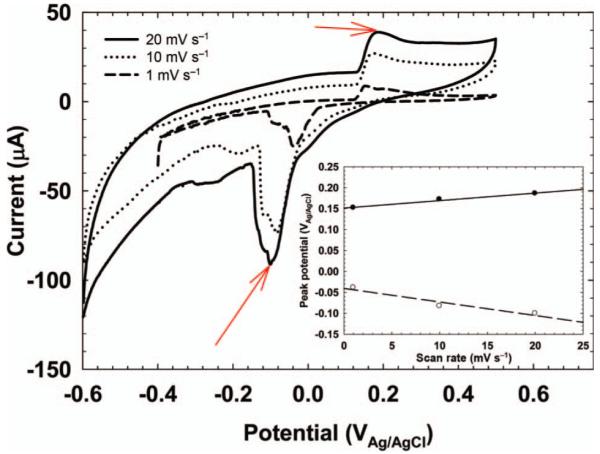
Example scan rate dependence of the peak potentials of the CV in Figure 3A. Red arrows show the peak potentials used for scan rate analysis. The inset shows that the peak potential is a linear function of scan rate.
Advanced techniques in CV can provide further information about the electrochemical responses of EABs under various conditions. These include the scan rate dependence of peak currents, altering the reversal potential of the CV scan, and altering the potential window of the CV scan (Bard and Faulkner 2001; Snider et al. 2011; Strycharz-Glaven et al. 2011). Figure 4 shows the scan rate dependence of the peak potential response of the result shown in Figure 3. The inset shows that the peaks highlighted by the red arrows have a linear scan rate dependence, indicating adsorbed species. Had a square root dependence been observed, this would have indicated a diffusing species (Bard and Faulkner 2001). Thus, scan rate dependence has been used frequently in the literature to show indirectly whether a diffusion process or an adsorption process controls electron transfer inside EABs (Fricke et al. 2008; Marsili et al. 2008b, 2010; Katuri et al. 2010; Strycharz et al. 2011).
One underutilized aspect of CV in EAB research is varying the initial potential and the potential window to study anodic and cathodic peak coupling. Figure 5A shows that scanning in the cathodic direction initially can mask the cathodic peak response. Only after the scan in the anodic direction is completed is the cathodic peak response observed. By simply manipulating the initial potential of the CV, anodic peaks can be coupled to cathodic peaks. Figure 5B shows further that different voltammograms can be obtained depending on the potential window used for the CV, highlighting the fact that the choice of CV parameters is critical and not arbitrary.
Figure 5.
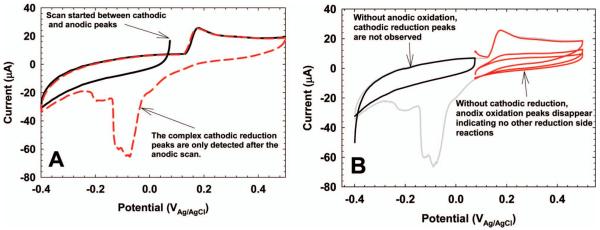
(A) Choosing the initial potential of the CV can yield information on anodic and cathodic peak coupling. (B) Choosing different specific potential windows can show different CV behaviors that may be interpreted in different ways.
Square wave voltammetry
Square wave voltammetry (SWV) is used less frequently than CV in EAB studies; however, it can detect low concentrations of electrochemically active species not easily detectable by CV. Additionally, SWV provides quick diagnostic information that can help identify whether an electrochemically active reaction is reversible or not. For EAB-relevant electrochemically active compounds such as riboflavin, a typical SWV will have the shape and characteristics shown in Figure 6. The red dashed trace is the difference in current between the forward and reverse currents, Idif. By using Idif, the background that increased exponentially in the forward and reverse currents is suppressed. The inset in Figure 6 focuses on the riboflavin peak response. The peak potential on Idif represents the midpoint potential of riboflavin, −277.7 mVAg/AgCl at pH 4.5. Visually, the midpoint potential on a SWV is the potential at the maximum current difference between the forward and reverse currents. The ratio of the magnitudes of the peak currents in the forward and reverse directions can be used to determine the electrochemical reversibility of riboflavin oxidation and reduction. This is because when the potential pulse straddles the standard reduction potential, the forward pulse produces an anodic current and the reverse pulse produces a cathodic current. The ratio of the peak forward current to the peak reverse current is 0.91, reflecting the quasi-reversible nature of the reaction (Nguyen et al. 2012b). The combination of the forward, reverse, and difference currents in SWV makes it a useful tool in analyzing electrochemically active species.
Figure 6.
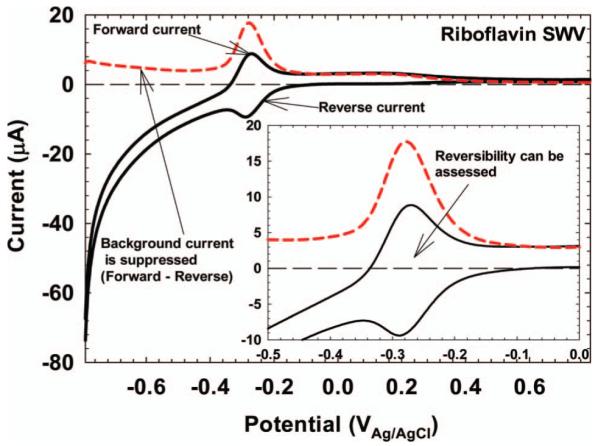
SWV of riboflavin in pH 4.5, 50 mM citric buffer at a glassy carbon electrode. The inset focuses on the electrochemical response. The midpoint potential was −277.7 mVAg/AgCl, very close to the theoretical value of −267.5 mVAg/AgCl at pH 4.5.
SWV analysis in EABs is not as straightforward as is shown in Figure 6. Figure 7 shows the SWV response of the S. oneidensis MR-1 biofilm described in Figure 3. The CV in Figure 7 is the same as that shown in Figure 3 and is only used for comparison. Because the CV is a complex response of multiple electrochemically active species, the SWV response is also complex. Unlike the SWV of riboflavin, with one distinct peak, the SWV of S. oneidensis MR-1 has multiple overlapping peaks that are difficult to separate. The current peak at −450 mVAg/AgCl is generally accepted as being flavin compounds excreted by S. oneidensis MR-1 (Marsili et al. 2008a). The forward and reverse currents for the SWV of S. oneidensis MR-1 are shown in Figure 8A. Above a potential of −400 mVAg/AgCl, both the forward and reverse currents are anodic. The same trend, but much larger, is shown for G. sulfurreducens in Figure 8B. This is unlike the forward and reverse currents shown for riboflavin in Figure 6, which straddle the zero current line in a quasi-reversible manner. The large anodic currents commonly seen in G. sulfurreducens and S. oneidensis MR-1 biofilms at positive polarization potentials are catalytic waves (Fricke et al. 2008; Marsili et al. 2010). The capability of SWV to detect catalytic waves of EABs while resolving the midpoint potentials of electrochemically active species under turnover conditions is invaluable to EAB electron transfer analysis.
Figure 7.
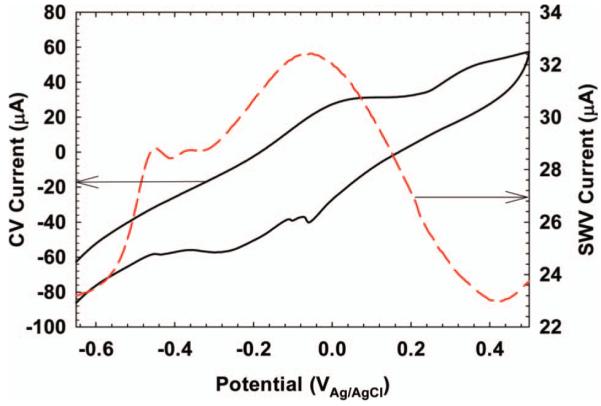
SWV of the S. oneidensis MR-1 biofilm described in Figure 4. The CV is the same as that in Figure 4 and is shown for comparison.
Figure 8.
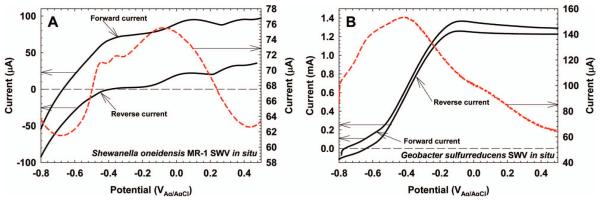
(A) Forward and reverse currents for S. oneidensis MR-1 in situ SWV. The difference current is the same as that in Figure 7. (B) Forward and reverse currents for G. sulfurreducens in situ SWV. Note the difference in the y-axis scales.
Electrochemical impedance spectroscopy
A more thorough review of electrochemical impedance spectroscopy (EIS) and its application in EABs has been written elsewhere and readers are referred there for detailed explanations (Manohar et al. 2008, 2010). EIS applies a sinusoidal potential waveform to measure the real impedance (resistance) and imaginary impedance (capacitance) of an electrochemical system. The choice of wave amplitude and polarization potential is critical since the potential waveform should oscillate between relevant potentials such as the midpoint potential of the EAB catalytic wave observed by CV and the OCP of the biofilm electrode after acclimatization. The effect is similar to assessing reversibility using the forward and reverse currents measured in SWV (Figure 8). Both anodic and cathodic currents must flow to measure charge transfer across the biofilm electrode. EIS has been used as a nondestructive electrochemical technique to study the development of charge transfer resistance in EABs (Manohar et al. 2008; Marsili et al. 2008b; Srikanth et al. 2008; Borole et al. 2010). Figure 9 shows bode and phase angle plots of G. sulfurreducens grown on glassy carbon (Marsili et al. 2008b). Initially, the electrode exhibited a capacitative behavior (phase angle ~90°) typical of a polarizable electrode. After growth for 72 h, the phase angle decreased to half the original maximum, highlighting the increase in a resistive element (phase angle ~0°) of the electrode surface. Two charge transfer events (time constants) were visible and show the complexity of electron transfer in G. sulfurreducens biofilms.
Figure 9.
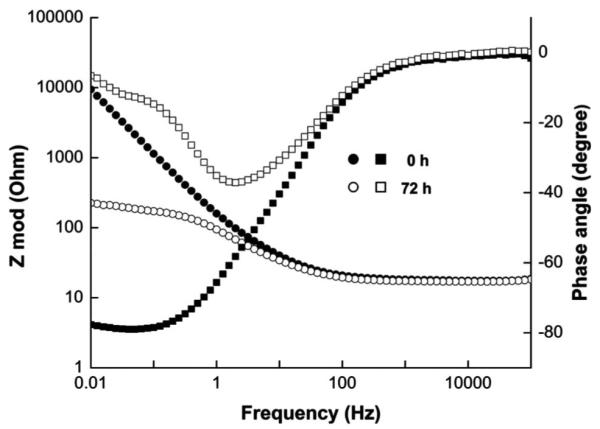
Bode (● and ○) and phase angle (■ and □) plots from EIS showing the complexity of electron transfer in G. sulfurreducens grown on glassy carbon electrodes (Figure 7 in reference). From Marsili et al. 2008b. Reproduced with permission from the American Society for Microbiology.
Limitations of electrochemical techniques
Electrochemical investigations in complex systems such as EABs require more physical and chemical evidence to determine if an observed electrochemical response was caused by a change in an experimental parameter. Additionally, the use of molecular techniques such as generation of mutants with different gene/protein expression levels provide a physiological link to electrochemical investigations. Electrochemical techniques such as CV and SWV accurately describe the nature of the electron transfer event in the presence of EABs (reversibility, mass transfer limitations, properties of redox couples, and reaction steps); however, they do not give any evidence on how EABs participate in the electron transfer or what aspect of EABs promotes electron transfer. Thus, the presence of redox couples in EABs does not necessarily imply that they participate in electron transfer. More importantly, the presence or absence of electrochemical activity (current peaks observed in CV or SWV) does not necessarily imply that it is or is not the source of long-term, sustainable current in EABs. An example of this is the ability of certain microorganisms to utilize soluble exogenous electron shuttles in their surroundings for extracellular electron transfer. For example, Milliken and May (2007) showed that Desulfitobacterium hafniense strain DCB2 could utilize exogenous quinone-like mediators to produce sustainable current in MFCs. In the authors laboratory, it has also been found that an iron-reducing, biofilm-forming Paenibacillus sp. could use exogenous flavins and anthroquinone-2,6-disulfonate to reduce ferrihydrate (Ahmed 2011). The electrochemical activity of these types of EABs would not be observable with CV in pure cultures in the laboratory, which highlights the importance of syntrophic interactions in mixed species EABs (Kiely et al. 2011). The interpretation of electrochemical investigations with CV has been a topic of intense debate in EAB research (Malvankar et al. 2011; Strycharz-Glaven et al. 2011).
Coupled techniques
The coupling of electrode polarization, CV, and SWV with methods that directly measure physical or chemical parameters varying inside EABs in situ addresses issues with the interpretation of electrochemical data. The goal of this coupling is to correlate the properties that vary within the EAB under various electrode-respiring conditions. Specifically, future EAB research and advanced techniques should focus on the variation that occurs within the biofilm and not just in the bulk solution. The correlation of variation in biofilms and bulk solution will advance the field significantly. This correlation can be done by: (1) directly measuring the kinetics of redox mediators inside biofilms; (2) resolving local concentrations of chemical species inside the biofilm; (3) resolving the physical location of electrochemically active species in situ; and (4) correlating limiting current with local biofilm parameters. There are several available tools and techniques that can be used, such as microelectrodes, spectroelectrochemical methods, and microscopy.
Microelectrodes
Microelectrodes have been used to study the stratification of various biofilm properties in biofilms and water-sediment interfaces since the early 1990s (Cronenberg and Vandenheuvel 1991; Glud et al. 1992; Vanhoudt et al. 1992; Zhang and Bishop 1994). For example, microelectrodes have been used to measure concentrations of oxygen, hydrogen, hydrogen sulfide, and carbon dioxide, as well as pH, redox potential, and local flow velocities (Lee and Debeer 1995; Yang and Lewandowski 1995; Xia et al. 1998; Yu and Bishop 1998; Beyenal et al. 2004). Voltammetric microelectrodes that use techniques such as cyclic voltammetry and pulse voltammetry have also been used to detect chemicals such as metal cations and flavins (Xu et al. 1998; Nguyen et al. 2012b). Because microelectrodes are minimally invasive and have dimensions that can be as small as 1-5 mm, they are well suited to studying changes in both the EAB and the bulk solution above the EAB during electrode respiration. A distinct advantage of microelectrodes is that they can correlate local biofilm properties with the bulk biofilm properties, electron transfer rates and electron transfer mechanisms. On the other hand, the data should be critically interpreted when a highly heterogeneous EAB is investigated because the results can vary between locations (Nguyen et al. 2012a).
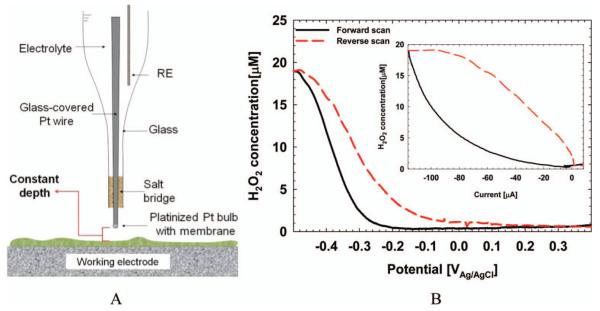
Microelectrodes can be used in conjunction with voltammetric techniques in two modes. The first, in which depth profiles are taken during constant polarization, is shown in Figure 10. A redox microelectrode was stepped down towards the bottom of a S. oneidensis biofilm. At each step, redox potential was measured, producing a redox potential depth profile inside the biofilm. In the presence of soluble redox mediators, redox potential is expected to increase towards the biofilm electrode. However, the redox potential profiles in Figure 10 show a decreasing redox potential profile, indicating that the S. oneidensis biofilm was not redox-controlled (Babauta et al. 2011). The second mode is shown in Figure 11. A hydrogen peroxide (H2O2) microelectrode (Figure 11A) was placed ~100 μm above a glassy carbon electrode in river water. The glassy carbon electrode simulated a cathode in a sediment microbial fuel cell in which oxygen reduction was the cathodic reaction. One by-product of oxygen reduction is H2O2, and a H2O2 microelectrode was used to detect H2O2 generation during a CV scan. In this case, the microelectrode was held at a constant distance from the biofilm electrode and the H2O2 concentration was monitored as a function of potential and current. Figure 11B shows that H2O2 was produced at potentials below −200 mVAg/AgCl. The inset in Figure 11B shows how the H2O2 concentration correlated with the measured current. The forward and reverse currents are well separated, demonstrating the effect of diffusion processes. It should be noted that no H2O2 could be detected accumulating in the bulk solution. It is obvious that microelectrode techniques are well suited to studying the chemistry near the biofilm electrode surface, which cannot be determined using other techniques.
Figure 10.
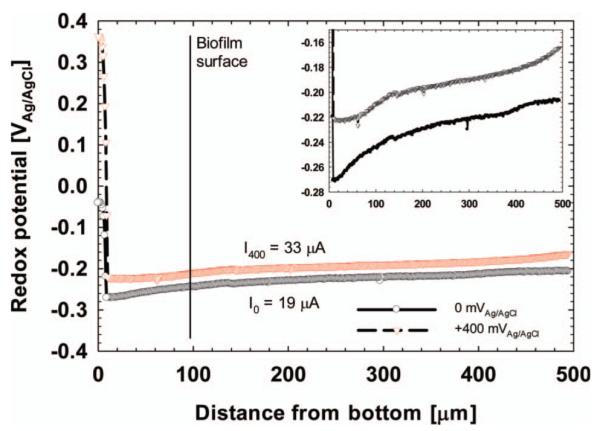
The redox potential inside a S. oneidensis MR-1 biofilm grown on a graphite electrode. Reprinted (adapted) with permission from Babauta et al. (2011). Copyright (2011) American Chemical Society.
Figure 11.
(A) Diagram of a H2O2 microelectrode. (B) H2O2 concentration measured ~100 μm above a glassy carbon electrode during a CV scan. The inset shows current vs H2O2 concentration.
Spectroelectrochemistry
Spectroelectrochemistry combines electrochemistry with spectroscopy; it correlates the change in the spectral signature, usually in the UV/vis/IR range, of electrochemically active compounds above an electrode set at a chosen polarization potential (Bard and Faulkner 2001). Spectroelectrochemical techniques have been used to identify the redox state of metallo-proteins such as heme-containing cytochromes in purified protein preparations (Bowden et al. 1982). The large literature covering redox titrations of cytochromes using absorbance measurements has made it possible to monitor the redox state of these proteins in EABs. For example, Nakamura et al. (2009) used evanescent wave UV/vis spectroscopy to compare the in vivo oxidation/reduction of c-type cytochromes (attached to whole cells) of Shewanella loihica PV-4 with the oxidation/reduction of purified c-type cytochromes. Busalmen et al. (2010) applied attenuated total reflection surface-enhanced infrared absorption spectroscopy (ATR-SEIS) to measure the redox state of c-type cytochromes on G. sulfurreducens cells attached to gold electrodes and were able to determine that c-type cytochromes were directly oxidized/reduced by the biofilm electrode. These spectroelectrochemical experiments provided detailed knowledge of the bacterial outer membrane/electrode interaction. A mini-review covering these techniques has been written for biological systems (Ataka et al. 2010).
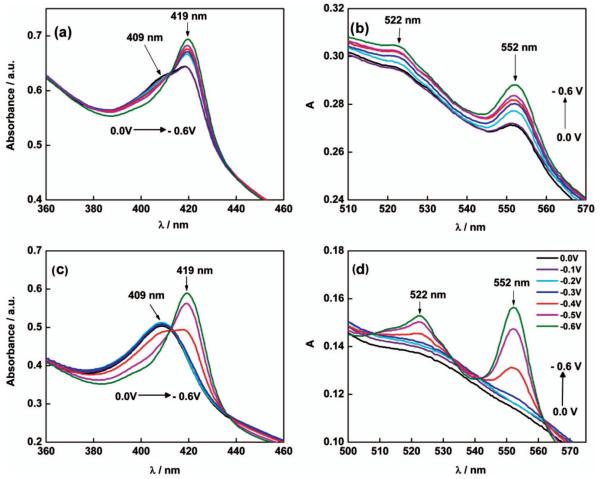
Only recently, however, has spectroelectrochemistry been used for in situ measurements of the redox state of cytochromes in thick, pre-grown EABs in which extracellular electron transfer through the biofilm matrix was studied. Liu et al. (2011) showed that the c-type cytochromes inside thick G. sulfurreducens biofilms probed under non-turnover conditions were completely reduced at polarization potentials below −350 mVSHE and completely oxidized at potentials above +100 mVSHE, demonstrating long-range extracellular electron transfer through the cytochrome network. At the same time, Jain et al. (2011) showed that the c-type cytochromes inside thick G. sulfurreducens biofilms probed under turnover conditions were only partially reduced (Figure 12A and B). Figure 12C and D support Liu et al.’s (2011) conclusion that in non-turnover conditions, cytochromes are nearly all in the oxidized state. These experiments tied the metabolic activity of G. sulfurreducens biofilms and cytochrome redox state to the biofilm electrode potential. The successful implementation of coupled techniques like spectroelectrochemistry will provide a more fundamental understanding of how electron transfer occurs in EABs in situ.
Figure 12.
Absorbance spectra of G. sulfurreducens biofilms exposed to different electrode potentials under turnover (a, b) and non-turnover (c,d) conditions. Reprinted from Jain et al. (2011), with permission from Elsevier.
Microscopy
The in situ imaging of biofilms using fluorescent microscopes is a standard technique in biofilm research (Lewandowski and Beyenal 2007). The commercial availability of optically transparent electrodes has allowed the use of in situ confocal scanning laser microscopy (CLSM) for EABs. The development of current with the biofilm has been observed for thick biofilms as well as for monolayers of cells (McLean et al. 2010). This coupling provides the ability to monitor biofilm parameters such as surface coverage, biovolume, and biofilm roughness with current. For example, Nevin et al. (2009) used CLSM to monitor the difference in biofilm structure between G. sulfurreducens biofilms grown on fumarate or an electrode. McLean et al. (2008) used CLSM to locate individual strains of S. oneidensis MR-1 in a mixed culture biofilm. Additionally, microscopic techniques allow the determination of single-cell electron transfer rates (McLean et al. 2010). It should be noted that single cell electron transfer rates are critical when electron transfer mechanisms are studied using various mutants. Mutants often exhibit a different growth rate than their wild type counterparts. Typically, mutants grow slower than wildtype. Therefore, when current generation is compared between a mutant and wild type, current should be quantified on a per cell basis. This would minimize the effect of growth rates on experimental observations. When chemical indicator probes are added to the influent growth medium, CLSM can correlate the chemical properties of EAB as well. Franks et al. (2009) successfully used CLSM in conjunction with a pH indicator probe, C-SNARF-4, to measure pH changes inside a G. sulfurreducens biofilm in a flow cell MFC. They were able to measure pH profiles that developed within the biofilm during electrode respiration, providing evidence that pH changes appreciably inside EABs. The use of microscopy would critically allow investigators to correlate biofilm structure with its function related to electron transfer.
Future techniques coupled to study electrochemically active biofilms
Any number of combinations of electrochemical techniques with analytical experiments can be designed, and these composite techniques will likely guide future EAB research because of the complexity of electron transfer inside EABs. Two potential examples include quartz crystal microbalance (QCM) and nuclear magnetic resonance (NMR). In QCM a quartz crystal is oscillated at selected frequencies and mass changes on a surface attached to the quartz crystal are measured (Bard and Faulkner 2001). The technique can easily detect the deposition of monolayers of material and can reach an atomic resolution of mass changes. When QCM is associated with an electrode and the biofilm electrode potential is controlled with a potentiostat, it becomes electrochemical quartz crystal microbalance (e-QCM), which has the capability of correlating mass change with current and could determine whether any adsorption reaction steps precede or follow electron transfer steps (Varela et al. 2000; Xie et al. 2010). However, e-QCM is typically used with solid surfaces; its use for thick biofilms could be limited because of their viscoelastic nature. It may find its use in probing thin biofilms. For example, Xie et al. (2010) used e-QCM to monitor the effect of electrode potential on Escherichia coli biofilm development. NMR uses high-strength magnetic fields and electromagnetic pulses to detect spin-active nuclei inside molecules. Some of the NMR biofilm reactors that are currently used for monitoring metabolites and diffusion coefficients could be modified to incorporate a three-electrode system (Majors et al. 2005, 2008; McLean et al. 2008; Renslow et al. 2010). Because of the electromagnetic shielding effects of conductive materials, the three electrodes would need to be oriented appropriately in order not to block the radio frequency pulse. Simultaneously performing electrochemical experiments and monitoring in situ metabolic reactions could play an important role in understanding the fundamental processes occurring in EABs and would allow for both mass and electron balances on the system.
Modeling electrochemically active biofilms
Along with the coupled techniques that could make critical contributions to the understanding of EAB extracellular electron transfer, mathematical modeling is expected to provide a theoretical basis for studying extracellular electron transfer. Originally, EAB modeling work had its roots in models developed specifically to describe the operation of MFCs and BESs: the goal was to link microbial processes to the power output of MFCs and optimize these processes based upon model predictions (Zhang and Halme 1995). Newer models now focus on the complex extracellular electron transfer mechanisms in EABs. The main goals of the models are to predict current and relate it to electron and proton transfer.
Predicting current generation from electrochemically active biofilms
Predicting the current generation from growing EABs through both mediated and conductive electron transfer mechanisms was a way to confirm the experimental results seen in the literature. Two models were developed that accounted for the growth kinetics of biofilms and mass transport inside biofilms during current generation (Marcus et al. 2007; Picioreanu et al. 2007). Each model was able to fit experimental data from Geobacter sulfurreducens biofilms successfully; together they highlighted the fact that both mediated electron transfer, modeled using Butler-Volmer kinetics (Equation 1), and conductive electron transfer, modeled using the Nernst-Monod equation (Equation 2), were possible. Furthermore, the conclusions on the limitations of current generation due to mass transport that were seen in the literature provided a theoretical, systematic basis for optimizing electrodes to be used in BESs (Rismani-Yazdi et al. 2008). However, further enhancement of the models to include pH effects and more sophisticated metabolic reactions would be necessary later.
The common form of the Butler-Volmer equation is given by Equation 1:
| (1) |
where n is the number of electrons transferred; I is the current density (A m−2); k0 is the standard heterogeneous rate constant (m s−1); Xred is the concentration of the reduced form of the redox couple at the biofilm electrode surface (mM); Xox is the concentration of the oxidized form of the redox couple at the biofilm electrode surface (mM); α is the charge transfer coefficient (unitless); f is the grouped term of the Faraday constant, F (s A mol−1), divided by the temperature, T (K), and the universal gas constant, R (J/K mol), thus nF/RT (1/V); E is the biofilm electrode potential (V); and is the standard reduction potential of the redox couple (V). The Butler-Volmer equation is useful for modeling mediated electron transfer mechanisms because it couples the concentration of the electron mediator in both the oxidized and the reduced form at the biofilm electrode surface and the biofilm electrode potential to the production of current. This equation is often coupled to the diffusion of electron mediators through biofilm to provide a method of calculating both current and depth profiles of mediator concentrations. Some of the parameters in this equation are critical for BESs. For example, the standard heterogeneous rate constant has been shown to control the dependence of current in sediment MFCs on temperature (Renslow et al. 2011a). This parameter is a function of both the biofilm electrode material properties and the redox couples present in the system. The charge transfer coefficient is also reliant on the redox couple properties. It is often assumed to be 0.5, which signifies a symmetric energy barrier for the forward and reverse redox reactions; however, some redox couples associated with EABs have transfer coefficients that deviate from this value (Verhagen and Hagen 1992). Finally, the standard reduction potential is critically important for EAB modeling because it can dictate the amount of energy that is available for a microorganism and also which terminal electron acceptors are available for that microorganism to utilize.
The Nernst-Monod equation is used to model conductive electron transfer:
| (2) |
where Imax is the limiting current density (A m−2); EKA is the potential at which the current is half the limiting current (V); S is the concentration of the electron-donating substrate (mM); and KS is the Monod half saturation constant for that substrate (mM). The Nernst-Monod equation is a special form of a multiplicative Monod equation, where the electron acceptor is assumed to be a solid electron acceptor, accessible via instantaneous conduction, as opposed to a soluble electron-accepting molecule. In this equation, EKA is the critical parameter because it defines where the inflection point on an ideal slow-scan CV will occur.
Predicting the role of proton transfer in current generation by electrochemically active biofilms
Understanding of the role of proton transfer in EABs respiring on electrodes has advanced significantly over the past few years because of published models. For example, Torres et al. (2008) and Franks et al. (2009) observed that current generation by EABs was affected by the total buffer strength and the pH of the bulk solution. Picioreanu et al. (2010) and Marcus et al. (2011) demonstrated the same buffer and pH trend in EABs using updated models to reffect proton transfer. However, the models demonstrated that the critical factor for proton transfer limitations in EABs was the mass transfer coefficient, which was arbitrarily set to provide two extreme cases. In other words, without sufficient experimental evidence on the mechanisms involved, the models could not exactly describe the experimental data. This was not a limitation of the models. As more experimental evidence is continually published on the nature of proton transfer in EABs, the models will provide an ability to visualize the possible outcomes of proton transfer in EABs (Patil et al. 2011). It is expected that the models will be continually updated to answer key questions raised about EABs. One of those key questions could be how proton transfer correlates with electron transfer mechanisms.
Predicting the mechanism of extracellular electron transfer in electrochemically active biofilms
In addition to the complete mathematical modeling of the entire anode, attention has been focused on fitting the experimental current-voltage dependence in EABs. Hamelers et al. (2011) developed the Butler-Volmer-Monod model (Equation 3) to simulate bio-anode polarization curves and cellular kinetics as a function of potential and substrate concentration. The Butler-Volmer-Monod model is given by:
| (3) |
where K1 and K2 are lumped parameters (unitless); ES/P is the thermodynamic electrode potential; and KM is the substrate affinity constant (mM). It is important to note that the substrate affinity constant is not identical to the Monod half saturation constant in the Nernst-Monod equation. The model demonstrated that the apparent Monod constant was a function of anode potential and that, therefore, it is only at sufficiently high overpotentials (large E – ES/P values) that Km is equivalent to KS. Both K1 and K2 are complex terms that appear to only be functions of electrode potential and the microorganism used. K1 can be understood as the ratio of the rate of biochemical substrate utilization to the rate of electrochemical exchange current density, whereas K2 can be understood as the ratio of the rate of product formation to the rate of substrate formation inside the microorganism.
Strycharz et al. (2011) developed a qualitative model to explain cyclic voltammetry curves obtained from G. sulfurreducens DL1 and variant strain KN400. This model showed that regardless of the exact electron transfer mechanism, mediated or conductive, electron transfer from cells to the biofilm electrode appears to be the primary bottleneck to current generation. As a refinement of conduction-based extracellular electron transfer models, superexchange has been modeled by Strycharz-Glaven et al. (2011) and theoretically studied by Polizzi et al. (2012). This is in direct contrast to the metal-like conduction evidence put out by Malvankar et al. (2011) for the same biofilm model, and some uncertainty exists between these models. Regardless of the outcome, the models put forth on extracellular electron transfer have brought to light some interesting limitations on how experimental evidence can be interpreted.
EAB models will need to create comprehensive pictures of extracellular electron transfer, cellular kinetics, community interactions, gene expression, and hydrodynamics to provide critical information. In particular, models that incorporate metabolic pathways and calculate metabolic fluxes will expand understanding of the link between extracellular electron transfer and EAB physiology (Mahadevan et al. 2011; Zomorrodi and Maranas 2012). Models that attempt to address a specific question in current understanding of EABs are necessary to bring insightful discussion to this field. Furthermore, experimental results should continue to be explained within the context of current mathematical models. It is expected that with the growing number of new advanced techniques being utilized, models will continue to be put forward. However, the current limitation is that most of them lack the proper experimental data to be tested. Moreover, some of them use unrealistic parameters that are impossible to observe physically. Future models should be restricted to physically meaningful domains and be tested against experimental data.
Facts and fiction
The direction in which this field of research will head is difficult to predict, as new applications of BESs are continually being published (Call et al. 2009; Cheng et al. 2009; Mehanna et al. 2010; Villano et al. 2011; Cusick et al. 2012). Interestingly, a majority of the researchers are still interested in demonstrating that it is possible to produce energy using different substrates and different microorganisms, without realizing that this was demonstrated nearly one hundred years ago! Although many publications still focus on MFC research, the current status of knowledge critically demonstrates that BESs are far from solving the energy demands of the future. However, BESs can be used as tools for discovering novel capabilities of bacteria in various environments and may eventually lead to promising applications. The authors believe there are four main issues that need to be addressed in this process, as follows.
Scaling up of the current density generated by electrochemically active biofilms
One of the limitations of MFCs, and of BESs generally, is that the current density does not scale up linearly with the active surface area of the biofilm electrode to meet the demand of high-capacity processes. The scaling up of MFCs has been partially addressed by methods such as reducing anodic and cathodic overpotentials, increasing solution conductivity, decreasing mass transfer resistances, decreasing electrode spacing, using novel air cathodes, and stacking (Ieropoulos et al. 2008; Dekker et al. 2009; Jiang et al. 2010; Logan 2010; Zhuang et al. 2010). While these improvements in MFC design and scalability have increased the maximum power density, they have not addressed the fundamental scalability of the current generation of anodes or cathodes inside these systems (Dewan et al. 2008). For example, Cheng and Logan (2011) showed that the scaling up of the maximum power generation in wastewater MFCs was more closely related to the cathode surface area (62% increase in power by doubling the cathode surface area) than to the anode surface area (12% increase in power by doubling the anode area). The disparity in the percentage increases in power highlights the incomplete fundamental knowledge of scaling up MFCs. There is room for improvement, which may involve using models specifically designed to optimize MFCs (Marcus et al. 2007; Picioreanu et al. 2007). Why MFC power production can only be improved at values <100% (increase in power per doubling of electrode area) is a question the authors believe to be at the heart of the scaling up of MFCs and BESs. In particular, the scalability of anodes in MFCs and BESs should be directly related to the extracellular electron transfer mechanisms that EABs utilize. Future scaling up studies need to focus on this aspect. The same outlook also applies to biocathodes. There is a critical need to address the individual contribution of each electrode to overall energy conversion. Otherwise, the energy conversion of these devices will continue to be suboptimal.
Translation of electrochemically active biofilm extracellular electron transfer research to bioelectrochemical systems
Figure 13 shows how potential losses restrict the amount of power available to an MFC. These losses are a large factor controlling the overall potential of an MFC when a current is drawn from it. Generally the OCP of an anode is around −500 mVSCE and the OCP of a cathode is +400 mVSCE (Dewan et al. 2010; Renslow et al. 2011a). Therefore, the typical maximum potential which would be expected is around 900 mV, although it may be possible to reach higher values. As soon as a load is applied to an MFC, and current starts to flow, potential losses at both the anode and the cathode affect the biofilm electrode potentials. The anode potential increases while the cathode potential decreases, reducing the cell potential. Depending on the relative sizes of the biofilm electrodes, anode potentials fall between −100 mVSCE and +100 mVSCE while cathode potentials typically stabilize between +200 mVSCE and 0 mVSCE (Dewan et al. 2010). Frequently the practical values of anodes in MFCs do not align with the polarization potentials used to cultivate EABs in the laboratory, yet many published research articles describe the usefulness of the data for advancing MFCs. For example, G. sulfurreducens biofilms are typically grown at 0 mVAg/AgCl or higher and have electrode potentials that cannot be observed in practical MFC applications. These EAB studies have significant relevance to studying electron transfer mechanisms, and this point is not debated. They provide a fundamental level of knowledge about EAB electrophysiology. What is missing, however, is the translation of that fundamental knowledge into practical use. Many EAB studies do not address the compatibility of their systems with what is observed in real devices, such as when an attempt is made to power an electronic device. Is what is done in the lab positively affecting what can be done in the field? A critical factor could be that EAB studies focusing on MFC/BES applications should test their electrodes in real MFCs/BESs.
Figure 13.
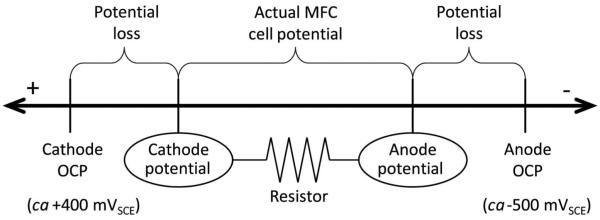
Potential losses at both the anode and the cathode restrict the amount of power that remains for the MFC when a resistor is connected. Activation, ohmic, and concentration losses reduce the anode and cathode potentials, lowering the cell potential from the maximum at OCP. The distances on the line are not drawn to scale.
Elucidating extracellular electron transfer mechanisms in electrochemically active biofilms
To date, the questions of whether electrons are transferred through conductive nanowires, bound redox mediators, or diffusing redox mediators and of how electrons enter these pathways have yet to be unequivocally answered. Also of importance is the role of extracellular polymeric substances (EPS) in electron transfer. For example, Cao et al. (2011) found redoxactive proteins in the EPS of Shewanella sp. HRCR-1, which were distinct from the redox-active proteins commonly found on the cell surface. EPS is known to facilitate oxidation/reduction reactions to minerals (Sand and Gehrke 2006; Gralnick and Newman 2007). How does EPS interact with the cells in EABs? There is a significant amount of knowledge missing on these electron transfer mechanisms that have been proposed to account directly for the path of electrons from inside cells to the biofilm electrode. While many publications in the literature propose different ideas and concepts, what is needed now is direct demonstration of key findings on the electron transfer processes. For example, having conductive nanowires in a biofilm does not necessarily mean that they are involved in electron transfer processes. The functions of key components of EABs in electron transfer could critically improve understanding of these systems and allow improvements in the devices in which EABs are used. In addition, new methods of measuring the same parameters reported in the past could add validity and create a functional base from which we can develop theory.
Correlating bulk measurement with measurements inside biofilms
EAB processes are an interfacial phenomenon: EABs interact with the electrode inside the biofilm diffusive and reactive layers at the electrode surface. These microscale layers are directly related to extracellular electron transfer, whereas diffusion processes above these layers are linked indirectly. Thus, it is expected that the surface concentrations of the redox-active compounds and local solution properties inside EABs are more relevant and critical than the corresponding values in the bulk. Correlating and fitting lines to bulk data may have little significance to the fundamental processes occurring inside EABs. Direct measurements inside EABs are preferred, such as measuring pH inside EABs or measuring the spectroelectrochemical properties of EABs (Franks et al. 2009; Babauta et al. 2011; Liu et al. 2011). This is especially important since the cell density inside some EABs is not uniformly distributed and predictions based on simple diffusion may not apply (Renslow et al. 2010).
How far has science really come in microbial fuel cell research related to electrochemically active biofilms?
Rismani-Yazdi et al. (2008) published a table on the MFC-related review papers from 2001 to 2007 that listed fifteen reviews. After running generic keyword searches using ‘review’ and another limiter, ‘microbial fuel cell,’ ‘extracellular electron transfer,’ ‘bioelectrochemical system,’ ‘microbial electrolysis cell,’ or ‘biofuel cell,’ the authors found that the number of reviews on MFCs, BESs, and enzymatic fuel cells increased significantly from 2008 to 2011. (See Table S1 in Supplementary material.) [Supplementary material is available via a multimedia link on the online article webpage.] Most of these reviews inferred that BES devices have promising applications for wastewater treatment, biosensors, creating value-added products, or producing sustainable energy, yet only one of the reviews puts forward relevant figures showing progress in producing useful power in MFCs (Rinaldi et al. 2008). It was noted previously that Logan and Regan (2006) put forward a similar figure. However, the present authors feel that the figure showing the exponential increase of power density in reported MFCs over the years from 1997 to 2009 needs to be reevaluated. There is a very clear trend across multiple experiments and MFC configurations that power densities reported as mW m−2 are related to electrode size (Dewan et al. 2008). The authors believe that even when MFCs and BESs are optimized experimentally via design considerations, a fundamental understanding of how to optimize EAB extracellular electron transfer to linearly scale with electrode size will still be lacking. MFC stacking will not address this limitation: instead it shows how inefficient MFCs still are as energy-converting devices. Although the authors are critical of what has been done to date with MFCs, they believe that MFCs and BESs have the potential to be used in high-throughput processes in the future. However, this will not occur until fundamental understanding of the mechanisms of extracellular electron transfer in mixed species EABs has progressed substantially (Kiely et al. 2011). Rather than focusing on new ways to apply MFCs and BESs, the focus should be on how to use these devices to elucidate mechanisms of extracellular electron transfer. It is to be hoped that the electrochemical techniques outlined here will be of some use in heading towards this goal.
Future directions in electrochemically active biofilm research
EAB research is new and there have been exciting discoveries related to electron transfer mechanisms. Many new techniques have been developed which can be used to understand electron transfer processes in EABs. This review has focused on these techniques and demonstrated the data generated in the authors’ laboratories. While knowledge of EAB electron transfer mechanisms is much more advanced than it was 5 years ago, the knowledge has yet to infiltrate the BES literature as a whole. The authors hope that the BES literature will benefit from their approach to describing how EAB extracellular electron transfer is studied. In the future they will be looking for BES studies that optimize both the design of the BES and the electron transfer expected from the EABs. The authors would also like to see BES studies address some of the questions concerning EAB extracellular electron transfer, that is, practical BES studies testing hypotheses developed in nonpractical EAB extracellular electron transfer studies.
Supplementary Material
Acknowledgements
This research was supported by the US Office of Naval Research (ONR), grant #N00014-09-1 0090, and National Science Foundation (NSF), grant #0954186. The authors gratefully acknowledge the financial support provided by the National Institutes of Health (NIH) Protein Biotechnology Training program, grant #T32-GM008336, for helping to fund Ryan Renslow and Jerome Babauta.
References
- Aelterman P, Freguia S, Keller J, Verstraete W, Rabaey K. The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol. 2008;78:409–418. doi: 10.1007/s00253-007-1327-8. [DOI] [PubMed] [Google Scholar]
- Ahmed B. Uranium immobilization in subsurface sediments [Doctoral thesis] Washington State University; Pullman (WA): 2011. p. 250. [Google Scholar]
- Arechederra R, Minteer SD. Organelle-based biofuel cells: immobilized mitochondria on carbon paper electrodes. Electrochim Acta. 2008;53:6698–6703. [Google Scholar]
- Arechederra RL, Minteer SD. Self-powered sensors. Anal Bioanal Chem. 2011;400:1605–1611. doi: 10.1007/s00216-011-4782-0. [DOI] [PubMed] [Google Scholar]
- Ataka K, Kottke T, Heberle J. Thinner, smaller, faster: IR techniques to probe the functionality of biological and biomimetic systems. Angew Chem Int Edit. 2010;49:5416–5424. doi: 10.1002/anie.200907114. [DOI] [PubMed] [Google Scholar]
- Babauta JT, Nguyen HD, Beyenal H. Redox and pH microenvironments within Shewanella oneidensis MR-1 biofilms reveal an electron transfer mechanism. Environ Sci Technol. 2011;45:6654–6660. doi: 10.1021/es200865u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bard AJ, Faulkner LR, editors. Electrochemical methods: fundamentals and applications. John Wiley & Sons, Inc.; New York (NY): 2001. p. 856. [Google Scholar]
- Beyenal H, Lewandowski Z. Mass-transport dynamics, activity, and structure of sulfate-reducing biofilms. AIChE Journal. 2001;47:1689–1697. [Google Scholar]
- Beyenal H, Lewandowski Z. Internal and external mass transfer in biofilms grown at various flow velocities. Biotechnol Prog. 2002;18:55–61. doi: 10.1021/bp010129s. [DOI] [PubMed] [Google Scholar]
- Beyenal H, Tanyolac A, Lewandowski Z. Measurement of local effective diffusivity in heterogeneous biofilms. Water Sci Technol. 1998;38:171–178. [Google Scholar]
- Beyenal H, Davis CC, Lewandowski Z. An improved Severinghaus-type carbon dioxide microelectrode for use in biofilms. Sensors Actuators B-Chem. 2004;97:202–210. [Google Scholar]
- Bird LJ, Bonnefoy V, Newman DK. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol. 2011;19:330–340. doi: 10.1016/j.tim.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Bond DR, Lovley DR. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microbiol. 2003;69:1548–1555. doi: 10.1128/AEM.69.3.1548-1555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borole AP, Aaron D, Hamilton CY, Tsouris C. Understanding long-term changes in microbial fuel cell performance using electrochemical impedance spectroscopy. Environ Sci Technol. 2010;44:2740–2744. doi: 10.1021/es9032937. [DOI] [PubMed] [Google Scholar]
- Bouhenni RA, Vora GJ, Biffinger JC, Shirodkar S, Brockma K, Ray R, Wu P, Johnson BJ, Biddle EM, Marshall MJ, et al. The role of Shewanella oneidensis MR-1 outer surface structures in extracellular electron transfer. Electroanalysis. 2010;22:856–864. [Google Scholar]
- Bowden EF, Hawkridge FM, Chlebowski JF, Bancroft EE, Thorpe, Blount HN. Cyclic voltammetry and derivative cyclic voltabsorptometry of purified horse heart cytochrome-C at tin-doped indium oxide optically transparent electrodes. J Am Chem Soc. 1982;104:7641–7644. [Google Scholar]
- Busalmen JP, Esteve-Nunez A, Berna A, Feliu JM. ATR-SEIRAs characterization of surface redox processes in G. sulfurreducens. Bioelectrochemistry. 2010;78:25–29. doi: 10.1016/j.bioelechem.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Call DF, Logan BE. A method for high throughput bioelectrochemical research based on small scale microbial electrolysis cells. Biosens Bioelectron. 2011;26:4526–4531. doi: 10.1016/j.bios.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Call DF, Wagner RC, Logan BE. Hydrogen production by Geobacter species and a mixed consortium in a microbial electrolysis cell. Appl Environm Microbiol. 2009;75:7579–7587. doi: 10.1128/AEM.01760-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B, Shi LA, Brown RN, Xiong YJ, Fredrickson JK, Romine MF, Marshall MJ, Lipton MS, Beyenal H. Extracellular polymeric substances from Shewanella sp. HRCR-1 biofilms: characterization by infrared spectroscopy and proteomics. Environ Microbiol. 2011;13:1018–1031. doi: 10.1111/j.1462-2920.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- Carmona-Martinez AA, Harnisch F, Fitzgerald LA, Biffinger JC, Ringeisen BR, Schroder U. Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants. Bioelectrochemistry. 2011;81:74–80. doi: 10.1016/j.bioelechem.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Cheng SA, Logan BE. Increasing power generation for scaling up single-chamber air cathode microbial fuel cells. Bioresource Technol. 2011;102:4468–4473. doi: 10.1016/j.biortech.2010.12.104. [DOI] [PubMed] [Google Scholar]
- Cheng SA, Kiely P, Logan BE. Pre-acclimation of a wastewater inoculum to cellulose in an aqueous-cathode MEC improves power generation in air-cathode MFCs. Bioresource Technol. 2011;102:367–371. doi: 10.1016/j.biortech.2010.05.083. [DOI] [PubMed] [Google Scholar]
- Cheng SA, Xing DF, Call DF, Logan BE. Direct biological conversion of electrical current into methane by electromethanogenesis. Environ Sci Techno. 2009;43:3953–3958. doi: 10.1021/es803531g. [DOI] [PubMed] [Google Scholar]
- Clauwaert P, Aelterman P, Pham TH, De Schamphelaire L, Carballa M, Rabaey K, Verstraete W. Minimizing losses in bio-electrochemical systems: the road to applications. Appl Microbiol Biotechnol. 2008;79:901–913. doi: 10.1007/s00253-008-1522-2. [DOI] [PubMed] [Google Scholar]
- Coursolle D, Baron DB, Bond DR, Gralnick JA. The Mtr respiratory pathway is essential for reducing flavins and electrodes in Shewanella oneidensis. J Bacteriol. 2010;192:467–474. doi: 10.1128/JB.00925-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronenberg CCH, Vandenheuvel JC. Determination of glucose diffusion-coefficients in biofilms with microelectrodes. Biosens Bioelectron. 1991;6:255–262. doi: 10.1016/0956-5663(91)80011-l. [DOI] [PubMed] [Google Scholar]
- Cusick RD, Kim Y, Logan BE. Energy capture from thermolytic solutions in microbial reverse-electrodialysis cells. Science. 2012;335:1474–1477. doi: 10.1126/science.1219330. [DOI] [PubMed] [Google Scholar]
- Debabov VG. Electricity from microorganisms. Microbiology. 2008;77:123–131. [PubMed] [Google Scholar]
- Dekker A, Ter Heijne A, Saakes M, Hamelers HVM, Buisman CJN. Analysis and improvement of a scaled-up and stacked microbial fuel cell. Environ Sci Technol. 2009;43:9038–9042. doi: 10.1021/es901939r. [DOI] [PubMed] [Google Scholar]
- Dewan A, Beyenal H, Lewandowski Z. Scaling up microbial fuel cells. Environ Sci Technol. 2008;42:7643–7648. doi: 10.1021/es800775d. [DOI] [PubMed] [Google Scholar]
- Dewan A, Beyenal H, Lewandowski Z. Intermittent energy harvesting improves the performance of microbial fuel cells. Environ Sci Technol. 2009;43:4600–4605. doi: 10.1021/es8037092. [DOI] [PubMed] [Google Scholar]
- Dewan A, Donovan C, Heo D, Beyenal H. Evaluating the performance of microbial fuel cells powering electronic devices. J Power Sources. 2010;195:90–96. [Google Scholar]
- Donovan C, Dewan A, Heo D, Beyenal H. Batteryless, wireless sensor powered by a sediment microbial fuel cell. Environ Sci Technol. 2008;42:8591–8596. doi: 10.1021/es801763g. [DOI] [PubMed] [Google Scholar]
- Donovan C, Dewan A, Peng HA, Heo D, Beyenal H. Power management system for a 2.5W remote sensor powered by a sediment microbial fuel cell. J Power Sources. 2011;196:1171–1177. [Google Scholar]
- Du ZW, Li HR, Gu TY. A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv. 2007;25:464–482. doi: 10.1016/j.biotechadv.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Eckermann AL, Feld DJ, Shaw JA, Meade TJ. Electrochemistry of redox-active self-assembled monolayers. Coordin Chem Rev. 2010;254:1769–1802. doi: 10.1016/j.ccr.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Naggar MY, Wanger G, Leung KM, Yuzvinsky TD, Southam G, Yang J, Lau WM, Nealson KH, Gorby YA. Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1. P Natl Acad Sci USA. 2010;107:18127–18131. doi: 10.1073/pnas.1004880107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein DA, Tender LM, Zeikus JG. Effect of electrode potential on electrode-reducing microbiota. Environ Sci Technol. 2006;40:6990–6995. doi: 10.1021/es061146m. [DOI] [PubMed] [Google Scholar]
- Fleming JT. Electronic interfacing with living cells. In: Belkin S, Gu MB, editors. Whole cell sensing systems I: Reporter cells and devices. Vol. 117. Springer-Verlag; Berlin, Heidelberg (Germany): 2010. pp. 155–178. Advances in Biochemical Engineering Biotechnology. [DOI] [PubMed] [Google Scholar]
- Franks AE, Nevin KP. Microbial fuel cells, a current review. Energies. 2010;3:899–919. [Google Scholar]
- Franks AE, Nevin KP, Jia HF, Izallalen M, Woodard TL, Lovley DR. Novel strategy for three-dimensional real-time imaging of microbial fuel cell communities: monitoring the inhibitory effects of proton accumulation within the anode biofilm. Energy Environ Sci. 2009;2:113–119. [Google Scholar]
- Fricke K, Harnisch F, Schroder U. On the use of cyclic voltammetry for the study of anodic electron transfer in microbial fuel cells. Energy Environ Sci. 2008;1:144–147. [Google Scholar]
- Glud RN, Ramsing NB, Revsbech NP. Photosynthesis and photosynthesis-coupled respiration in natural biofilms quantified with oxygen microsensors. J Phycol. 1992;28:51–60. [Google Scholar]
- Gooding J, Yang WR. The self-assembled monolayer modification of electrodes - some recent advances in biological application. Actual Chimique. 2008;320-21:85–89. [Google Scholar]
- Gralnick JA, Newman DK. Extracellular respiration. Molec Microbiol. 2007;65:1–11. doi: 10.1111/j.1365-2958.2007.05778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guven G, Prodanovic R, Schwaneberg U. Protein engineering - an option for enzymatic biofuel cell design. Electroanalysis. 2010;22:765–775. [Google Scholar]
- Hallenbeck PC, Ghosh D, Skonieczny MT, Yargeau V. Microbiological and engineering aspects of biohydrogen production. Indian J Microbiol. 2009;49:48–59. doi: 10.1007/s12088-009-0010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelers HV, Ter Heijne A, Sleutels TH, Jeremiasse AW, Strik DP, Buisman CJ. New applications and performance of bioelectrochemical systems. Appl Microbiol Biotechnol. 2010;85:1673–1685. doi: 10.1007/s00253-009-2357-1. [DOI] [PubMed] [Google Scholar]
- Hamelers HVM, Ter Heijne A, Stein N, Rozendal RA, Buisman CJN. Butler-Volmer-Monod model for describing bio-anode polarization curves. Bioresource Technol. 2011;102:381–387. doi: 10.1016/j.biortech.2010.06.156. [DOI] [PubMed] [Google Scholar]
- Harnisch F, Schroder U. From MFC to MXC: chemical and biological cathodes and their potential for microbial bioelectrochemical systems. Chem Soc Rev. 2010;39:4433–4448. doi: 10.1039/c003068f. [DOI] [PubMed] [Google Scholar]
- Haruyama T. Design and fabrication of a molecular interface on an electrode with functional protein molecules for bioelectronic properties. Electrochemistry. 2010;78:888–895. [Google Scholar]
- Huang LP, Cheng SA, Chen GH. Bioelectrochemical systems for efficient recalcitrant wastes treatment. J Chem Technol Biotechnol. 2011a;86:481–491. [Google Scholar]
- Huang LP, Regan JM, Quan X. Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresource Technol. 2011b;102:316–323. doi: 10.1016/j.biortech.2010.06.096. [DOI] [PubMed] [Google Scholar]
- Ieropoulos I, Greenman J, Melhuish C. Microbial fuel cells based on carbon veil electrodes: stack configuration and scalability. Int J Energy Res. 2008;32:1228–1240. [Google Scholar]
- Inoue K, Leang C, Franks AE, Woodard TL, Nevin KP, Lovley DR. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ Microbiol Rep. 2011;3:211–217. doi: 10.1111/j.1758-2229.2010.00210.x. [DOI] [PubMed] [Google Scholar]
- Ito J, Petzold CJ, Mukhopadhyay A, Heazlewood JL. The role of proteomics in the development of cellulosic biofuels. Curr Proteom. 2010;7:121–134. [Google Scholar]
- Ivanov I, Vidakovic-Koch T, Sundmacher K. Recent advances in enzymatic fuel cells: experiments and modeling. Energies. 2010;3:803–846. [Google Scholar]
- Jadhav GS, Ghangrekar MM. Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresource Technol. 2009;100:717–723. doi: 10.1016/j.biortech.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Jain A, Gazzola G, Panzera A, Zanoni M, Marsii E. Visible spectroelectrochemical characterization of Geobacter sulfurreducens biofilms on optically transparent indium tin oxide electrode. Electrochim Acta. 2011;56:10776–10785. [Google Scholar]
- Jiang DQ, Li XA, Raymond D, Mooradain J, Li BK. Power recovery with multi-anode/cathode microbial fuel cells suitable for future large-scale applications. Int J Hydrogen Energ. 2010;35:8683–8689. [Google Scholar]
- Kannan AM, Renugopalakrishnan V, Filipek S, Li P, Audette GF, Munukutla L. Bio-batteries and bio-fuel cells: leveraging on electronic charge transfer proteins. J Nanosci Nanotechnol. 2009;9:1665–1678. doi: 10.1166/jnn.2009.si03. [DOI] [PubMed] [Google Scholar]
- Kassongo J, Togo CA. Performance improvement of whey-driven microbial fuel cells by acclimation of indigenous anodophilic microbes. Afr J Biotechnol. 2011;10:7846–7852. [Google Scholar]
- Katuri KP, Kavanagh P, Rengaraj S, Leech D. Geobacter sulfurreducens biofilms developed under different growth conditions on glassy carbon electrodes: insights using cyclic voltammetry. Chem Commun. 2010;46:4758–4760. doi: 10.1039/c003342a. [DOI] [PubMed] [Google Scholar]
- Kiely PD, Regan JM, Logan BE. The electric picnic: synergistic requirements for exoelectrogenic microbial communities. Curr Opin Biotechnol. 2011;22:378–385. doi: 10.1016/j.copbio.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Kim JR, Min B, Logan BE. Evaluation of procedures to acclimate a microbial fuel cell for electricity production. Appl Microbiol Biotechnol. 2005;68:23–30. doi: 10.1007/s00253-004-1845-6. [DOI] [PubMed] [Google Scholar]
- Kissinger PT, Heineman WR, editors. Laboratory techniques in electroanalytical chemistry. Marcel Dekker, Inc; New York (NY): 1996. p. 1008. [Google Scholar]
- Kraft B, Strous M, Tegetmeyer HE. Microbial nitrate respiration - genes, enzymes and environmental distribution. J Biotechnol. 2011;155:104–117. doi: 10.1016/j.jbiotec.2010.12.025. [DOI] [PubMed] [Google Scholar]
- Landoulsi J, Cooksey KE, Dupres V. Review - Interactions between diatoms and stainless steel: focus on biofouling and biocorrosion. Biofouling. 2011;27:1105–1124. doi: 10.1080/08927014.2011.629043. [DOI] [PubMed] [Google Scholar]
- Larrosa-Guerrero A, Scott K, Katuri KP, Godinez C, Head IM, Curtis T. Open circuit versus closed circuit enrichment of anodic biofilms in MFC: effect on performance and anodic communities. Appl Microbiol Biotechnol. 2010;87:1699–1713. doi: 10.1007/s00253-010-2624-1. [DOI] [PubMed] [Google Scholar]
- Lee HS, Rittmann BE. Characterization of energy losses in an upflow single-chamber microbial electrolysis cell. Int J Hydrogen Energ. 2010;35:920–927. [Google Scholar]
- Lee HS, Torres CI, Rittmann BE. Effects of substrate diffusion and anode potential on kinetic parameters for anode-respiring bacteria. Environ Sci Technol. 2009b;43:7571–7577. doi: 10.1021/es9015519. [DOI] [PubMed] [Google Scholar]
- Lee HS, Vermaas WFJ, Rittmann BE. Biological hydrogen production: prospects and challenges. Trends Biotechnol. 2010;28:262–271. doi: 10.1016/j.tibtech.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Lee HS, Torres CI, Parameswaran P, Rittmann BE. Fate of H(2) in an upflow single-chamber microbial Electrolysis cell using a metal-catalyst-free cathode. Environ Sci Technol. 2009a;43:7971–7976. doi: 10.1021/es900204j. [DOI] [PubMed] [Google Scholar]
- Lee JW, Kjeang E. A perspective on microfluidic biofuel cells. Biomicrofluidics. 2010;4(4):041301. doi: 10.1063/1.3515523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WC, Debeer D. Oxygen and pH microprofiles above corroding mild-steel covered with a biofilm. Biofouling: 1995:273–280. [Google Scholar]
- Lefebvre O, Uzabiaga A, Chang IS, Kim BH, Ng HY. Microbial fuel cells for energy self-sufficient domestic wastewater treatment-a review and discussion from energetic consideration. Appl Microbiol Biotechnol. 2011;89:259–270. doi: 10.1007/s00253-010-2881-z. [DOI] [PubMed] [Google Scholar]
- Lewandowski Z, Beyenal H. Fundamentals of biofilm research. CRC Press; Boca Raton (FL): 2007. p. 480. [Google Scholar]
- Li FX, Sharma Y, Lei Y, Li BK, Zhou QX. Microbial fuel cells: the effects of configurations, electrolyte solutions, and electrode materials on power generation. Appl Biochem Biotechnol. 2010;160:168–181. doi: 10.1007/s12010-008-8516-5. [DOI] [PubMed] [Google Scholar]
- Li WW, Sheng GP, Liu XW, Yu HQ. Recent advances in the separators for microbial fuel cells. Bioresource Technol. 2011;102:244–252. doi: 10.1016/j.biortech.2010.03.090. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kim H, Franklin RR, Bond DR. Linking spectral and electrochemical analysis to monitor c-type cytochrome redox status in living Geobacter sulfurreducens biofilms. Chemphyschem. 2011;12:2235–2241. doi: 10.1002/cphc.201100246. [DOI] [PubMed] [Google Scholar]
- Liu Y, Harnisch F, Fricke K, Sietmann R, Schroder U. Improvement of the anodic bioelectrocatalytic activity of mixed culture biofilms by a simple consecutive electrochemical selection procedure. Biosens Bioelectron. 2008;24:1006–1011. doi: 10.1016/j.bios.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kim E, Ghodssi R, Rubloff GW, Culver JN, Bentley WE, Payne GF. Biofabrication to build the biology-device interface. Biofabrication. 2010;2(2):022002. doi: 10.1088/1758-5082/2/2/022002. [DOI] [PubMed] [Google Scholar]
- Logan BE. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl Microbiol Biotechnol. 2010;85:1665–1671. doi: 10.1007/s00253-009-2378-9. [DOI] [PubMed] [Google Scholar]
- Logan BE, Regan JM. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol. 2006;14:512–518. doi: 10.1016/j.tim.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Logan BE, Call D, Cheng S, Hamelers HVM, Sleutels THJA, Jeremiasse AW, Rozendal RA. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ Sci Technol. 2008;42:8630–8640. doi: 10.1021/es801553z. [DOI] [PubMed] [Google Scholar]
- Logan BE, Hamelers B, Rozendal RA, Schrorder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K. Microbial fuel cells: methodology and technology. Environ Sci Technol. 2006;40:5181–5192. doi: 10.1021/es0605016. [DOI] [PubMed] [Google Scholar]
- Mahadevan R, Palsson BO, Lovley DR. In situ to in silico and back: elucidating the physiology and ecology of Geobacter spp. using genome-scale modelling. Nature Rev Microbiol. 2011;9:39–50. doi: 10.1038/nrmicro2456. [DOI] [PubMed] [Google Scholar]
- Majors PD, McLean JS, Scholten JCM. NMR bioreactor development for live in-situ microbial functional analysis. J Magn Reson. 2008;192:159–166. doi: 10.1016/j.jmr.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Majors PD, McLean JS, Pinchuk GE, Fredrickson JK, Gorby YA, Minard KR, Wind RA. NMR methods for in situ biofilm metabolism studies. J Microbiol Meth. 2005;62:337–344. doi: 10.1016/j.mimet.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, Johnson JP, et al. Tunable metallic-like conductivity in microbial nanowire networks. Nature Nanotechnol. 2011;6:573–579. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- Mandler D, Kraus-Ophir S. Self-assembled monolayers (SAMs) for electrochemical sensing. J Solid State Electr. 2011;15:1535–1558. [Google Scholar]
- Manohar AK, Mansfeld F. The internal resistance of a microbial fuel cell and its dependence on cell design and operating conditions. Electrochim Acta. 2009;54:1664–1670. [Google Scholar]
- Manohar AK, He Z, Mansfeld F. The use of electrochemical impedance spectroscopy (EIS) for the evaluation of the electrochemical properties of bioelectrochemical systems. In: Rabaey K, Angenent LT, Schröder U, Keller J, editors. Bioelectrochemical systems: from extracellular electron transfer to biotechnological application. IWA Publishing; London (UK): 2010. pp. 169–183. [Google Scholar]
- Manohar AK, Bretschger O, Nealson KH, Mansfeld F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry. 2008;72:149–154. doi: 10.1016/j.bioelechem.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Marcus AK, Torres CI, Rittmann BE. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol Bioeng. 2007;98:1171–1182. doi: 10.1002/bit.21533. [DOI] [PubMed] [Google Scholar]
- Marcus AK, Torres CI, Rittmann BE. Analysis of a microbial electrochemical cell using the proton condition in biofilm (PCBIOFILM) model. Bioresource Technol. 2011;102:253–262. doi: 10.1016/j.biortech.2010.03.100. [DOI] [PubMed] [Google Scholar]
- Marsili E, Sun J, Bond DR. Voltammetry and growth physiology of Geobacter sulfurreducens biofilms as a function of growth stage and imposed electrode potential. Electroanalysis. 2010;22:865–874. [Google Scholar]
- Marsili E, Rollefson JB, Baron DB, Hozalski RM, Bond DR. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl Environ Microbiol. 2008b;74:7329–7337. doi: 10.1128/AEM.00177-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, Bond DR. Shewanella secretes flavins that mediate extracellular electron transfer. P Natl Acad Sci USA. 2008a;105:3968–3973. doi: 10.1073/pnas.0710525105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JS, Majors PD, Reardon CL, Bilskis CL, Reed SB, Romine MF, Fredrickson JK. Investigations of structure and metabolism within Shewanella oneidensis MR-1 biofilms. J Microbiol Meth. 2008;74:47–56. doi: 10.1016/j.mimet.2008.02.015. [DOI] [PubMed] [Google Scholar]
- McLean JS, Wanger G, Gorby YA, Wainstein M, McQuaid J, Ishii SI, Bretschger O, Beyenal H, Nealson KH. Quantification of electron transfer rates to a solid phase electron acceptor through the stages of biofilm formation from single cells to multicellular communities. Environ Sci Technol. 2010;44:2721–2727. doi: 10.1021/es903043p. [DOI] [PubMed] [Google Scholar]
- Mehanna M, Kiely PD, Call DF, Logan BE. Microbial electrodialysis cell for simultaneous water desalination and hydrogen gas production. Environ Sci Technol. 2010;44:9578–9583. doi: 10.1021/es1025646. [DOI] [PubMed] [Google Scholar]
- Meunier CF, Yang XY, Rooke JC, Su BL. Biofuel cells based on the immobilization of photosynthetically active bioentities. Chemcatchem. 2011;3:476–488. [Google Scholar]
- Milliken CE, May HD. Sustained generation of electricity by the spore-forming, Gram-positive, Desulfitobacterium hafniense strain DCB2. Appl Microbiol Biotechnol. 2007;73:1180–1189. doi: 10.1007/s00253-006-0564-6. [DOI] [PubMed] [Google Scholar]
- Moehlenbrock MJ, Minteer SD. Extended lifetime biofuel cells. Chem Soc Rev. 2008;37:1188–1196. doi: 10.1039/b708013c. [DOI] [PubMed] [Google Scholar]
- Mohapatra BR, Dinardo O, Gould WD, Koren DW. Biochemical and genomic facets on the dissimilatory reduction of radionuclides by microorganisms - a review. Miner Eng. 2010;23:591–599. [Google Scholar]
- Nakamura R, Ishii K, Hashimoto K. Electronic absorption spectra and redox properties of C type cytochromes in living microbes. Angew Chem Int Edit. 2009;48:1606–1608. doi: 10.1002/anie.200804917. [DOI] [PubMed] [Google Scholar]
- Namour P, Jaffrezic-Renault N. Sensors for measuring biodegradable and total organic matter in water. Trac-Trend Anal Chem. 2010;29:848–857. [Google Scholar]
- Nealson KH, Finkel SE. Electron flow and biofilms. MRS Bulletin. 2011;36:380–384. [Google Scholar]
- Nevin KP, Kim BC, Glaven RH, Johnson JP, Woodard TL, Methe BA, DiDonato RJ, Covalla SF, Franks AE, Liu A, et al. Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. Plos One. 2009;4(5):e5628. doi: 10.1371/journal.pone.0005628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HD, Renslow R, Babauta J, Ahmed B, Beyenal H. A voltammetric flavin microelectrode for use in biofilms. Sensor Actuat B-Chem. 2012b;161:929–937. doi: 10.1016/j.snb.2011.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HD, Cao B, Mishra B, Boyanou MI, Kemner KM, Fredrickson JK, Beyenal H. Microscale geochemical gradients in Hanford 300 Area sediment biofilms and influence of uranium. Water Res. 2012a;46:227–234. doi: 10.1016/j.watres.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Oldham KB, Myland JC. Fundamentals of electrochemical science. Academic Press, Inc.; San Diego (CA): 1994. p. 496. [Google Scholar]
- Ong YT, Ahmad AL, Zein SHS, Tan SH. A review on carbon nanotubes in an environmental protection and green engineering perspective. Braz J Chem Eng. 2010;27:227–242. [Google Scholar]
- Osman MH, Shah AA, Walsh FC. Recent progress and continuing challenges in bio-fuel cells. Part II: microbial. Biosens Bioelectron. 2010;26:953–963. doi: 10.1016/j.bios.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Osman MH, Shah AA, Walsh FC. Recent progress and continuing challenges in bio-fuel cells. Part I: Enzymatic cells. Biosens Bioelectron. 2011;26:3087–3102. doi: 10.1016/j.bios.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Pant D, Van Bogaert G, Diels L, Vanbroekhoven K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresource Technol. 2010;101:1533–1543. doi: 10.1016/j.biortech.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Parameswaran P, Torres CI, Lee HS, Rittmann BE, Krajmalnik-Brown R. Hydrogen consumption in microbial electrochemical systems (MXCs): the role of homo-acetogenic bacteria. Bioresource Technol. 2011;102:263–271. doi: 10.1016/j.biortech.2010.03.133. [DOI] [PubMed] [Google Scholar]
- Pasco NF, Weld RJ, Hay JM, Gooneratne R. Development and applications of whole cell biosensors for ecotoxicity testing. Anal Bioanal Chem. 2011;400:931–945. doi: 10.1007/s00216-011-4663-6. [DOI] [PubMed] [Google Scholar]
- Patil SA, Harnisch F, Koch C, Hubschmann T, Fetzer I, Carmona-Martinez AA, Muller S, Schroder U. Electroactive mixed culture derived biofilms in microbial bioelectrochemical systems: the role of pH on biofilm formation, performance and composition. Bioresource Technol. 2011;102:9683–9690. doi: 10.1016/j.biortech.2011.07.087. [DOI] [PubMed] [Google Scholar]
- Peng L, You SJ, Wang JY. Electrode potential regulates cytochrome accumulation on Shewanella oneidensis cell surface and the consequence to bioelectrocatalytic current generation. Biosens Bioelectron. 2010;25:2530–2533. doi: 10.1016/j.bios.2010.03.039. [DOI] [PubMed] [Google Scholar]
- Picioreanu C, van Loosdrecht MCM, Curtis TP, Scott K. Model based evaluation of the effect of pH and electrode geometry on microbial fuel cell performance. Bioelectrochemistry. 2010;78:8–24. doi: 10.1016/j.bioelechem.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Picioreanu C, Head IM, Katuri KP, van Loosdrecht MCM, Scott K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007;41:2921–2940. doi: 10.1016/j.watres.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Polizzi NF, Skourtis SS, Beratan DN. Physical constraints on charge transport through bacterial nanowires. Faraday Discuss. 2012;155:43–61. doi: 10.1039/c1fd00098e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomareva ON, Arlyapov VA, Alferov VA, Reshetilov AN. Microbial biosensors for detection of biological oxygen demand (a review) Appl Biochem Microbiol. 2011;47:1–11. [PubMed] [Google Scholar]
- Pugh S, McKenna R, Moolick R, Nielsen DR. Advances and opportunities at the interface between microbial bioenergy and nanotechnology. Can J Chem Eng. 2011;89:2–12. [Google Scholar]
- Qian F, Morse DE. Miniaturizing microbial fuel cells. Trends Biotechnol. 2011;29:62–69. doi: 10.1016/j.tibtech.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W. Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol. 2004;70:5373–5382. doi: 10.1128/AEM.70.9.5373-5382.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaey K, Rodriguez J, Blackall LL, Keller J, Gross P, Batstone D, Verstraete W, Nealson KH. Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J. 2007;1:9–18. doi: 10.1038/ismej.2007.4. [DOI] [PubMed] [Google Scholar]
- Rachinski S, Carubelli A, Mangoni AP, Mangrich AS. Microbial fuel cells used in the production of electricity from organic waste: a perspective of future. Quim Nova. 2010;33:1773–1778. [Google Scholar]
- Ramanavicius A, Ramanaviciene A. Hemoproteins in design of biofuel cells. Fuel Cells. 2009;9:25–36. [Google Scholar]
- Rasouli M, Phee LSJ. Energy sources and their development for application in medical devices. Expert Rev Med Dev. 2010;7:693–709. doi: 10.1586/erd.10.20. [DOI] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, Lovley DR. Extracellular electron transfer via microbial nanowires. Nature. 2005;435:1098–1101. doi: 10.1038/nature03661. [DOI] [PubMed] [Google Scholar]
- Renslow R, Lewandowski Z, Beyenal H. Biofilm image reconstruction for assessing structural parameters. Biotechnol Bioeng. 2011b;108:1383–1394. doi: 10.1002/bit.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslow R, Donovan C, Shim M, Babauta J, Nannapaneni S, Schenk J, Beyenal H. Oxygen reduction kinetics on graphite cathodes in sediment microbial fuel cells. Phys Chem Chem Phys. 2011a;13:21573–21584. doi: 10.1039/c1cp23200b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslow RS, Majors PD, McLean JS, Fredrickson JK, Ahmed B, Beyenal H. In situ effective diffusion coefficient profiles in live biofilms using pulsed-field gradient nuclear magnetic resonance. Biotechnol Bioeng. 2010;106:928–937. doi: 10.1002/bit.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter H, McCarthy K, Nevin KP, Johnson JP, Rotello VM, Lovley DR. Electricity generation by Geobacter sulfurreducens attached to gold electrodes. Langmuir. 2008;24:4376–4379. doi: 10.1021/la703469y. [DOI] [PubMed] [Google Scholar]
- Richter H, Nevin KP, Jia HF, Lowy DA, Lovley DR, Tender LM. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energ Environ Sci. 2009;2:506–516. [Google Scholar]
- Rinaldi A, Mecheri B, Garavaglia V, Licoccia S, Di Nardo P, Traversa E. Engineering materials and biology to boost performance of microbial fuel cells: a critical review. Energ Environ Sci. 2008;1:417–429. [Google Scholar]
- Rismani-Yazdi H, Carver SM, Christy AD, Tuovinen IH. Cathodic limitations in microbial fuel cells: an overview. J Power Sources. 2008;180:683–694. [Google Scholar]
- Rodrigo MA, Canizares P, Garcia H, Linares JJ, Lobato J. Study of the acclimation stage and of the effect of the biodegradability on the performance of a microbial fuel cell. Bioresource Technol. 2009;100:4704–4710. doi: 10.1016/j.biortech.2009.04.073. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Schroder U. Photomicrobial solar and fuel cells. Electroanalysis. 2010;22:844–855. [Google Scholar]
- Rosenbaum M, Aulenta F, Villano M, Angenent LT. Cathodes as electron donors for microbial metabolism: which extracellular electron transfer mechanisms are involved? Bioresource Technol. 2011;102:324–333. doi: 10.1016/j.biortech.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Rozendal RA, Sleutels THJA, Hamelers HVM, Buisman CJN. Effect of the type of ion exchange membrane on performance, ion transport, and pH in biocatalyzed electrolysis of wastewater. Water Sci Technol. 2008;57:1757–1762. doi: 10.2166/wst.2008.043. [DOI] [PubMed] [Google Scholar]
- Rubenwolf S, Kerzenmacher S, Zengerle R, von Stetten F. Strategies to extend the lifetime of bioelectrochemical enzyme electrodes for biosensing and biofuel cell applications. Appl Microbiol Biotechnol. 2011;89:1315–1322. doi: 10.1007/s00253-010-3073-6. [DOI] [PubMed] [Google Scholar]
- Sand W, Gehrke T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res Microbiol. 2006;157:49–56. doi: 10.1016/j.resmic.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Schaetzle O, Barrier F, Baronian K. Bacteria and yeasts as catalysts in microbial fuel cells: electron transfer from micro-organisms to electrodes for green electricity. Energ Environ Sci. 2008;1:607–620. [Google Scholar]
- Shantaram A, Beyenal H, Raajan R, Veluchamy A, Lewandowski Z. Wireless sensors powered by microbial fuel cells. Environ Sci Technol. 2005;39:5037–5042. doi: 10.1021/es0480668. [DOI] [PubMed] [Google Scholar]
- Sharma V, Kundu PP. Biocatalysts in microbial fuel cells. Enzyme Microb Technol. 2010;47:179–188. [Google Scholar]
- Snider RM, Guissepi-Elie A, Strycharz-Glaven S, Tender L. Abstracts of Papers of the American Chemical Society. American Chemical Society; Washington (DC): 2011. 241. On the conductive nature of biofilms of Geobacter sulfurreducens. Abstract No. 371-BIOT. [Google Scholar]
- Srikanth S, Mohan SV, Sarma PN. Positive anodic poised potential regulates microbial fuel cell performance with the function of open and closed circuitry. Bioresource Technol. 2010;101:5337–5344. doi: 10.1016/j.biortech.2010.02.028. [DOI] [PubMed] [Google Scholar]
- Srikanth S, Marsili E, Flickinger MC, Bond DR. Electrochemical characterization of Geobacter sulfurreducens cells immobilized on graphite paper electrodes. Biotechnol Bioeng. 2008;99:1065–1073. doi: 10.1002/bit.21671. [DOI] [PubMed] [Google Scholar]
- Stams AJM, de Bok FAM, Plugge CM, van Eekert MHA, Dolfing J, Schraa G. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol. 2006;8:371–382. doi: 10.1111/j.1462-2920.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- Strik DPBTB, Timmers RA, Helder M, Steinbusch KJJ, Hamelers HVM, Buisman CJN. Microbial solar cells: applying photosynthetic and electrochemically active organisms. Trends Biotechnol. 2011;29:41–49. doi: 10.1016/j.tibtech.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Strycharz SM, Malanoski AP, Snider RM, Yi H, Lovley DR, Tender LM. Application of cyclic voltammetry to investigate enhanced catalytic current generation by biofilm-modified anodes of Geobacter sulfurreducens strain DL1 vs. variant strain KN400. Energ Environ Sci. 2011;4:896–913. [Google Scholar]
- Strycharz-Glaven SM, Snider RM, Guiseppi-Elie A, Tender LM. On the electrical conductivity of microbial nanowires and biofilms. Energ Environ Sci. 2011;4:4366–4379. [Google Scholar]
- Su LA, Jia WZ, Hou CJ, Lei Y. Microbial biosensors: a review. Biosens Bioelectron. 2011;26:1788–1799. doi: 10.1016/j.bios.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Torres CI, Marcus AK, Rittmann BE. Proton transport inside the biofilm limits electrical current generation by anode-respiring bacteria. Biotechnol Bioeng. 2008;100:872–881. doi: 10.1002/bit.21821. [DOI] [PubMed] [Google Scholar]
- Torres CI, Marcus AK, Lee HS, Parameswaran P, Krajmalnik-Brown R, Rittmann BE. A kinetic perspective on extracellular electron transfer by anode-respiring bacteria. FEMS Microbiol Rev. 2010;34:3–17. doi: 10.1111/j.1574-6976.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- Torres CI, Krajmalnik-Brown R, Parameswaran P, Marcus AK, Wanger G, Gorby YA, Rittmann BE. Selecting anode-respiring bacteria based on anode potential: phylogenetic, electrochemical, and microscopic characterization. Environ Sci Technol. 2009;43:9519–9524. doi: 10.1021/es902165y. [DOI] [PubMed] [Google Scholar]
- Vaddiraju S, Tomazos I, Burgess DJ, Jain FC, Papadimitrakopoulos F. Emerging synergy between nano-technology and implantable biosensors: a review. Biosens Bioelectron. 2010;25:1553–1565. doi: 10.1016/j.bios.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoudt P, Lewandowski Z, Little B. Iridium oxide pH microelectrode. Biotechnol Bioeng. 1992;40:601–608. doi: 10.1002/bit.260400507. [DOI] [PubMed] [Google Scholar]
- Varela H, Malta M, Torresi RM. Low cost in situ techniques in electrochemistry: the quartz crystal microbalance. Quim Nova. 2000;23:664–679. [Google Scholar]
- Velasquez-Orta SB, Head IM, Curtis TP, Scott K, Lloyd JR, von Canstein H. The effect of flavin electron shuttles in microbial fuel cells current production. Appl Microbiol Biotechnol. 2010;85:1373–1381. doi: 10.1007/s00253-009-2172-8. [DOI] [PubMed] [Google Scholar]
- Verhagen MFJM, Hagen WR. Electron-transfer mechanisms of flavin adenine-dinucleotide at the Glassy-Carbon electrode - a model study for protein electrochemistry. J Electroanal Chem. 1992;334:339–350. [Google Scholar]
- Villano M, Monaco G, Aulenta F, Majone M. Electrochemically assisted methane production in a biofilm reactor. J Power Sources. 2011;196:9467–9472. [Google Scholar]
- Wang HY, Bernarda A, Huang CY, Lee DJ, Chang JS. Micro-sized microbial fuel cell: a mini-review. Bioresource Technol. 2011;102:235–243. doi: 10.1016/j.biortech.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Wang X, Feng YJ, Ren NQ, Wang HM, Lee H, Li N, Zhao QL. Accelerated start-up of two-chambered microbial fuel cells: effect of anodic positive poised potential. Electrochim Acta. 2009;54:1109–1114. [Google Scholar]
- Wei JC, Liang P, Huang X. Recent progress in electrodes for microbial fuel cells. Bioresource Technol. 2011;102:9335–9344. doi: 10.1016/j.biortech.2011.07.019. [DOI] [PubMed] [Google Scholar]
- Wesley MJ, Lerner RN, Kim ES, Islam S, Liu Y. Biological fixed film. Water Environ Res. 2011;83:1150–1186. [Google Scholar]
- Willner I, Yan YM, Willner B, Tel-Vered R. Integrated enzyme-based biofuel cells - a review. Fuel Cells. 2009;9:7–24. [Google Scholar]
- Wrana N, Sparling R, Cicek N, Levin DB. Hydrogen gas production in a microbial electrolysis cell by electrohydrogenesis. J Clean Prod. 2010;18:S105–S111. [Google Scholar]
- Xia FH, Beyenal H, Lewandowski Z. An electrochemical technique to measure local flow velocity in biofilms. Water Res. 1998;32:3631–3636. [Google Scholar]
- Xie XH, Li EL, Tang ZK. EQCM and EIS study of the effect of potential of zero charge on Escherichia coli biofilm development. Int J Electrochem Sci. 2010;5:1018–1025. [Google Scholar]
- Xu K, Dexter SC, Luther GW. Voltammetric micro-electrodes for biocorrosion studies. Corrosion. 1998;54:814–823. [Google Scholar]
- Xu X, Ying YB. Microbial biosensors for environmental monitoring and food analysis. Food Rev Int. 2011;27:300–329. [Google Scholar]
- Yang SN, Lewandowski Z. Measurement of local mass-transfer coefficient in biofilms. Biotechnol Bioeng. 1995;48:737–744. doi: 10.1002/bit.260480623. [DOI] [PubMed] [Google Scholar]
- Yang XM, Beyenal H, Harkin G, Lewandowski Z. Quantifying biofilm structure using image analysis. J Microbiol Meth. 2000;39:109–119. doi: 10.1016/s0167-7012(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Yang XM, Beyenal H, Harkin G, Lewandowski Z. Evaluation of biofilm image thresholding methods. Water Res. 2001;35:1149–1158. doi: 10.1016/s0043-1354(00)00361-4. [DOI] [PubMed] [Google Scholar]
- Yi HN, Nevin KP, Kim BC, Franks AE, Klimes A, Tender LM, Lovley DR. Selection of a variant of Geobacter sulfurreducens with enhanced capacity for current production in microbial fuel cells. Biosens Bioelectron. 2009;24:3498–3503. doi: 10.1016/j.bios.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Yu T, Bishop PL. Stratification of microbial metabolic processes and redox potential change in an aerobic biofilm studied using microelectrodes. Water Sci Technol. 1998;37:195–198. [Google Scholar]
- Zhang L, Zhu X, Li J, Liao Q, Ye DD. Biofilm formation and electricity generation of a microbial fuel cell started up under different external resistances. J Power Sources. 2011;196:6029–6035. [Google Scholar]
- Zhang TC, Bishop PL. Experimental-determination of the dissolved-oxygen boundary-layer and mass-transfer resistance near the fluid-biofilm interface. Water Sci Technol. 1994;30:47–58. [Google Scholar]
- Zhang XC, Halme A. Modeling of a microbial fuel-cell process. Biotechnol Lett. 1995;17:809–814. [Google Scholar]
- Zhao F, Slade RCT, Varcoe JR. Techniques for the study and development of microbial fuel cells: an electrochemical perspective. Chem Soc Rev. 2009;38:1926–1939. doi: 10.1039/b819866g. [DOI] [PubMed] [Google Scholar]
- Zhou JP, McCreanor PT, Montalto F, Erdal ZK. Sustainability. Water Environ Res. 2011a;83:1414–1438. [Google Scholar]
- Zhou MH, Chi ML, Luo JM, He HH, Jin T. An overview of electrode materials in microbial fuel cells. J Power Sources. 2011b;196:4427–4435. [Google Scholar]
- Zhuang L, Feng CH, Zhou SG, Li YT, Wang YQ. Comparison of membrane- and cloth-cathode assembly for scalable microbial fuel cells: construction, performance and cost. Process Biochem. 2010;45:929–934. [Google Scholar]
- Zomorrodi AR, Maranas CD. OptCom: a multi-level optimization framework for the metabolic modeling and analysis of microbial communities. PLoS Comput Biol. 2012;8(2):e1002363. doi: 10.1371/journal.pcbi.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.