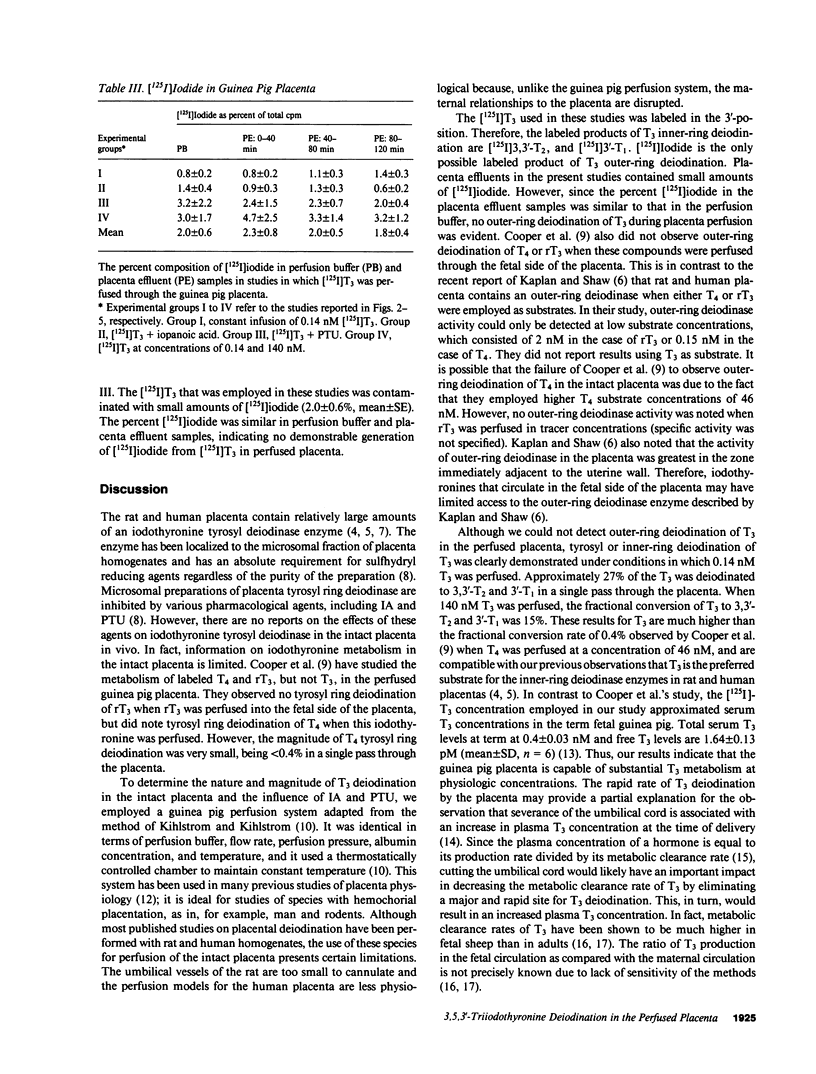
Abstract
Broken cell preparations of rat and human placentas contain an inner (tyrosyl)-ring iodothyronine deiodinase enzyme with greatest activity when the substrate is 3,5,3'-triiodothyronine (T3). This report describes the deiodination of T3 in the intact placenta and the effect of sodium iopanoate (IA) and propylthiouracil (PTU) on T3 deiodination. Under nembutal anesthesia, the placenta of 60-65-d-old pregnant guinea pigs was surgically exposed, a single umbilical artery and the umbilical vein were cannulated, and the fetus was removed. In a temperature-controlled chamber (37 degrees C), the fetal side of the placenta was perfused through the umbilical artery at a rate of 1 ml/min with 3% bovine serum albumin Krebs-Henseleit buffer containing 0.14 nM outer ring labeled [125I]T3. Placenta effluent fractions were collected at timed intervals from the umbilical vein cannula throughout a 120-min perfusion period. The contents of the perfusion buffer and the various effluent fractions were analyzed for their iodothyronine content by high pressure liquid chromatography. In five experiments, the percent composition of 125I-labeled iodothyronines in the perfusion buffer and placenta effluent was 95.3 +/- 1.0 (mean +/- SE) and 70.2 +/- 2.1 for T3 (P less than 0.01), 2.5 +/- 0.7 and 20.1 +/- 1.8 for 3,3'-T2 (P less than 0.01), and 0 and 8.2 +/- 0.9 for 3'-T1. There was no difference between the percent [125I]iodide in the perfusion buffer and in the placenta effluents. When placentas were perfused with IA and [125I]T3, after perfusion with [125I]T3 alone, there was a significant increase (P less than 0.01) in the percent [125I]T3 in the placenta effluents, and a significant decrease in [125I]3,3'-T2 (P less than 0.01) and [125I]3'-T1 (P less than 0.01). In contrast, PTU did not affect the composition of labeled iodothyronines in the placenta effluents, despite the fact that the addition of PTU significantly (P less than 0.001) inhibits the inner-ring deiodination of [125I]T3 in human or guinea pig placenta microsomes in the presence of low (0.25 mM) concentrations of dithiothreitol. The present studies demonstrate that T3 is actively deiodinated in the inner ring to 3,3'-T2 by the intact guinea pig placenta. A portion of 3,3'-T2 is further deiodinated in the inner ring to generate 3'-T1. No outer ring deiodination of T3 was seen under the conditions employed. IA, but not PTU, inhibits T3 deiodination in the placenta perfused in situ. We conclude that the placenta is probably a site for fetal T3 metabolism.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird D. T., Horton R., Longcope C., Tait J. F. Steroid dynamics under steady-state conditions. Recent Prog Horm Res. 1969;25:611–664. doi: 10.1016/b978-0-12-571125-8.50017-x. [DOI] [PubMed] [Google Scholar]
- Banovac K., Bzik L., Tislarić D., Sekso M. Conversion of thyroxine to triiodothyronine and reverse triiodothyronine in human placenta and fetal membranes. Horm Res. 1980;12(5):253–259. doi: 10.1159/000179128. [DOI] [PubMed] [Google Scholar]
- Chopra I. J., Sack J., Fisher D. A. 3,3',5'-Triiodothyronine (reverse T3) and 3,3',5-triiodothyronine (T3) in fetal and adult sheep: studies of metabolic clearance rates, production rates, serum binding, and thyroidal content relative to thyroxine. Endocrinology. 1975 Nov;97(5):1080–1088. doi: 10.1210/endo-97-5-1080. [DOI] [PubMed] [Google Scholar]
- Cooper E., Gibbens M., Thomas C. R., Lowy C., Burke C. W. Conversion of thyroxine to 3,3',5'-triiodothyronine in the guinea pig placenta: in vivo studies. Endocrinology. 1983 May;112(5):1808–1815. doi: 10.1210/endo-112-5-1808. [DOI] [PubMed] [Google Scholar]
- Fay M., Roti E., Fang S. L., Wright G., Braverman L. E., Emerson C. H. The effects of propylthiouracil, iodothyronines, and other agents on thyroid hormone metabolism in human placenta. J Clin Endocrinol Metab. 1984 Feb;58(2):280–286. doi: 10.1210/jcem-58-2-280. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Dussault J. H., Erenberg A., Lam R. W. Thyroxine and triiodothyronine metabolism in maternal and fetal sheep. Pediatr Res. 1972 Dec;6(12):894–899. doi: 10.1203/00006450-197212000-00007. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Shaw E. A. Type II iodothyronine 5'-deiodination by human and rat placenta in vitro. J Clin Endocrinol Metab. 1984 Aug;59(2):253–257. doi: 10.1210/jcem-59-2-253. [DOI] [PubMed] [Google Scholar]
- Kihlström I., Kihlström J. E. An improved technique for perfusion of the guinea pig placenta in situ giving viable conditions demonstrated by placental transport of amino acids (L- and D-alanine). Biol Neonate. 1981;39(3-4):150–159. doi: 10.1159/000241420. [DOI] [PubMed] [Google Scholar]
- LOUROS N., MIRAS C., VRETTOS A. LA D'SIODATION IN VITRO DE LA THYROXINE PAR LE PLACENTA HUMAIN. Ann Endocrinol (Paris) 1964 Jul-Aug;25:417–422. [PubMed] [Google Scholar]
- Marchant B., Brownlie B. E., Hart D. M., Horton P. W., Alexander W. D. The placental transfer of propylthiouracil, methimazole and carbimazole. J Clin Endocrinol Metab. 1977 Dec;45(6):1187–1193. doi: 10.1210/jcem-45-6-1187. [DOI] [PubMed] [Google Scholar]
- McCann U. D., Shaw E. A., Kaplan M. M. Iodothyronine deiodination reaction types in several rat tissues: effects of age, thyroid status, and glucocorticoid treatment. Endocrinology. 1984 May;114(5):1513–1521. doi: 10.1210/endo-114-5-1513. [DOI] [PubMed] [Google Scholar]
- Rajatanavin R., Young R. A., Braverman L. E. Effect of chloride on serum thyroxine binding in familial dysalbuminemic hyperthyroxinemia. J Clin Endocrinol Metab. 1984 Feb;58(2):388–391. doi: 10.1210/jcem-58-2-388. [DOI] [PubMed] [Google Scholar]
- Roti E., Fang S. L., Braverman L. E., Emerson C. H. Rat placenta is an active site of inner ring deiodination of thyroxine and 3,3',5-triiodothyronine. Endocrinology. 1982 Jan;110(1):34–37. doi: 10.1210/endo-110-1-34. [DOI] [PubMed] [Google Scholar]
- Roti E., Fang S. L., Green K., Emerson C. H., Braverman L. E. Human placenta is an active site of thyroxine and 3,3'5-triiodothyronine tyrosyl ring deiodination. J Clin Endocrinol Metab. 1981 Sep;53(3):498–501. doi: 10.1210/jcem-53-3-498. [DOI] [PubMed] [Google Scholar]
- Sack J., Beaudry M., DeLamater P. V., Oh W., Fisher D. A. Umbilical cord cutting triggers hypertriiodothyroninemia and nonshivering thermogenesis in the newborn lamb. Pediatr Res. 1976 Mar;10(3):169–169. doi: 10.1203/00006450-197603000-00005. [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Shyh T. P., Chopra I. J., Solomon D. H., Huang H. W., Chu P. C. Comparison sodium ipodate (oragrafin) and propylthiouracil in early treatment of hyperthyroidism. J Clin Endocrinol Metab. 1982 Mar;54(3):630–634. doi: 10.1210/jcem-54-3-630. [DOI] [PubMed] [Google Scholar]





