Abstract
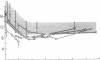
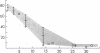
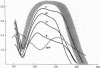
To explain the transient anemia and poikilocytosis seen during infancy in hereditary elliptocytosis (HE), we resealed erythrocyte (RBC) ghosts from affected children or their elliptocytic parents with 2,3-diphosphoglycerate (DPG) (0-8 mM), a compound that dissociates membrane skeletons, then measured ghost mechanical stability in the ektacytometer. Without added 2,3-DPG, ghost mechanical stability was subnormal in infantile poikilocytosis (IP) and HE but was even more abnormal in hereditary pyropoikilocytosis (HPP). Addition of 2,3-DPG (2.55 mM) to IP or HE ghosts, decreased their stability to that of HPP ghosts (without 2,3-DPG). Nonphysiological 2,3-DPG levels (6-8 mM) were required to elicit a similar effect in normal ghosts. The data suggest that free 2,3-DPG, present in neonatal RBC as a consequence of diminished binding to HbF, may render HE susceptible to in vivo fragmentation. The developmental switch from fetal to adult hemoglobin, by diminishing available free 2,3-DPG, may explain the abatement of poikilocytosis and hemolytic anemia that accompanies maturation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Bennett V. Deficient red-cell spectrin in severe, recessively inherited spherocytosis. N Engl J Med. 1982 May 13;306(19):1155–1161. doi: 10.1056/NEJM198205133061906. [DOI] [PubMed] [Google Scholar]
- Austin R. F., Desforges J. F. Hereditary elliptocytosis: an unusual presentation of hemolysis in the newborn associated with transient morphologic abnormalities. Pediatrics. 1969 Aug;44(2):196–200. [PubMed] [Google Scholar]
- Bunn H. F., Briehl R. W. The interaction of 2,3-diphosphoglycerate with various human hemoglobins. J Clin Invest. 1970 Jun;49(6):1088–1095. doi: 10.1172/JCI106324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn H. F., Ransil B. J., Chao A. The interaction between erythrocyte organic phosphates, magnesium ion, and hemoglobin. J Biol Chem. 1971 Sep 10;246(17):5273–5279. [PubMed] [Google Scholar]
- Carpentieri U., Gustavson L. P., Haggard M. E. Pyknocytosis in a neonate: an unusual presentation of hereditary elliptocytosis. Clin Pediatr (Phila) 1977 Jan;16(1):76–78. doi: 10.1177/000992287701600114. [DOI] [PubMed] [Google Scholar]
- Chasis J. A., Mohandas N. Erythrocyte membrane deformability and stability: two distinct membrane properties that are independently regulated by skeletal protein associations. J Cell Biol. 1986 Aug;103(2):343–350. doi: 10.1083/jcb.103.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. R., Mohandas N., Shohet S. B. Osmotic gradient ektacytometry: comprehensive characterization of red cell volume and surface maintenance. Blood. 1983 May;61(5):899–910. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Hajjar K. A. Structural and functional analysis of spectrin from neonatal erythrocytes. Biochim Biophys Acta. 1985 Mar 1;827(3):460–465. doi: 10.1016/0167-4838(85)90233-x. [DOI] [PubMed] [Google Scholar]
- Holroyde C. P., Oski F. A., Gardner F. H. The "pocked" erythrocyte. Red-cell surface alterations in reticuloendothelial immaturity of the neonate. N Engl J Med. 1969 Sep 4;281(10):516–520. doi: 10.1056/NEJM196909042811002. [DOI] [PubMed] [Google Scholar]
- Keitt A. S. Reduced nicotinamide adenine dinucleotide-linked analysis of 2,3-diphosphoglyceric acid: spectrophotometric and fluorometric procedures. J Lab Clin Med. 1971 Mar;77(3):470–475. [PubMed] [Google Scholar]
- Knowles W. J., Bologna M. L. Isolation of the chemical domains of human erythrocyte spectrin. Methods Enzymol. 1983;96:305–313. doi: 10.1016/s0076-6879(83)96028-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane P. A., Shew R. L., Iarocci T. A., Mohandas N., Hays T., Mentzer W. C. Unique alpha-spectrin mutant in a kindred with common hereditary elliptocytosis. J Clin Invest. 1987 Mar;79(3):989–996. doi: 10.1172/JCI112911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. A molecular defect of spectrin in a subset of patients with hereditary elliptocytosis. Alterations in the alpha-subunit domain involved in spectrin self-association. J Clin Invest. 1984 Jun;73(6):1688–1695. doi: 10.1172/JCI111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- MacDougall L. G., Moodley G., Quirk M. The pyropoikilocytosis-elliptocytosis syndrome in a black South African infant: clinical and hematological features. Am J Pediatr Hematol Oncol. 1982 Fall;4(3):344–349. [PubMed] [Google Scholar]
- Matovcik L. M., Mentzer W. C. The membrane of the human neonatal red cell. Clin Haematol. 1985 Feb;14(1):203–221. [PubMed] [Google Scholar]
- Mentzer W. C., Turetsky T., Mohandas N., Schrier S., Wu C. S., Koenig H. Identification of the hereditary pyropoikilocytosis carrier state. Blood. 1984 Jun;63(6):1439–1446. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Oski F. A., Delivoria-Papadopoulos M. The red cell, 2,3-diphosphoglycerate, and tissue oxygen release. J Pediatr. 1970 Dec;77(6):941–956. doi: 10.1016/s0022-3476(70)80076-2. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Palek J., Lux S. E. Red cell membrane skeletal defects in hereditary and acquired hemolytic anemias. Semin Hematol. 1983 Jul;20(3):189–224. [PubMed] [Google Scholar]
- Pearson H. A. Life-span of the fetal red blood cell. J Pediatr. 1967 Feb;70(2):166–171. doi: 10.1016/s0022-3476(67)80410-4. [DOI] [PubMed] [Google Scholar]
- Prchal J. T., Castleberry R. P., Parmley R. T., Crist W. M., Malluh A. Hereditary pyropoikilocytosis and elliptocytosis: clinical, laboratory, and ultrastructural features in infants and children. Pediatr Res. 1982 Jun;16(6):484–489. doi: 10.1203/00006450-198206000-00017. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Casaly J. 2,3-Diphosphoglycerate and ATP dissociate erythrocyte membrane skeletons. J Biol Chem. 1980 Oct 25;255(20):9955–9960. [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Waugh R. E. Effects of 2,3-diphosphoglycerate on the mechanical properties of erythrocyte membrane. Blood. 1986 Jul;68(1):231–238. [PubMed] [Google Scholar]
- Zarkowsky H. S. Heat-induced erythrocyte fragmentation in neonatal elliptocytosis. Br J Haematol. 1979 Apr;41(4):515–518. doi: 10.1111/j.1365-2141.1979.tb05889.x. [DOI] [PubMed] [Google Scholar]