Inflammation is a fundamental protective response in higher eukaryotes to a variety of external stimuli such as environmental toxins, pathogens, or allergens. These stimuli are encountered by immune cells such as mast cells (MCs), which mediate the initial defense reaction against external “invaders”. MCs reside throughout vascularized tissues and are especially prominent near body surfaces defining the border between the external and internal environments. MCs are considered the main “effector” cells in allergic disorders and tissue remodeling. However, more recent evidence suggests that MCs are also involved in inflammatory reactions associated with obesity, atherosclerosis, autoimmune disorders, and cancer (1). MCs derive from hematopoietic stem cells, circulate and migrate as precursors, and terminally mature in their target tissues (2). There, MCs reside as quiescent cells until activated by antigen-presenting immunoglobulin E or other nonimmunological stimuli. This triggers the release of various effector molecules from MCs, which mediate the immune response (3). These mediators include histamine, proteases, various growth factors, and chemokines, which are stored in cytosolic granules in quiescent MCs and are acutely secreted upon activation. Additionally, MC activation triggers de novo synthesis of lipid-derived signaling molecules. The most prominent are eicosanoids comprising leukotrienes and prostaglandins, which are synthesized by lipoxygenases and cyclooxygenases (COX), respectively (4). The common precursor of all eicosanoids is the polyunsaturated omega-6 FA arachidonic acid (20:4 ω6, AA).
The bulk of cellular AA is found in membrane glycerophospholipids predominantly esterified to the sn-2 position on the glycerol backbone (5). In the classical synthesis pathway for eicosanoids, phospholipases A2 (PLA2) hydrolyze the ester bond to release AA. The mammalian PLA2 superfamily comprises more than 30 enzymes subdivided in six groups (6). Group IVA PLA2 (cPLA2α) is considered to be the primary phospholipase for AA release in mice and in humans (Fig. 1). The fact that cPLA2α deficiency leads to attenuation, but not loss, of eicosanoid production suggests that other PLA2s also contribute to relevant PLA2 activity (7, 8). An alternative pathway for the provision of AA involves the sequential degradation of glycerophospholipids by phospholipase C (PLC) and diacylglycerol lipases (DAGL) to generate 2-arachidonoyl-glycerol (2-AG) (9). 2-AG is then cleaved by monoacyglycerol lipase (MGL) producing free glycerol and AA (10) (Fig. 1). Pharmacological or genetic inactivation of MGL in mice proved its critical role in inflammation (11, 12). Additionally, 2-AG can undergo direct oxygenation by COX-2 without prior hydrolysis resulting in prostaglandin glycerol esters with distinct biological activities (13). Apparently, the pathways responsible for AA release differ in a tissue- and cell-specific manner.
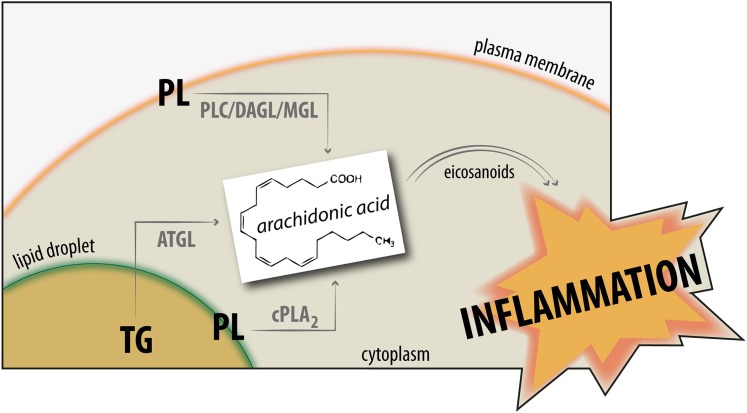
Fig. 1.
Schematic model of arachidonic acid (AA) release and action. Membrane-bound phospholipids (PLs) are hydrolyzed by enzymes of the PLA2 superfamily releasing free AA for subsequent eicosanoid production, which then mediates the inflammation response. AA may also be generated by alternative pathways: phospholipase C (PLC) and diacylglycerol lipase (DAGL) generate 2-arachidonoyl-glycerol (2-AG), which is degraded by monoacylglycerol lipase (MGL) releasing AA. Alternatively, triacylglycerols (TGs) stored within cytosolic lipid droplets are hydrolyzed by adipose triglyceride lipase (ATGL) and release AA. Apparently, the pathway that is responsible for AA release along the PLC/DAGL/MGL route is most prominent in the brain and the liver, while the pathway along the ATGL route may predominate in TG-rich lipid droplet-containing inflammatory cells, such as mast cells.
In the current issue of The Journal of Lipid Research, Dichlberger et al. (14) propose a third pathway for the provision of AA for eicosanoid biosynthesis. The authors show that adipose triglyceride lipase (ATGL)-mediated hydrolysis of lipid droplet (LD)-associated TGs is crucially involved in the production of nonesterified AA in MCs (Fig. 1). Silencing of ATGL in human MCs caused an increase in neutral lipids in LDs and a concomitant decrease in eicosanoid production similar to silencing of cPLA2α. ATGL belongs to a protein family of nine members containing a patatin domain. Several members of this family are phospholipases, which led to the family designation patatin-like phospholipase domain-containing protein A1 to A9 (PNPLA1-9) (15). Unlike other family members, ATGL preferentially hydrolyzes TGs playing a crucial role in TG catabolism (lipolysis) in adipose and many nonadipose tissues (16, 17). ATGL requires a coactivator termed comparative gene identification 58 (CGI-58) for maximal TG hydrolase activity (18). In addition to its TG hydrolase activity, ATGL also exhibits low but detectable phospholipase A2 activity as well as transacylase activity (19, 20). While classical metabolic lipases are characterized by a catalytic triad in the active site and predominantly hydrolyze FA at the sn-1(3) position of TGs, ATGL comprises a catalytic dyad, and the enzyme preferentially cleaves acyl-chains at the sn-2 position similar to its PNPLA relatives with PLA2 activity (21). The substrate selectivity of ATGL extends to the sn-1 position upon activation by its coactivator CGI-58. ATGL catalyzes the hydrolysis of saturated and unsaturated long-chain FAs from TGs in vitro with a slight preference for unsaturated FAs in vivo.
Interestingly, Dichlberger and colleagues have previously shown that a considerable amount of AA is found esterified in the TG pool of human MCs (22). The current study now provides compelling evidence that ATGL releases AA from LD-associated TGs as a crucial step for eicosanoid biosynthesis in MCs. This is provocative, because it underscores a crucial role of neutral lipid metabolism in the function of immune cells. It adds to emerging evidence of an important contribution of LDs and LD metabolism in various cell types of the immune system including macrophages, granulocytes, lymphocytes, and MCs (5). For example, AA converting lipoxygenases and COX as well as other enzymes involved in eicosanoid biosynthesis have been shown to locate to LDs. Thus, LDs are considered to represent a site of prostaglandin synthesis. ATGL and CGI-58 are expressed in all these cells suggesting a functional role of lipolysis, but few data are available on their role in inflammation. In macrophages, ATGL deficiency impairs phagocytosis and attenuates the development of atherosclerosis (23, 24). The role of ATGL in other immune cells has not yet been addressed. Humans with a mutation in the gene coding for ATGL (PNPLA2) are diagnosed by neutral lipid accumulation in granulocytes and lymphocytes (Jordans anomaly) (25). Yet, whether and how this affects the immune system of affected individuals is not known.
In MCs, additional mechanistic studies are required to clarify whether ATGL-generated AA can be directly utilized for eicosanoid biosynthesis or whether preceding AA reesterification into glycerophospholipids and subsequent rerelease by cPLA2α is required before oxygenation. Additionally, it has to be shown whether the TG hydrolase of ATGL accounts for AA release in MCs, as hypothesized, or whether ATGL’s minor phospholipase activity contributes to AA production. Future work will also have to address whether loss of ATGL in animal models either by genetic or pharmacologic inactivation of the enzyme affects AA release, eicosanoid production, and inflammation. ATGL-deficient mice may be employed in various inflammatory disease models, such as cancer, to provide further insights into the biological significance of this enzyme not only in MCs but also in other inflammatory/immune cells.
In summary, evidence emerges that lipolysis meets inflammation: besides their established role in energy metabolism, neutral lipid hydrolases also may participate in inflammatory signaling processes. Dichlberger et al. provide the first evidence that ATGL plays such a role triggering inflammation via MCs.
Acknowledgments
The authors thank Robert Zimmermann and Ulrike Taschler for discussion and Thomas O. Eichmann for figure preparation.
Footnotes
This work was supported by the SFB LIPOTOX F30 funded by the Austrian Science Fund (FWF).
References
- 1.Galli S. J., Grimbaldeston M., Tsai M. 2008. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat. Rev. Immunol. 8: 478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maaninka K., Lappalainen J., Kovanen P. T. 2013. Human mast cells arise from a common circulating progenitor. J. Allergy Clin. Immunol. 132: 463–9.e3. [DOI] [PubMed] [Google Scholar]
- 3.Theoharides T. C., Kempuraj D., Tagen M., Conti P., Kalogeromitros D. 2007. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 217: 65–78. [DOI] [PubMed] [Google Scholar]
- 4.Boyce J. A. 2007. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 217: 168–185. [DOI] [PubMed] [Google Scholar]
- 5.Bozza P. T., Bakker-Abreu I., Navarro-Xavier R. A., Bandeira-Melo C. 2011. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot. Essent. Fatty Acids. 85: 205–213. [DOI] [PubMed] [Google Scholar]
- 6.Dennis E. A., Cao J., Hsu Y-H., Magrioti V., Kokotos G. 2011. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 111: 6130–6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uozumi N., Shimizu T. 2002. Roles for cytosolic phospholipase A2alpha as revealed by gene-targeted mice. Prostaglandins Other Lipid Mediat. 68–69: 59–69. [DOI] [PubMed] [Google Scholar]
- 8.Adler D. H., Cogan J. D., Phillips J. A., Schnetz-Boutaud N., Milne G. L., Iverson T., Stein J. A., Brenner D. A., Morrow J. D., Boutaud O., et al. 2008. Inherited human cPLA(2alpha) deficiency is associated with impaired eicosanoid biosynthesis, small intestinal ulceration, and platelet dysfunction. J. Clin. Invest. 118: 2121–2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao Y., Vasilyev D. V., Goncalves M. B., Howell F. V., Hobbs C., Reisenberg M., Shen R., Zhang M-Y., Strassle B. W., Lu P., et al. 2010. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J. Neurosci. 30: 2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinh T. P., Carpenter D., Leslie F. M., Freund T. F., Katona I., Sensi S. L., Kathuria S., Piomelli D. 2002. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc. Natl. Acad. Sci. USA. 99: 10819–10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nomura D. K., Morrison B. E., Blankman J. L., Long J. Z., Kinsey S. G., Marcondes M. C. G., Ward A. M., Hahn Y. K., Lichtman A. H., Conti B., et al. 2011. Endocannabinoid hydrolysis generates brain prostaglandins that promote neuroinflammation. Science. 334: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao Z., Mulvihill M. M., Mukhopadhyay P., Xu H., Erdélyi K., Hao E., Holovac E., Haskó G., Cravatt B. F., Nomura D. K., Pacher P. 2013. Monoacylglycerol lipase controls endocannabinoid and eicosanoid signaling and hepatic injury in mice. Gastroenterology. 144: 808–817.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sang N., Zhang J., Chen C. 2006. PGE2 glycerol ester, a COX-2 oxidative metabolite of 2-arachidonoyl glycerol, modulates inhibitory synaptic transmission in mouse hippocampal neurons. J. Physiol. 572: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dichlberger A., Schlager S., Maaninka K., Schneider W. J., Kovanen P. T. 2014. Adipose triglyceride lipase regulates eicosanoid production in activated human mast cells. J. Lipid Res. 55: 2471–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kienesberger P. C., Oberer M., Lass A., Zechner R. 2009. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J. Lipid Res. 50(Suppl): S63–S68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 17.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., et al. 2006. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 312: 734–737. [DOI] [PubMed] [Google Scholar]
- 18.Lass A., Zimmermann R., Haemmerle G., Riederer M., Schoiswohl G., Schweiger M., Kienesberger P., Strauss J. G., Gorkiewicz G., Zechner R. 2006. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 3: 309–319. [DOI] [PubMed] [Google Scholar]
- 19.Notari L., Baladron V., Aroca-Aguilar J. D., Balko N., Heredia R., Meyer C., Notario P. M., Saravanamuthu S., Nueda M. L., Sanchez-Sanchez F., et al. 2006. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J. Biol. Chem. 281: 38022–38037. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. 2004. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 279: 48968–48975. [DOI] [PubMed] [Google Scholar]
- 21.Eichmann T. O., Kumari M., Haas J. T., Farese R. V., Jr, Zimmermann R., Lass A., Zechner R. 2012. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 287: 41446–41457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dichlberger A., Schlager S., Lappalainen J., Käkelä R., Hattula K., Butcher S. J., Schneider W. J., Kovanen P. T. 2011. Lipid body formation during maturation of human mast cells. J. Lipid Res. 52: 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., et al. 2010. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285: 20192–20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lammers B., Chandak P. G., Aflaki E., Van Puijvelde G. H. M., Radovic B., Hildebrand R. B., Meurs I., Out R., Kuiper J., Van Berkel T. J. C., et al. 2011. Macrophage adipose triglyceride lipase deficiency attenuates atherosclerotic lesion development in low-density lipoprotein receptor knockout mice. Arterioscler. Thromb. Vasc. Biol. 31: 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schweiger M., Lass A., Zimmermann R., Eichmann T. O., Zechner R. 2009. Neutral lipid storage disease: genetic disorders caused by mutations in adipose triglyceride lipase/PNPLA2 or CGI-58/ABHD5. Am. J. Physiol. Endocrinol. Metab. 297: E289–E296. [DOI] [PubMed] [Google Scholar]