Abstract
Readmission within 30 days after hospital discharge for common cardiovascular conditions such as heart failure and acute myocardial infarction is extremely common among older persons. To incentivize investment in reducing preventable rehospitalizations, the United States federal government has directed increasing financial penalties to hospitals with higher-than-expected 30-day readmission rates. Uncertainty exists, however, regarding the best approaches to reducing these adverse outcomes. In this review, we summarize the literature on predictors of 30-day readmission, the utility of risk prediction models, and strategies to reduce short-term readmission after hospitalization for heart failure and acute myocardial infarction. We report that few variables have been found to consistently predict the occurrence of 30-day readmission and that risk prediction models lack strong discriminative ability. We additionally report that the literature on interventions to reduce 30-day rehospitalization has significant limitations due to heterogeneity, susceptibility to bias, and lack of reporting on important contextual factors and details of program implementation. New information is characterizing the period after hospitalization as a time of high generalized risk, which has been termed the post-hospital syndrome. This framework for characterizing inherent post-discharge instability suggests new approaches to reducing readmissions.
Keywords: Readmission, Rehospitalization, Heart failure, Acute myocardial infarction, Care transitions, Post-hospital syndrome, Elderly
Introduction
Short-term readmission after hospitalization for common cardiac conditions such as heart failure (HF) and acute myocardial infarction (AMI) is extremely common in older adults. Data from the Medicare population has demonstrated that one in four patients surviving hospitalization for HF is readmitted within 30 days [1-6]. Similarly, one in five older persons surviving hospitalization for AMI is readmitted in the month after discharge [1-3, 5, 6].
To reduce costs [7] and improve healthcare quality [8-11], the United States government has put increasing pressure on hospitals to decrease preventable readmissions. Starting in 2009, Medicare publicly reported hospital 30-day risk-standardized readmission rates for HF, AMI, and pneumonia on its Hospital Compare website [12]. In 2012, following the passage of the Affordable Care Act, the federal government began directing financial penalties toward hospitals with higher-than-expected 30-day readmission rates for these three conditions [12]. These penalties have increased each year since 2012; this year, total Medicare payments may be reduced up to 3% for hospitals with the highest 30-day readmission rates for publicly reported conditions [12].
Despite these changes, the scientific literature has not provided clear guidance on the best ways to reduce preventable rehospitalizations. Studies identifying risk factors have found inconsistent results; published predictive models have had only poor to fair discriminative capacity; and intervention studies have generally been limited by their small sample size and local context-dependent factors. Findings have been heterogeneous and are difficult to generalize.
Our aim in this review was to summarize literature pertaining to strategies to reduce 30-day readmissions among older patients hospitalized with HF or AMI. In particular, we considered research on predictors of readmission, models for risk stratification, and interventions to reduce rehospitalization. Given the inconsistencies and limitations arising from this traditional deterministic approach to understanding rehospitalization, we also broadened the scope of our evaluation to consider insights derived from the study of national Medicare data, including evidence it provides of a transient period of acquired risk following hospitalization, which has been called the post-hospital syndrome [13].
Risk Prediction
Efforts to develop a deterministic understanding of rehospitalization have been difficult, as no specific patient or hospital factors have been shown to consistently predict 30-day readmission after hospitalization for HF or AMI. A systematic review of 112 studies describing the association between traditional patient characteristics and readmission after hospitalization for HF found that demographic characteristics, comorbid conditions, and markers of HF severity such as left ventricular ejection fraction and New York Heart Association class were associated with readmission in only a minority of cases [14]. Although higher levels of admission cardiac troponin and B-type natriuretic peptide were associated with readmission risk, these cardiac biomarkers were measured in fewer than one in six of the included studies. Similarly, a systematic review of 35 studies describing the association between patient characteristics and readmission after AMI found no consistent findings across studies [15]. These results may relate to the fact that examined covariates have generally not included common conditions and syndromes found in the elderly. With the exception of a recent finding linking unrecognized mild cognitive impairment with readmission after HF hospitalization [16], the association of frailty, mobility disability, impaired functional status, and sensory impairment with proximate outcomes after hospitalization has yet to be examined in older persons with either HF or AMI.
Social factors have also not been conclusively related to short-term readmission despite increased attention to this topic in recent years. For example, a systematic review of social factors in HF readmission found inconsistent associations between short-term readmission and patient socioeconomic status as measured by health insurance and yearly income, social support as measured by marital status, and high-risk behavior as measured by smoking status, cocaine use, and non-adherence in both medication use and physician follow-up [17]. This may be because the relationship between social factors and readmission is complex and is apparent only in the setting of significant medical or functional needs [18]. These relationships may be further influenced by environmental factors. For example, increasing U.S. state-level income inequality may be associated with higher risk of readmission even after controlling for patient income and education [19].
Finally, hospital care strategies, hospital structural characteristics, and local care resources have only rarely been conclusively linked with readmission outcomes after hospitalization for HF or AMI. For example, while considerable attention has been drawn to the topic of higher readmission rates at hospitals providing care to minority and safety-net populations [20], readmission rates may be similar [21, 22] or only marginally higher at these institutions [5, 23]. Moreover, large numbers of minority-serving and safety-net hospitals have low rates of rehospitalization [5, 23]. Controversy also exists within the epidemiologic literature as to whether specific discharge and transitional care practices such as patient discharge education [24], early outpatient follow-up [25-27], and improved communication between hospital and outpatient provider [28, 29] are associated with lower readmission, possibly because the quality of education, communication, and follow-up visits have been difficult to model [30]. Isolated studies have shown that higher readmission rates are associated with lower hospital case volume, lack of availability of advanced cardiac services, and lower nurse-to-patient ratios [31, 32].
In summary, a review of the literature demonstrates that in a large number of studies, no specific patient or hospital factors have been shown to consistently predict 30-day readmission after hospitalization for HF or AMI. These findings suggest that readmission cannot be easily understood through the exclusive use of a simple deterministic framework. High-risk patients are unlikely to have few conserved characteristics. Strategies to reduce rehospitalization may therefore be unsuccessful if they rely on simple rules for assigning risk.
Risk Stratification
As with risk prediction, accurate risk stratification for rehospitalization has been difficult. In contrast with models predicting short-term mortality among hospitalized patients [33-35], no risk prediction model for 30-day readmission after hospitalization for HF or AMI has demonstrated dependable model discrimination and calibration. Discrimination is defined by the area under the receiver operating characteristic (ROC) curve, and measures how well a model can separate those who will and will not have the outcome of interest. In this case, if the predicted risks for readmitted patients are all higher than for patients who are not readmitted, we say that the model discriminates perfectly (c-statistic of 1). Conversely, if risk prediction is no better than chance, the c-statistic is 0.5. Models are typically considered reasonable when the c-statistic is greater than 0.7 and strong when the c-statistic is greater than 0.8 [36]. Model calibration, on the other hand, reflects the degree to which predicted and observed event rates are similar across the overall population of study. Calibration is typically measured by the Hosmer-Lemeshow test or other goodness-of-fit tests. One can therefore have a test that broadly produces accurate estimates of overall readmission risk for subgroups within a population (high calibration) while failing to identify whether particular patients will or will not be rehospitalized (poor discrimination).
For 30-day readmission after HF hospitalization, only two models have generated c-statistics greater than 0.6 after study in both derivation and validation cohorts. The model developed by Amarasingham et al. is an automated model incorporating data from the electronic health record at the time of hospitalization [37]. Final variables include the Tabak mortality score that includes age, vital signs, and various laboratory values [38], demographic characteristics, specific comorbidities, and markers of social instability and lower socioeconomic status. The model has a c-statistic of 0.72 and does not demonstrate a lack of fit between predicted and observed events. A second model by Hammill et al. combines claims-based demographic and comorbidity data with clinical data including vital signs, laboratory values, and measured left ventricular ejection fraction [39]. The model does not include markers of social deprivation. Model calibration was good, but discrimination was poor, with a resulting c-statistic of 0.6. Although a third model by Hummel et al. generated a c-statistic of 0.74 by adding information on past hospitalization history to an extended CMS readmission model containing information on patient demographics, comorbidities, vital signs, and diagnostic test results (www.readmissionscore.org/heart_failure.php) [40], this expanded model has not been validated in a second patient cohort. While other models for HF readmission exist, they were designed to predict different outcomes such as long-term readmission up to one year after hospitalization [41-43], HF-related readmission rather than all-cause readmission [41], or the combined endpoint of readmission plus death [44, 45]. Many of these models have not reported measures of discrimination or results from a validation cohort [43, 44, 46].
In contrast to the widespread focus on readmission after hospitalization for HF, very few risk prediction models exist for 30-day readmission after hospitalization for AMI. One of these models builds upon the CMS readmission model by adding information on patient symptoms, vital signs, and laboratory testing results to the demographic and comorbidity information found in the original claims-based model for profiling readmission rates across hospitals (www.readmissionscore.org/heart_attack.php) [47, 48]. No published models for 30-day readmission after AMI have extended the discriminative ability of the CMS model by incorporating data on previous healthcare use or social factors.
More generally, the discriminative ability of predictive models for short-term readmission that have been created for a range of common medical conditions increases with the granularity and comprehensiveness of the included data. Models derived purely from administrative data available at the time of hospitalization have the lowest discriminative ability but are the least onerous to use. These models were originally designed to measure hospital 30-day readmission rates and are as accurate for hospital profiling as models incorporating detailed clinical information available at the time of hospital presentation [47, 49]. These more parsimonious models are therefore the current gold standard for measuring hospital performance and are used by the Centers for Medicare & Medicaid Services in the U.S. However, these claims-based models are significantly less effective for quantifying an individual patient’s risk of rehospitalization [48, 50]. In contrast, models with higher discriminative capacity for short-term readmission for individual patients have used both administrative and clinical data derived throughout hospitalization, data incorporating functional variables, and data incorporating social determinants of health. Unsurprisingly, a recent systematic review of risk prediction models for 30-day readmission involving patients admitted for a broad range of medical conditions found that discrimination was higher for models incorporating data from the entire hospitalization rather than data available only at the time of admission (c-statistic range 0.68-0.83 versus 0.56-0.72, respectively) [48]. In addition, when different models were compared within the same sample population, the inclusion of variables describing self-rated health, the ability to independently perform activities of daily living, support with activities of daily living, residential stability, and other social factors improved model discrimination to a significant extent [37, 51]. However, additional resources may be needed for the continued collection of data throughout hospitalization and the assessment of patients’ self-rated health, functional status, and social attributes.
In summary, our ability to predict which patients will be readmitted is limited. In contrast, we are extremely proficient in predicting short-term mortality among patients hospitalized with common cardiopulmonary conditions. This distinction provides evidence that readmission has complex determinants beyond traditional characterizations of medical complexity and acuity of illness. Given the difficulties inherent in risk prediction, strategies to reduce rehospitalization may be more successful when applied more generally across all discharged patients.
Strategies to Reduce Readmission
Interventions to reduce 30-day readmission after hospitalization for HF and AMI have primarily focused on improving transitional care from the hospital back to the community. However, the literature in this area has significant limitations. The great majority of studies involve a small number of participants at single centers, a shortcoming that is particularly important because the success or failure of complex interventions may be highly context-dependent or may rest on the talents and motivations of a small number of health providers. Yet very few studies report on potentially important contextual factors such as patients’ socioeconomic characteristics [17], available organizational resources to reduce readmissions, and prevailing organizational culture related to collaboration and problem-solving [52]. In addition, information is rarely provided on the specifics of implementation such as required staff resources, training requirements, oversight strategies, and integration into current processes of care. These topics are critical to programmatic development and success in the real world. And, perhaps most fundamentally, there is concern that many studies may be subject to bias due to lack of blinding, as study investigators conduct outcome assessments, thereby increasing the risk of confirmation bias and selective outcome reporting.
Moreover, very few studies have specifically examined the impact of transitional care strategies on 30-day readmission as opposed to readmission over longer time intervals. The few papers that do examine 30-day readmission describe single-center cohorts and assess only modest interventions such as pre-discharge patient education and counseling [53, 54] or assessment for discharge readiness [55]. These relatively limited interventions did not result in a significant reduction in 30-day rates of rehospitalization.
Meta-analyses of studies over longer time horizons suggest that multidisciplinary interventions with both pre- and post-discharge components may be effective in reducing long-term readmissions after HF hospitalization. However, many of the included studies are subject to the limitations described previously and are very heterogeneous with regard to their intervention content, delivery personnel, method of communication with patients, intensity and complexity of patient contact, and associated environmental context [56]. However, their associated findings may still have generalizable relevance for short-term rehospitalization. For example, a meta-analysis of 18 randomized controlled trials of comprehensive discharge planning and post-discharge support of older patients with systolic HF found that over a pooled mean observation period of eight months, the number needed to treat to prevent one readmission was 12 [57]. Although specific inpatient and post-discharge interventions varied by study, readmission reductions were seen with many different post-discharge strategies, including home visits, frequent phone contact, and extended home-care services. Similarly, a meta-analysis of 10 randomized controlled trials of chronic care management following HF hospitalization [58] found that program delivery via multidisciplinary teams and in-person contact resulted in fewer readmissions over a period of 3–12 months. In contrast, care delivered by a single expert – in this case, a registered nurse with expertise in heart failure – or delivered via phone was less effective. Analogously, singular interventions including high-quality telemonitoring strategies in the post-discharge period [59] have not been effective in reducing readmission after hospitalization for HF.
Intervention studies to reduce readmission following hospitalization for AMI are significantly fewer in number and lower in quality. Only two assess strategies for reducing short-term readmission [60, 61]. Both involve outpatient nursing follow-up in fewer than 70 patients, and both failed to find a reduction in readmission in the intervention arms. Furthermore, a meta-analysis including these studies, as well as four other randomized trials [62-65], failed to link hospital-initiated discharge support, patient and family education, and community-based support to lower readmission after AMI [66]. However, the included study by Young et al. had the most comprehensive intervention bundle, involving six home visits by a cardiac-trained nurse, a standardized nurse checklist, referral criteria for specialty care, communication with the family physician, and patient education, and was associated with a lower number of all-cause readmission days and emergency department encounters per 1,000 follow-up days [62].
Outside of HF and AMI, data from other admitting conditions provide limited support for broad-based and longitudinal transitional care interventions. A 2011 systematic review of studies from 1993 to 2011 examined the role of pre-discharge interventions (patient education, medication reconciliation, discharge planning, and scheduling follow-up before discharge), post-discharge interventions (follow-up telephone calls, patient-activated hotlines, timely communication with ambulatory providers, timely follow-up with ambulatory providers, and home visits), and bridging interventions (transition coaches, physician continuity across inpatient and outpatient settings, and patient-centered discharge instructions) on 30-day readmission among patients admitted with a range of medical conditions [67]. The study found that no single intervention alone was associated with reductions in 30-day readmissions. However, among the randomized studies included in the systematic review, multicomponent interventions were more likely to be successful [68-70].
Three other systematic reviews on topics related to care transitions and readmission with component studies, predominantly from the previous decade, have corroborated these results [71-73]. A study of patient handovers from hospital to primary care found that multicomponent interventions to improve the quality of information shared with outpatient providers, the extent of communication between settings, and the degree to which post-discharge care was coordinated showed benefit in reducing hospital use after discharge, though efficacy could not be traced to specific interventions [71]. Similarly, a systematic review of hospital-initiated transitional care strategies for general medical patients found that a bridging strategy incorporating both pre-discharge and post-discharge interventions with a dedicated transition provider who contacted patients both before and after discharge could reduce readmission or ED visits, though the strength of the association was low [72]. Finally, a Cochrane systematic review of 24 randomized trials investigating discharge planning, defined as the development of an individualized discharge plan for a patient with or without further post-discharge support, found that the relative risk of 90-day readmission was 0.82 in the setting of intervention [73]. In all cases, however, the quality of evidence in favor of specific interventions or a specific intervention bundle was relatively weak.
Given these limitations in the current literature, data from observational studies of large groups of hospitals are particularly important, though they should be considered exploratory in nature. In their Web-based survey of over 500 hospitals participating in a national quality initiative to reduce readmission, Bradley et al. found that hospitals with the lowest 30-day risk-standardized readmission rates were more likely to partner with community physicians and local hospitals to reduce readmission, have nurses responsible for medication reconciliation, arrange follow-up appointments before discharge, have a process in place to send all discharge papers or electronic summaries directly to the patient’s primary physician, and assign staff to follow up on test results that return after the patient is discharged [74]. Although risk reduction from the implementation of each individual strategy was relatively low, hospitals that implemented more strategies had significantly lower risk-standardized readmission rates (0.34% reduction for each additional strategy). Analogously, a survey of 100 hospitals from a different national quality initiative found that hospitals with lower 30-day readmission rates had modestly higher performance on a composite endpoint measuring discharge and transitional care practices [75]. The composite endpoint incorporated the presence of at least one mechanism to remind providers to discharge patients on evidence-based therapies, day-of-discharge education, written discharge materials with medication information, provision of a weight scale at discharge, referral to a disease management program, primary care provider notification about hospital admission, scheduled follow-up appointments for patients, initial follow-up within 14 days of discharge, and electronic transmission of prescriptions directly to pharmacies or delivery of prescriptions directly to patients.
In summary, the great majority of studies examining strategies to reduce 30-day readmission are limited by single-center designs, small sample size, lack of description of important contextual factors, and lack of description of implementation specifics. This information impairs interpretation of study findings, including their generalizability to local contexts. Therefore, while improved transitional care has face validity for reducing rehospitalization, the exact manners for optimally doing so remain unknown.
Insights from National Medicare Data
Given the difficulties in identifying consistent predictors of risk, developing accurate models for risk stratification, and creating generalizable knowledge about effective interventions, we have looked to national Medicare data for further insights. These data are derived from administrative claims filed by healthcare providers for services rendered to Medicare beneficiaries. The strength of the data resides both in its broad generalizability, as it includes all older persons with traditional Medicare health insurance, and its comprehensive accounting of healthcare utilization. From 2007 to 2009, this database included over 440,000 yearly hospitalizations for HF, with approximately 110,000 associated 30-day readmissions, as well as 180,000 yearly hospitalizations for AMI, with approximately 35,000 associated 30-day readmissions [1].
Using this national database, we characterized the diagnoses and timing of 30-day readmissions among elderly persons initially hospitalized for HF and AMI [1]. We found that patients are readmitted with a diverse spectrum of conditions that usually differ from the cause of the initial hospitalization (Table 1). For example, only 1 in every 10 patients readmitted within 30 days of an AMI is rehospitalized for a second heart attack. Moreover, almost 50% of 30-day readmissions after AMI are for non-cardiovascular conditions. We also found that while a disproportionately high number of readmissions occur soon after hospitalization, readmissions occur frequently throughout the month after hospital discharge (Table 2). For example, almost 40% of 30-day readmissions after hospitalization for HF occur during days 16 to 30 after hospitalization. Taken together, these findings imply that the entire 30-day period after discharge is one of heightened vulnerability for rehospitalization due to a wide range of illnesses.
Table 1. The 10 Most Common Readmission Diagnoses Among Older Patients Initially Hospitalized with Heart Failure and Acute Myocardial Infarction.
| Rank | Heart failure | Acute myocardial infarction |
|---|---|---|
|
| ||
| Readmission diagnosis (% of 30-day readmissions) |
Readmission diagnosis (% of 30-day readmissions) |
|
| 1 | Heart failure (35.22%) | Heart failure (19.27%) |
| 2 | Renal disorders (8.11%) | Acute myocardial infarction (9.95%) |
| 3 | Pneumonia (4.98%) | Renal disorders (5.28%) |
| 4 | Arrhythmias and conduction disorders (4.04%) |
Arrhythmias and conduction disorders (4.95%) |
| 5 | Septicemia/shock (3.55%) | Pneumonia (4.89%) |
| 6 | Cardiorespiratory failure (3.50%) | Chronic angina and coronary artery disease (4.85%) |
| 7 | Chronic obstructive pulmonary disease/asthma (3.14%) |
Septicemia/shock (3.96%) |
| 8 | Chronic angina and coronary artery disease (2.36%) |
Complications of care (3.86%) |
| 9 | Acute myocardial infarction (2.32%) | Cardio-respiratory failure (3.14%) |
| 10 | Complications of care (2.18%) | Gastrointestinal hemorrhage (3.09%) |
Table 2. Readmission Timing Among Older Patients Initially Hospitalized with Heart Failure or Acute Myocardial Infarction.
| Time period after discharge |
Heart failure | Acute myocardial infarction |
|---|---|---|
|
| ||
| Percentage of 30-day readmissions occurring during time period |
Percentage of 30-day readmissions occurring during time period |
|
| First 3 days | 13.4% | 19.1% |
| First 7 days | 31.4% | 40.1% |
| First 15 days | 61.0% | 67.6% |
This generalized and extended period of risk after hospital discharge has been termed post-hospital syndrome. This condition is an acquired and transient period of vulnerability after hospitalization during which the patient is susceptible to a wide range of medical disorders and events [13]. Why might this syndrome exist, and does it represent a coherent clinical condition? In addition to the acute illness resulting in hospitalization, hospital care and the hospital environment might contribute to post-hospital syndrome by imposing numerous additional stresses on the patient. These include unfamiliar environments, sleep disturbance and deprivation [77], imposed caloric restrictions [78], pain [79], increased immobility due to bed rest [80, 81], adverse effects of commonly used pharmacotherapies started in the hospital [82], nosocomial infections [83], and medical errors [84]. These stresses are frequently experienced by patients who are not only acutely ill, but who also have impaired physiologic reserves due to comorbid conditions and common geriatric syndromes such as cognitive impairment, functional disability, mobility impairment, and frailty. What is indisputable is that recently hospitalized patients are particularly vulnerable to both recurrent disease and secondary illnesses.
It follows that healthcare providers studying rehospitalization should focus not just on the initial condition triggering hospitalization and the transitional care process, but also on the factors during hospitalization and the early recovery period that contribute to further vulnerability after discharge. Our understanding of how to mitigate contributors to physiologic stress during hospitalization must improve. There are multiple potential therapeutic approaches to reduce these stresses that can be investigated. For example, one might experiment with personalizing the living experience while the patient is in the hospital by encouraging family members to bring in favorite and familiar items such as photos and bedding. One can minimize sleep disruptions by reducing nighttime awakenings for vital signs checks, blood draws, and other tests. One might reduce noise and light exposure by disabling unnecessary alarms and turning off lights. One can work doubly hard to improve nutritional intake by minimizing NPO orders and liberalizing caloric restrictions originally designed for younger and healthier outpatients. And one can focus on mobilizing patients as much as possible immediately upon hospital admission rather than just prior to discharge. These and many other interventions would be appropriate for study while the patient is hospitalized. After hospital discharge, one can direct additional resources to rebuilding physiologic reserves while thinking holistically about the patient rather than narrowly focusing on the specific disease process that resulted in the initial hospitalization. Patients likely require longitudinal attention rather than post-discharge care that is limited to a single ambulatory visit, as the risk of deterioration is high for at least the first full month after hospitalization.
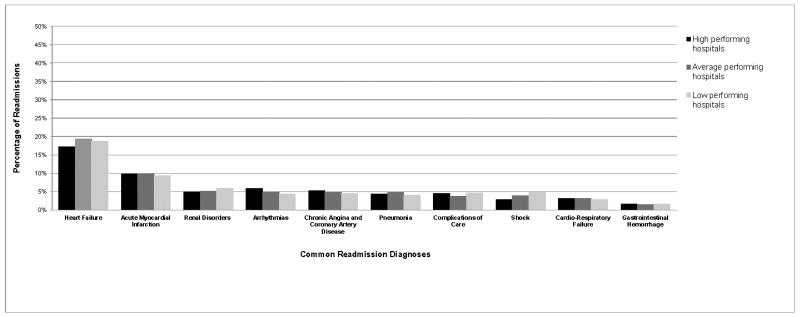
There is further evidence in favor of a broad and longitudinal approach to reducing readmissions from our examination of United States hospitals with the lowest 30-day readmission rates after hospitalization for HF and AMI [85]. Through the study of more than 4,000 acute care hospitals caring for Medicare beneficiaries, we found that the highest-performing institutions had achieved their especially low rates of rehospitalization by reducing readmissions across the entire spectrum of diagnostic categories throughout the month after discharge. These top-performing hospitals were not particularly good at reducing readmissions for specific diagnostic categories (e.g., renal disorders after HF hospitalization or bleeding complications after AMI) or specific time periods (e.g., the first or fourth weeks after hospital discharge). Rather, the highest performers had lowered readmissions for all diagnoses and time periods in the month after hospitalization. Representative data demonstrating similarity in readmission diagnoses across hospital performance groups for patients readmitted within 30 days of AMI is shown in Figure 1. This finding suggests that lower readmission rates might be best achieved through use of general strategies and capacities that lower readmission risk globally rather than for specific diagnoses or time periods after admission, as this approach would be consistent with readmission patterns at top hospitals in the United States.
Figure 1.
The 10 most common readmission diagnoses for high-, average-, and low-performing hospitals after initial hospitalization for acute myocardial infarction. Numbers are rounded to the nearest percentage. High-performing hospitals have 30-day risk standardized readmission rates lower than the national average rate, with >95% probability. Low-performing hospitals have 30-day risk standardized readmission rates higher than the national average rate, with >95% probability. All remaining hospitals are considered average.
Reproduced from Dharmarajan K, Hsieh AF, Lin Z, et al. Hospital readmission performance and patterns of readmission: retrospective cohort study of Medicare admissions. BMJ (Clinical research ed.). 2013;347:f6571, with permission from BMJ Publishing Group Ltd.
Ultimately, further characterization of the underlying cause of post-hospital syndrome will require additional complementary research from multiple disciplines. From an epidemiologic perspective, better understanding is needed of the length of time during which elevated risk persists, the rate at which risk declines over time, and the differences in risk trajectories by patient factors, including the initial admitting condition. From a physiologic perspective, additional insights are needed on the perturbations caused by acute illness and hospitalization on diverse biologic systems, including endocrine, immunologic, neurologic, and musculoskeletal domains. And at a more basic level, better understanding is required of the genetic and cellular factors that modulate the recovery process and increase or decrease specific patients’ risk of deterioration in the post-acute care period. This information may not only help in risk prediction but could possibly help guide targeted interventions that lessen preventable harms from hospitalization and hasten recovery after discharge.
Conclusions
Predictors and strategies to reduce 30-day readmission after hospitalization for HF and AMI remain limited. Consistent markers of risk, effective discriminative models for risk stratification, and generalizable knowledge on effective interventions have yet to be determined. In light of these challenges, it has become clear that readmission may not be readily explained by simple deterministic understanding of risk. Alternative frameworks are needed. The post-hospital syndrome describes a period of vulnerability, and is in need of further explication.
As we move forward, an integrated view of patient risk and recovery after hospitalization is needed, one that incorporates a deep understandings of baseline health, the acute illness process, hospitalization, and the transition to the community or post-acute care facility. With older patients in particular, we will need to understand how common geriatric conditions and syndromes relate to care needs during each step within this continuum. Ultimately, this desire to improve care across geographic and institutional boundaries should promote patient-centered outcomes that are more longitudinal and enduring rather than restricted to the hospital.
Acknowledgements
Dr. Dharmarajan is supported by grant K23AG048331-01 from the National Institute on Aging and the American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. Dr. Krumholz is supported by grant 1U01HL105270-04 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not represent the official views of the NIA or NHLBI.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Kumar Dharmarajan declares that he has no conflict of interest.
Harlan M. Krumholz works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. Dr. Krumholz is also the chair of a cardiac scientific advisory board for UnitedHealth and is the recipient of research grants from both Johnson & Johnson and Medtronic, through Yale University, to develop methods of clinical trial data-sharing.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
Kumar Dharmarajan, Section of Cardiovascular Medicine, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT; Center for Outcomes Research and Evaluation, Yale-New Haven Hospital, New Haven, CT.
Harlan M. Krumholz, Section of Cardiovascular Medicine, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT; Center for Outcomes Research and Evaluation, Yale-New Haven Hospital, New Haven, CT; Robert Wood Johnson Foundation Clinical Scholars Program, Department of Internal Medicine, Yale University School of Medicine, New Haven, CT; Department of Health Policy and Management, Yale University School of Public Health, New Haven, CT.
References
Papers of particular interest, published recently, have been highlighted as:
*Of importance
**Of major importance
- 1**.Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA: the journal of the American Medical Association. 2013 Jan 23;309(4):355–363. doi: 10.1001/jama.2012.216476. This paper describes the clinical epidemiology of 30-day readmissions after hospitalization for three common cardiopulmonary conditions and finds that older patients are vulnerable to readmission from a broad spectrum of conditions for the entire month after hospital discharge.
- 2.Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circulation. Cardiovascular quality and outcomes. 2009 Sep;2(5):407–413. doi: 10.1161/CIRCOUTCOMES.109.883256. [DOI] [PubMed] [Google Scholar]
- 3.Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure. Update on publicly reported outcomes measures based on the 2010 release. Circulation. Cardiovascular quality and outcomes. 2010 Sep;3(5):459–467. doi: 10.1161/CIRCOUTCOMES.110.957613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circulation. Heart failure. 2010 Jan;3(1):97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Medicare & Medicaid Services . Medicare Hospital Quality Chartbook 2011: Performance Report on Readmission Measures for Acute Myocardial Infarction, Heart Failure, and Pneumonia. Centers for Medicare & Medicaid Services; Washington, DC: 2011. [Google Scholar]
- 6.Centers for Medicare & Medicaid Services . Medicare Hospital Quality Chartbook 2012: Performance Report on Outcome Measures. Centers for Medicare & Medicaid Services; Washington, DC: 2012. [Google Scholar]
- 7.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. The New England journal of medicine. 2009 Apr 2;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 8.Ashton CM, Kuykendall DH, Johnson ML, Wray NP, Wu L. The association between the quality of inpatient care and early readmission. Annals of internal medicine. 1995 Mar 15;122(6):415–421. doi: 10.7326/0003-4819-122-6-199503150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Ashton CM, Wray NP. A conceptual framework for the study of early readmission as an indicator of quality of care. Social science & medicine (1982) 1996 Dec;43(11):1533–1541. doi: 10.1016/s0277-9536(96)00049-4. [DOI] [PubMed] [Google Scholar]
- 10.Ashton CM, Del Junco DJ, Souchek J, Wray NP, Mansyur CL. The association between the quality of inpatient care and early readmission: a meta-analysis of the evidence. Medical care. 1997 Oct;35(10):1044–1059. doi: 10.1097/00005650-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 11.van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011 Apr 19;183(7):E391–402. doi: 10.1503/cmaj.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kocher RP, Adashi EY. Hospital readmissions and the Affordable Care Act: paying for coordinated quality care. JAMA: the journal of the American Medical Association. 2011 Oct 26;306(16):1794–1795. doi: 10.1001/jama.2011.1561. [DOI] [PubMed] [Google Scholar]
- 13**.Krumholz HM. Post-hospital syndrome—an acquired, transient condition of generalized risk. The New England journal of medicine. 2013 Jan 10;368(2):100–102. doi: 10.1056/NEJMp1212324. This perspective piece introduces the concept of the post-hospital syndrome and its implications for both hospital care and transitional care.
- 14.Ross JS, Mulvey GK, Stauffer B, et al. Statistical models and patient predictors of readmission for heart failure: a systematic review. Archives of internal medicine. 2008 Jul 14;168(13):1371–1386. doi: 10.1001/archinte.168.13.1371. [DOI] [PubMed] [Google Scholar]
- 15.Desai MM, Stauffer BD, Feringa HH, Schreiner GC. Statistical models and patient predictors of readmission for acute myocardial infarction: a systematic review. Circulation. Cardiovascular quality and outcomes. 2009 Sep;2(5):500–507. doi: 10.1161/CIRCOUTCOMES.108.832949. [DOI] [PubMed] [Google Scholar]
- 16.Dodson JA, Truong TT, Towle VR, Kerins G, Chaudhry SI. Cognitive impairment in older adults with heart failure: prevalence, documentation, and impact on outcomes. The American journal of medicine. 2013 Feb;126(2):120–126. doi: 10.1016/j.amjmed.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. Journal of general internal medicine. 2013 Feb;28(2):269–282. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic status, Medicaid coverage, clinical comorbidity, and rehospitalization or death after an incident heart failure hospitalization: Atherosclerosis Risk in Communities cohort (1987 to 2004) Circulation. Heart failure. 2011 May;4(3):308–316. doi: 10.1161/CIRCHEARTFAILURE.110.959031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenauer PK, Lagu T, Rothberg MB, et al. Income inequality and 30 day outcomes after acute myocardial infarction, heart failure, and pneumonia: retrospective cohort study. BMJ (Clinical research ed.) 2013;346:f521. doi: 10.1136/bmj.f521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA: the journal of the American Medical Association. 2011 Feb 16;305(7):675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blum AB, Egorova NN, Sosunov EA, et al. Impact of socioeconomic status measures on hospital profiling in new york city. Circulation. Cardiovascular quality and outcomes. 2014 May;7(3):391–397. doi: 10.1161/CIRCOUTCOMES.113.000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernheim SM. Measuring quality and enacting policy: readmission rates and socioeconomic factors. Circulation. Cardiovascular quality and outcomes. 2014 May;7(3):350–352. doi: 10.1161/CIRCOUTCOMES.114.001037. [DOI] [PubMed] [Google Scholar]
- 23.Ross JS, Bernheim SM, Lin Z, et al. Based on key measures, care quality for Medicare enrollees at safety-net and non-safety-net hospitals was almost equal. Health affairs (Project Hope) 2012 Aug;31(8):1739–1748. doi: 10.1377/hlthaff.2011.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jha AK, Orav EJ, Epstein AM. Public reporting of discharge planning and rates of readmissions. The New England journal of medicine. 2009 Dec 31;361(27):2637–2645. doi: 10.1056/NEJMsa0904859. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA: the journal of the American Medical Association. 2010 May 5;303(17):1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 26.Hess CN, Shah BR, Peng SA, Thomas L, Roe MT, Peterson ED. Association of early physician follow-up and 30-day readmission after non-ST-segment-elevation myocardial infarction among older patients. Circulation. 2013 Sep 10;128(11):1206–1213. doi: 10.1161/CIRCULATIONAHA.113.004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashiwagi DT, Burton MC, Kirkland LL, Cha S, Varkey P. Do timely outpatient follow-up visits decrease hospital readmission rates? American journal of medical quality: the official journal of the American College of Medical Quality. 2012 Jan-Feb;27(1):11–15. doi: 10.1177/1062860611409197. [DOI] [PubMed] [Google Scholar]
- 28.Bell CM, Schnipper JL, Auerbach AD, et al. Association of communication between hospital-based physicians and primary care providers with patient outcomes. Journal of general internal medicine. 2009 Mar;24(3):381–386. doi: 10.1007/s11606-008-0882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. Journal of general internal medicine. 2002 Mar;17(3):186–192. doi: 10.1046/j.1525-1497.2002.10741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz LI, Jenq GY, Brewster UC, et al. Comprehensive quality of discharge summaries at an academic medical center. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2013 Aug;8(8):436–443. doi: 10.1002/jhm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joynt KE, Jha AK. Who has higher readmission rates for heart failure, and why? Implications for efforts to improve care using financial incentives. Circulation. Cardiovascular quality and outcomes. 2011 Jan 1;4(1):53–59. doi: 10.1161/CIRCOUTCOMES.110.950964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McHugh MD, Ma C. Hospital nursing and 30-day readmissions among Medicare patients with heart failure, acute myocardial infarction, and pneumonia. Medical care. 2013 Jan;51(1):52–59. doi: 10.1097/MLR.0b013e3182763284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA: the journal of the American Medical Association. 2003 Nov 19;290(19):2581–2587. doi: 10.1001/jama.290.19.2581. [DOI] [PubMed] [Google Scholar]
- 34.Fine MJ, Auble TE, Yealy DM, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. The New England journal of medicine. 1997 Jan 23;336(4):243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 35.Hersh AM, Masoudi FA, Allen LA. Postdischarge environment following heart failure hospitalization: expanding the view of hospital readmission. Journal of the American Heart Association. 2013 Apr;2(2):e000116. doi: 10.1161/JAHA.113.000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hosmer DW, Lemeshow S, editors. Applied Logistic Regression. 2nd Edition John Wiley & Sons; New York, NY: 2000. [Google Scholar]
- 37.Amarasingham R, Moore BJ, Tabak YP, et al. An automated model to identify heart failure patients at risk for 30-day readmission or death using electronic medical record data. Medical care. 2010 Nov;48(11):981–988. doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 38.Tabak YP, Johannes RS, Silber JH. Using automated clinical data for risk adjustment: development and validation of six disease-specific mortality predictive models for pay-for-performance. Medical care. 2007 Aug;45(8):789–805. doi: 10.1097/MLR.0b013e31803d3b41. [DOI] [PubMed] [Google Scholar]
- 39.Hammill BG, Curtis LH, Fonarow GC, et al. Incremental value of clinical data beyond claims data in predicting 30-day outcomes after heart failure hospitalization. Circulation. Cardiovascular quality and outcomes. 2011 Jan 1;4(1):60–67. doi: 10.1161/CIRCOUTCOMES.110.954693. [DOI] [PubMed] [Google Scholar]
- 40.Hummel SL, Katrapati P, Gillespie BW, Defranco AC, Koelling TM. Impact of Prior Admissions on 30-Day Readmissions in Medicare Heart Failure Inpatients. Mayo Clinic proceedings. 2014 Mar 29; doi: 10.1016/j.mayocp.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philbin EF, DiSalvo TG. Prediction of hospital readmission for heart failure: development of a simple risk score based on administrative data. Journal of the American College of Cardiology. 1999 May;33(6):1560–1566. doi: 10.1016/s0735-1097(99)00059-5. [DOI] [PubMed] [Google Scholar]
- 42.Krumholz HM, Chen YT, Wang Y, Vaccarino V, Radford MJ, Horwitz RI. Predictors of readmission among elderly survivors of admission with heart failure. American heart journal. 2000 Jan;139(1 Pt 1):72–77. doi: 10.1016/s0002-8703(00)90311-9. [DOI] [PubMed] [Google Scholar]
- 43.Yamokoski LM, Hasselblad V, Moser DK, et al. Prediction of rehospitalization and death in severe heart failure by physicians and nurses of the ESCAPE trial. Journal of cardiac failure. 2007 Feb;13(1):8–13. doi: 10.1016/j.cardfail.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 44.Chin MH, Goldman L. Correlates of early hospital readmission or death in patients with congestive heart failure. The American journal of cardiology. 1997 Jun 15;79(12):1640–1644. doi: 10.1016/s0002-9149(97)00214-2. [DOI] [PubMed] [Google Scholar]
- 45.Au AG, McAlister FA, Bakal JA, Ezekowitz J, Kaul P, van Walraven C. Predicting the risk of unplanned readmission or death within 30 days of discharge after a heart failure hospitalization. American heart journal. 2012 Sep;164(3):365–372. doi: 10.1016/j.ahj.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Felker GM, Leimberger JD, Califf RM, et al. Risk stratification after hospitalization for decompensated heart failure. Journal of cardiac failure. 2004 Dec;10(6):460–466. doi: 10.1016/j.cardfail.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Krumholz HM, Lin Z, Drye EE, et al. An administrative claims measure suitable for profiling hospital performance based on 30-day all-cause readmission rates among patients with acute myocardial infarction. Circulation. Cardiovascular quality and outcomes. 2011 Mar;4(2):243–252. doi: 10.1161/CIRCOUTCOMES.110.957498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA: the journal of the American Medical Association. 2011 Oct 19;306(15):1688–1698. doi: 10.1001/jama.2011.1515. This paper describes risk prediction models for readmission both within and after the first 30 days after hospitalization.
- 49.Keenan PS, Normand SL, Lin Z, et al. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circulation. Cardiovascular quality and outcomes. 2008 Sep;1(1):29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 50.Kansagara D, Englander H, Salanitro A, et al. Risk Prediction Models for Hospital Readmission: A Systematic Review. Washington, DC: 2011. [PubMed] [Google Scholar]
- 51.Coleman EA, Min SJ, Chomiak A, Kramer AM. Posthospital care transitions: patterns, complications, and risk identification. Health services research. 2004 Oct;39(5):1449–1465. doi: 10.1111/j.1475-6773.2004.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Curry LA, Spatz E, Cherlin E, et al. What distinguishes top-performing hospitals in acute myocardial infarction mortality rates? A qualitative study. Annals of internal medicine. 2011 Mar 15;154(6):384–390. doi: 10.7326/0003-4819-154-6-201103150-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jaarsma T, Halfens R, Huijer Abu-Saad H, et al. Effects of education and support on self-care and resource utilization in patients with heart failure. European heart journal. 1999 May;20(9):673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 54.Rainville EC. Impact of pharmacist interventions on hospital readmissions for heart failure. American journal of health-system pharmacy: AJHP: official journal of the American Society of Health-System Pharmacists. 1999 Jul 1;56(13):1339–1342. [PubMed] [Google Scholar]
- 55.McDonald K, Ledwidge M, Cahill J, et al. Elimination of early rehospitalization in a randomized, controlled trial of multidisciplinary care in a high-risk, elderly heart failure population: the potential contributions of specialist care, clinical stability and optimal angiotensin-converting enzyme inhibitor dose at discharge. European journal of heart failure. 2001 Mar;3(2):209–215. doi: 10.1016/s1388-9842(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 56.Krumholz HM, Currie PM, Riegel B, et al. A taxonomy for disease management: a scientific statement from the American Heart Association Disease Management Taxonomy Writing Group. Circulation. 2006 Sep 26;114(13):1432–1445. doi: 10.1161/CIRCULATIONAHA.106.177322. [DOI] [PubMed] [Google Scholar]
- 57.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA: the journal of the American Medical Association. 2004 Mar 17;291(11):1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 58.Sochalski J, Jaarsma T, Krumholz HM, et al. What works in chronic care management: the case of heart failure. Health affairs (Project Hope) 2009 Jan-Feb;28(1):179–189. doi: 10.1377/hlthaff.28.1.179. [DOI] [PubMed] [Google Scholar]
- 59.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. The New England journal of medicine. 2010 Dec 9;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kotowycz MA, Cosman TL, Tartaglia C, Afzal R, Syal RP, Natarajan MK. Safety and feasibility of early hospital discharge in ST-segment elevation myocardial infarction—a prospective and randomized trial in low-risk primary percutaneous coronary intervention patients (the Safe-Depart Trial) American heart journal. 2010 Jan;159(1):117, e111–116. doi: 10.1016/j.ahj.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 61.Robertson KA, Kayhko K. Cost analysis of an intensive home follow-up program for first-time post-myocardial infarction patients and their families. Dynamics (Pembroke, Ont.) 2001 Winter;12(4):25–31. [PubMed] [Google Scholar]
- 62.Young W, Rewa G, Goodman SG, et al. Evaluation of a community-based inner-city disease management program for postmyocardial infarction patients: a randomized controlled trial. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2003 Oct 28;169(9):905–910. [PMC free article] [PubMed] [Google Scholar]
- 63.Costa e Silva R, Pellanda L, Portal V, Maciel P, Furquim A, Schaan B. Transdisciplinary approach to the follow-up of patients after myocardial infarction. Clinics (Sao Paulo, Brazil) 2008 Aug;63(4):489–496. doi: 10.1590/S1807-59322008000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinclair AJ, Conroy SP, Davies M, Bayer AJ. Post-discharge home-based support for older cardiac patients: a randomised controlled trial. Age and ageing. 2005 Jul;34(4):338–343. doi: 10.1093/ageing/afi116. [DOI] [PubMed] [Google Scholar]
- 65.Hanssen TA, Nordrehaug JE, Eide GE, Hanestad BR. Does a telephone follow-up intervention for patients discharged with acute myocardial infarction have long-term effects on health-related quality of life? A randomised controlled trial. Journal of clinical nursing. 2009 May;18(9):1334–1345. doi: 10.1111/j.1365-2702.2008.02654.x. [DOI] [PubMed] [Google Scholar]
- 66.Prvu Bettger J, Alexander KP, Dolor RJ, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Annals of internal medicine. 2012 Sep 18;157(6):407–416. doi: 10.7326/0003-4819-157-6-201209180-00004. [DOI] [PubMed] [Google Scholar]
- 67*.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Annals of internal medicine. 2011 Oct 18;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. This article summarizes the broad spectrum of interventions tested to lower 30-day readmissions following hospitalization for a wide range of medical conditions.
- 68.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Archives of internal medicine. 2006 Sep 25;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 69.Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Annals of internal medicine. 1994 Jun 15;120(12):999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 70.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Annals of internal medicine. 2009 Feb 3;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Annals of internal medicine. 2012 Sep 18;157(6):417–428. doi: 10.7326/0003-4819-157-6-201209180-00006. [DOI] [PubMed] [Google Scholar]
- 72.Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy: a systematic review. Annals of internal medicine. 2013 Mar 5;158(5 Pt 2):433–440. doi: 10.7326/0003-4819-158-5-201303051-00011. [DOI] [PubMed] [Google Scholar]
- 73.Shepperd S, Lannin NA, Clemson LM, McCluskey A, Cameron ID, Barras SL. Discharge planning from hospital to home. The Cochrane database of systematic reviews. 2013;1:CD000313. doi: 10.1002/14651858.CD000313.pub4. [DOI] [PubMed] [Google Scholar]
- 74.Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circulation. Cardiovascular quality and outcomes. 2013 Jul;6(4):444–450. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kociol RD, Peterson ED, Hammill BG, et al. National survey of hospital strategies to reduce heart failure readmissions: findings from the Get With the Guidelines–Heart Failure registry. Circulation. Heart failure. 2012 Nov;5(6):680–687. doi: 10.1161/CIRCHEARTFAILURE.112.967406. [DOI] [PubMed] [Google Scholar]
- 76.Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA: the journal of the American Medical Association. 2014 Jun 4;311(21):2169–70. doi: 10.1001/jama.2014.3695. [DOI] [PubMed] [Google Scholar]
- 77.Yoder JC, Staisiunas PG, Meltzer DO, Knutson KL, Arora VM. Noise and sleep among adult medical inpatients: far from a quiet night. Archives of internal medicine. 2012 Jan 9;172(1):68–70. doi: 10.1001/archinternmed.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sullivan DH, Sun S, Walls RC. Protein-energy undernutrition among elderly hospitalized patients: a prospective study. JAMA: the journal of the American Medical Association. 1999 Jun 2;281(21):2013–2019. doi: 10.1001/jama.281.21.2013. [DOI] [PubMed] [Google Scholar]
- 79.Donovan M, Dillon P, McGuire L. Incidence and characteristics of pain in a sample of medical-surgical inpatients. Pain. 1987 Jul;30(1):69–78. doi: 10.1016/0304-3959(87)90084-4. [DOI] [PubMed] [Google Scholar]
- 80.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA: the journal of the American Medical Association. 2007 Apr 25;297(16):1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 81.Gill TM, Allore HG, Holford TR, Guo Z. Hospitalization, restricted activity, and the development of disability among older persons. JAMA: the journal of the American Medical Association. 2004 Nov 3;292(17):2115–2124. doi: 10.1001/jama.292.17.2115. [DOI] [PubMed] [Google Scholar]
- 82.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011 Mar 3;364(9):797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Loo VG, Bourgault AM, Poirier L, et al. Host and pathogen factors for Clostridium difficile infection and colonization. The New England journal of medicine. 2011 Nov 3;365(18):1693–1703. doi: 10.1056/NEJMoa1012413. [DOI] [PubMed] [Google Scholar]
- 84.Brennan TA, Leape LL, Laird NM, et al. Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. The New England journal of medicine. 1991 Feb 7;324(6):370–376. doi: 10.1056/NEJM199102073240604. [DOI] [PubMed] [Google Scholar]
- 85*.Dharmarajan K, Hsieh AF, Lin Z, et al. Hospital readmission performance and patterns of readmission: retrospective cohort study of Medicare admissions. BMJ (Clinical research ed.) 2013;347:f6571. doi: 10.1136/bmj.f6571. This article describes readmission patterns at hospitals in the United States with the highest and lowest 30-day readmission rates and finds that top-performing hospitals have lowered readmissions broadly across diagnostic categories and time periods after hospitalization.