Abstract
Human mast cells (MCs) contain TG-rich cytoplasmic lipid droplets (LDs) with high arachidonic acid (AA) content. Here, we investigated the functional role of adipose TG lipase (ATGL) in TG hydrolysis and the ensuing release of AA as substrate for eicosanoid generation by activated human primary MCs in culture. Silencing of ATGL in MCs by siRNAs induced the accumulation of neutral lipids in LDs. IgE-dependent activation of MCs triggered the secretion of the two major eicosanoids, prostaglandin D2 (PGD2) and leukotriene C4 (LTC4). The immediate release of PGD2 from the activated MCs was solely dependent on cyclooxygenase (COX) 1, while during the delayed phase of lipid mediator production, the inducible COX-2 also contributed to its release. Importantly, when ATGL-silenced MCs were activated, the secretion of both PGD2 and LTC4 was significantly reduced. Interestingly, the inhibitory effect on the release of LTC4 was even more pronounced in ATGL-silenced MCs than in cytosolic phospholipase A2-silenced MCs. These data show that ATGL hydrolyzes AA-containing TGs present in human MC LDs and define ATGL as a novel regulator of the substrate availability of AA for eicosanoid generation upon MC activation.
Keywords: arachidonic acid, leukotrienes, lipid droplets, lipolysis and fatty acid metabolism, phospholipases/A2, prostaglandins
Mast cells (MCs), traditionally known as potent effector cells in IgE-dependent allergic disorders (1), also exert a wide range of pathophysiological functions in inflammatory diseases including atherosclerosis, rheumatoid arthritis, and asthma (2). For the MCs to act as disease-causing or disease-modifying effector cells, they need to be activated. Upon activation by immunological (classically IgE-mediated) or nonimmunological stimuli, MCs immediately exocytose a fraction of their cytoplasmic secretory granules, which contain a host of biologically active preformed mediators, notably histamine, heparin, and serine neutral proteases (3, 4). MC activation also initiates the synthesis of lipid mediators, cytokines, and chemokines, which are released from the cytoplasmic nongranule compartments of the activated MCs within minutes to hours (5).
The lipid mediators secreted by activated MCs include prostaglandins (PGs), leukotrienes (LTs), and platelet-activating factor, together referred to as eicosanoids (i.e., molecules derived from the oxidative metabolism of arachidonic acid [AA]) (5). As a source of endogenous AA, MCs utilize various glycerolipid pools. Traditionally, the major glycerolipid pool of AA within MCs, as in other inflammatory cells, has been assumed to reside in the membrane phospholipids (PLs) in which AA is mainly esterified in the sn-2 position and is released from them by members of the phospholipase family, in particular by group IV cytosolic phospholipase A2 (cPLA2) (6). Interestingly, human lung MCs have been found to contain large amounts of AA in their cytoplasmic TG-rich lipid droplets (LDs) (7), and, in fact, ∼50% of the total cellular AA in these cells is esterified into TGs (8). We have recently demonstrated that cultured human MCs derived from CD34+ progenitors in peripheral blood preferentially incorporate exogenously applied AA into the large TG pool of their LDs (9). Interestingly, tandem mass spectrometric compositional analysis of the AA-containing TGs revealed species in which up to three arachidonyl moieties had been esterified to the glycerol backbone. These findings confirmed that in human MCs, the TGs of the cytoplasmic LDs serve as a major storage site for AA and triggered our interest in the mechanisms regulating the subsequent fate of this particular AA pool.
In this regard, the LDs of MCs are also considered to be important in AA mobilization and subsequent eicosanoid generation (10). From the liberated AA, the PGs and LTs are formed by the action of cyclooxygenases (COXs) and lipoxygenases (LOs), respectively (11). The presence of a COX in human lung MC LDs has been visualized by immunogold labeling, demonstrating that MC LDs potentially serve as an active site for PG biosynthesis (12). For an efficient utilization of the TG pool in LDs as a source of AA, human MCs need to express appropriate lipolytic enzymes that mediate the hydrolysis of AA from TGs. A candidate for this function is the neutral TG hydrolase, adipose TG lipase (ATGL; also termed patatin-like phospholipase domain-containing protein A2 [PNPLA2]), which mediates the initial step in TG hydrolysis resulting in the formation of an FFA and a diacylglycerol (DAG) molecule (13). ATGL is expressed in most tissues of the body with the highest expression and activity being found in white and brown adipose tissues (14). Interestingly, ATGL mRNA is also expressed in macrophages and macrophage-derived foam cells of both murine and human origin (15). Thus, implications of the ATGL in immune response, inflammation, and atherosclerosis are emerging (16). However, besides macrophages, no information is available about the presence of ATGL in any other cell types belonging to the immune system.
Based on these considerations and on our previous findings in cultured human MCs, we examined the expression and function of ATGL in MCs. Particularly, we wished to test the hypothesis that ATGL plays a functional role in the hydrolysis of AA present in the TG pool of human MC LDs (i.e., that its activity would be required for providing the substrate for eicosanoid biosynthesis in these cells). We demonstrate that ATGL is expressed in human MCs and that siRNA-mediated silencing of ATGL greatly reduces the amounts of prostaglandin D2 (PGD2) and leukotriene C4 (LTC4) released upon activation of MCs. Thus, ATGL expression in human MCs is necessary for the immediate generation of lipid mediators via both the PG and the LT pathways. These novel observations show that AA-containing TGs in LDs play an important role in providing AA as a substrate for eicosanoid metabolism in human MCs, and that ATGL is an important regulator of this pathway.
METHODS
Cell culture
Mature human MCs were generated exactly as described previously (9). Briefly, CD34+ progenitor cells were isolated from fresh buffy coats prepared from the peripheral blood of healthy blood donors (Finnish Red Cross Blood Transfusion Service, Helsinki, Finland). The isolated CD34+ cells were cultured for at least 6 weeks at 37°C under serum-free conditions in Iscove’s modified Dulbecco’s medium supplemented with BIT 9500 serum substitute (StemCell Technologies), l-glutamine (2 mM), 2-mercaptoethanol (0.1 mM), penicillin (100 U/ml), streptomycin (100 µg/ml), and recombinant human stem cell factor (also termed KITL, KIT ligand) (100 ng/ml; PeproTech, Rocky Hill, NJ). All experiments were carried out with mature MCs (i.e., with cells that had been allowed to differentiate for 6 to 8 weeks) (17).
MC activation
For immunological activation via IgE receptor cross-linking, MCs were first sensitized for 3 h with human IgE (1 µg/ml, DiaTec), washed twice with PBS, resuspended in fresh medium, and then activated by incubation in the presence of polyclonal rabbit anti-human IgE (1 µg/ml, Millipore) for 5, 10, 30, 60, 120, and 240 min.
ATGL and cPLA2 knockdown with siRNA
MCs were maintained in fresh culture medium 24 h prior to experiments. Then, 2 × 106 cells were transfected with 100 nM total siRNA targeted at ATGL (also known as PNPLA2) mRNA (Hs_PNPLA2_5, Hs_PNPLA2_6, Hs_LOC100507839_2, and Hs_LOC100507839_3 siRNAs at 1:1:1:1 molar ratio; QIAGEN) and/or 25 nM total siRNA targeted at cPLA2 mRNA (Hs_PLA2G4A_8, Hs_PLA2G4A_9, Hs_PLA2G4A_6, and Hs_PLA2G4A_7 siRNAs at 1:1:1:1 molar ratio; QIAGEN) or with 100 nM AllStars Negative Control siRNA AF488 (QIAGEN) using HiPerfect transfection reagent (QIAGEN) according to the manufacturer’s instructions. Twenty hours after transfection, the cells were subjected to immunological activation as described previously.
Quantitative RT-PCR
Total RNA was isolated from cultured human MCs (RNA NucleoSpin II, Macherey Nagel), and cDNA was generated by RT-PCR using M-MLV reverse transcriptase and random hexamers (both from Promega). For quantitative RT-PCR, the cDNA was amplified in duplicates using either TaqMan Universal PCR Master Mix (Applied Biosystems) or Power SYBR Green PCR Master Mix (Applied Biosystems) with gene-specific oligonucleotides and fluorogenic TaqMan probes on an ABI PRISM 7500 sequence detector system (Applied Biosystems). Specific oligonucleotides and probes were designed for the following genes: ALOX5 (sense: 5′-CTCAAGCAACACCGACGTAAA-3′ antisense: 5′-CCTTGTGGCATTTGGCATCG-3′), ATGL (sense: 5′-CAGACGGCGAGAATGTCATT-3′ antisense: 5′-AAATGCCACCATCCACGTAG-3′), cPLA2 (sense: 5′-GATGAAACTCTAGGGACAGCAAC-3′ antisense: 5′-CTGGGCATGAGCAAACTTCAA-3′), COX-1 (sense: 5′-CACAGTGCGCTCCAACCTTA-3′ antisense: 5′-TGGAGAAAGACTCCCAGCTGA-3′ probe: 5′-FAM-CTTATCCCCAGTCCCCCCACCTACAACTC-BHQ1-3′), COX-2 (sense: 5′-CGAGGGCCAGCTTTCAC-3′ antisense: 5′-GGCGCAGTTTGTCTAG-3′ probe: 5′-FAM-TGATTTAAGTGGCCC-BHQ1-3′), LTC4S (sense: 5′-AGTCCTGCTGCAAGCCTACTT-3′ antisense: 5′-AGGAACAGCGGGAAGTACTCG-3′), HPGDS (sense: 5′-ATGCGCCTCATCTTATGCAAG-3′ antisense: 5′-GGTTGTCTAACAGGTCAGGCT-3′), and GAPDH (sense: 5′-GTCAACGGATTTGGTCGTATTGG-3′ antisense: 5′-GGCAACAATATCCACTTTACCAGAGT-3′ probe: 5′-FAM-TGGTCACCAGGGCTGCTT-BHQ1-3′). For data normalization, GAPDH was used as an endogenous control, and the relative units for gene expression were calculated by using the 2−ΔΔCT method (18).
Western blotting
For the preparation of total cell lysates, MCs were washed twice with PBS, lysed in cell lysis buffer (25 mM Tris/HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% NP-40, 5% glycerol) containing complete protease inhibitor cocktail (Roche). The proteins in total cell lysates were separated by SDS-PAGE under reducing condition and then transferred onto a nitrocellulose membrane (Hybond-C Extra; Amersham Biosciences). Nonspecific binding sites were blocked by incubating the membrane with 5% nonfat dry milk in 1× TBS-T buffer (150 mM NaCl, 10 mM Tris, 0.1% Tween-20, pH 8) for 1 h at room temperature. Immunodetection was performed using rabbit anti-human ATGL (1:300 dilution; #2138, Cell Signaling), goat anti-human COX-1 (0.5 µg/ml; sc-1752; Santa Cruz Biotechnology Inc.), rabbit anti-human COX-2 (1:300 dilution; 160107; Cayman Chemicals), mouse anti-human hematopoietic prostaglandin D2 synthase (HPGDS) (0.1 µg/ml; MAB6487; R and D Systems), or mouse anti-human GAPDH (0.5 µg/ml; G8795, Sigma), followed by incubation, according to the primary antibody, with either HRP-labeled goat anti-mouse IgG (1:2,000 dilution; 0447; DAKO), HRP-labeled rabbit anti-goat IgG (1:2,000 dilution; 0449; DAKO), or HRP-labeled goat anti-rabbit IgG (1:10,000 dilution; A0545; Sigma-Aldrich). The signals were detected using an enhanced chemiluminescence method (PIERCE).
Quantification of MC lipid mediators
The amounts of PGD2 and LTC4 released into culture media were analyzed using commercial enzyme immunoassays (Prostaglandin D2-MOX EIA Kit and Leukotriene C4 EIA Kit, Cayman Chemicals) according to the manufacturer’s protocols. For blocking the COX-1-mediated generation of PGD2, MCs were incubated with 200 µM aspirin for 3 h, washed twice with PBS, resuspended in fresh medium, and activated as described previously.
LD visualization and quantification
MC LDs were visualized by Oil Red O staining and quantified by flow cytometry as described previously (9). Briefly, MCs were sedimented (Cytospin, Shandon Instruments) onto glass slides (15 × 103 cells/slide) and fixed with 10% neutral buffered formalin solution (Sigma). The cells were then stained with Oil Red O for 30 min and counterstained with Mayer’s hematoxylin. Coverslips were mounted with aqueous medium to preserve the Oil Red O staining (Aquamount, DAKO). Images were captured with a Nikon Eclipse E600 microscope (original magnification, 20×). For LD quantification, MCs were fixed with 4% paraformaldehyde and probed with allophycocyanin (APC)-labeled mouse monoclonal anti-human CD117 (4 µg/ml, BD Pharmingen) or APC-labeled mouse IgG1 isotype control (BD Pharmingen). Subsequently, the MCs were stained with the fluorescent dye Bodipy 493/503 (10 µg/ml, Molecular Probes) to stain intracellular LDs and analyzed (1 × 104 cells/measurement) using a FACSAria II flow cytometer (BD Biosciences).
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0 software. Statistical significance between two groups was determined by one-way or two-way ANOVA, followed by the Bonferroni post hoc test. Data are shown as the means ± SEM. The following levels of statistical significance were used: * P < 0.05, ** P < 0.01, and *** P < 0.001.
RESULTS
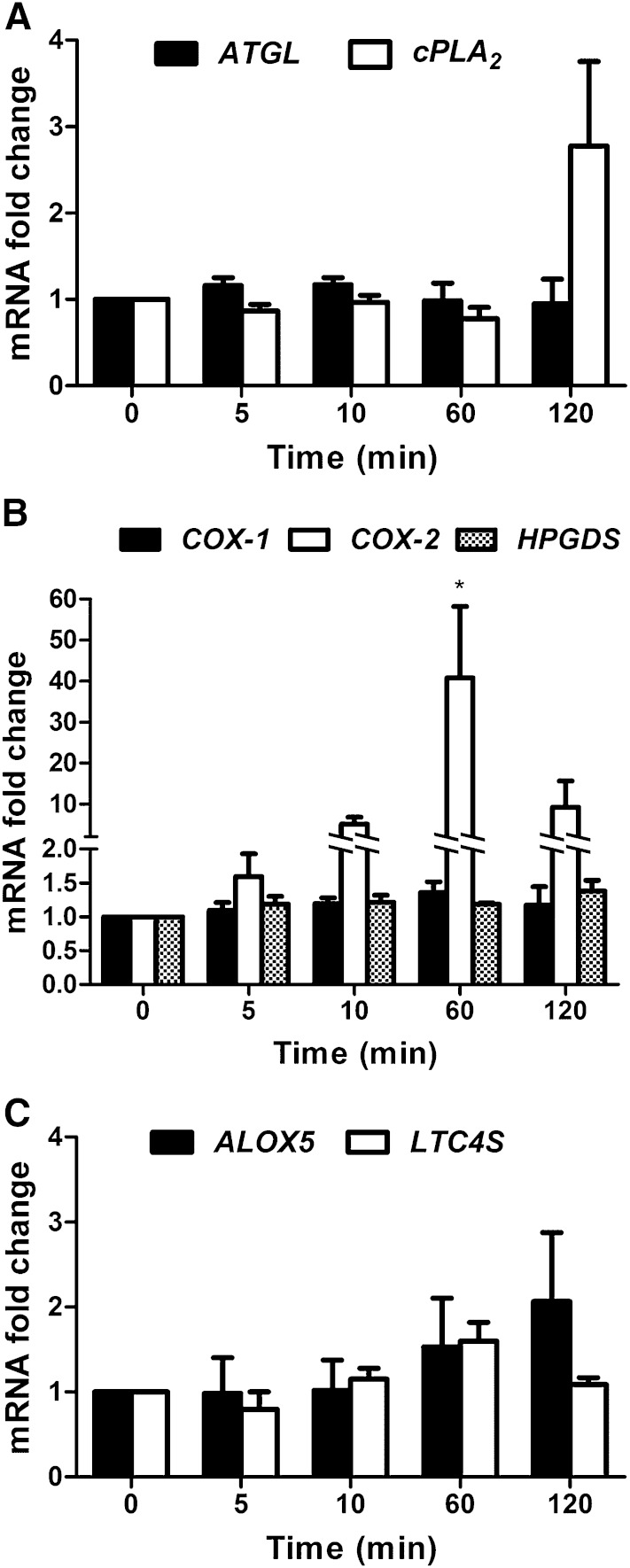
Because the intracellular lipolytic enzyme ATGL is a prime candidate for hydrolyzing AA from TGs, we first analyzed its expression and regulation in cultured mature human MCs derived from blood CD34+ progenitors. In addition, we also investigated the expression of cPLA2, which has been traditionally considered to be the major enzyme mediating the release of AA from membrane PLs. For this purpose, the cultured MCs were immunologically activated by IgE-triggered cross-linking of the cell surface IgE receptors (see Methods), and the ATGL and cPLA2 mRNA levels were measured at different time points (over 5 to 120 min) after activation (Fig. 1A). Compared with the resting MCs, no significant changes were observed in ATGL or cPLA2 mRNA levels after MC activation. Based on our interest in MC eicosanoid metabolism, we also analyzed the expression levels of the major enzymes of the PGD2 synthesis pathway, COX-1, COX-2, and the HPGDS, which catalyzes PGD2 synthesis in MCs. The COX-1 and HPGDS mRNA levels did not change significantly in the stimulated MCs (Fig. 1B). Interestingly, COX-2 mRNA levels were low in unstimulated MCs but dramatically increased at 60 min postactivation and then sharply declined (Fig. 1B). These results agree with the well-established fact that COX-1, as a constitutively expressed enzyme of the PG pathway, is responsible for the basal PG generation (11), whereas COX-2 acts as the inducible isoform under inflammatory conditions (19). Moreover, we analyzed the gene expression of the major enzymes of the LTC4 pathway, arachidonate-lipoxygenase 5 (ALOX5) and LTC4 synthase (LTC4S). As demonstrated in Fig. 1C, the mRNA expression levels of neither ALOX5 nor LTC4 changed significantly. Taken together, these findings demonstrate that human MCs express ATGL and cPLA2, as well as all the major enzymes of the PGD2 and LTC4 pathways, and that upon immunological activation of these cells, only the expression of COX-2 is significantly increased.
Fig. 1.
Regulation of ATGL, cPLA2, COX-1, COX-2, HPGDS, ALOX5, and LTC4S transcript levels in immunologically activated human MCs. For immunological activation, MCs were first sensitized in the presence of IgE (1 μg/ml) for 3 h at 37°C and subsequently activated by adding anti-IgE (1 μg/ml). Untreated MCs were used as a control. The activated cells were collected at 5, 10, 60, and 120 min, and RNA was isolated and analyzed for the mRNA expression of ATGL and cPLA2 (A); COX-1, COX-2, and HPGDS (B); and ALOX5 and LTC4S (C) by quantitative PCR (qPCR). For data normalization, GAPDH was used as an endogenous control. Transcript expression levels are shown as fold change and represent the mean ± SEM of three to four different donors. * P < 0.05.
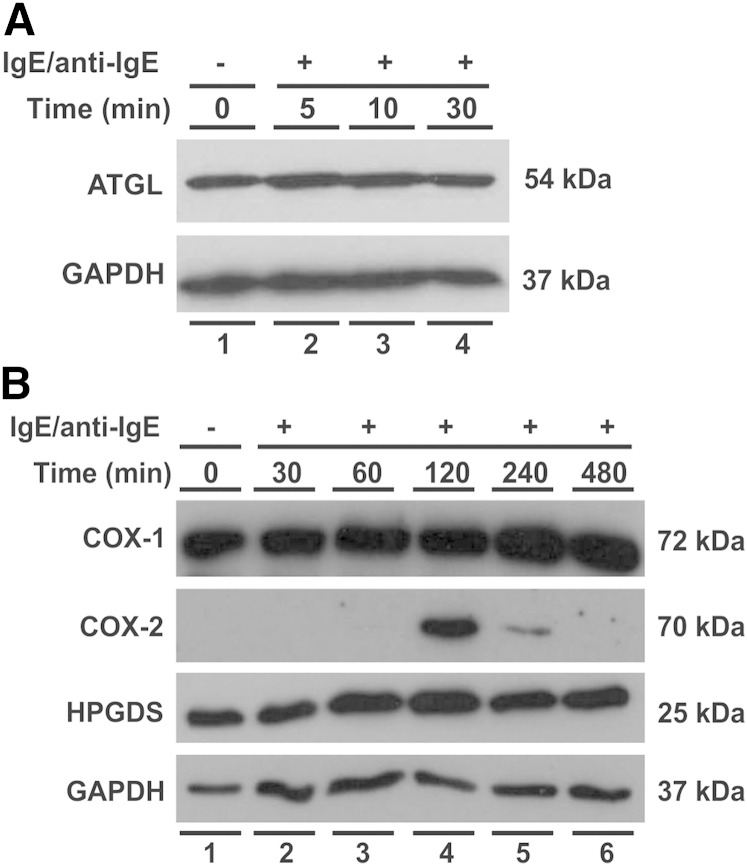
To substantiate these findings at the protein level, we next analyzed ATGL and major enzymes of the PGD2 pathway in unstimulated and stimulated MCs by immunoblotting. The 54 kDa immunoreactive band representing ATGL was clearly present in unstimulated cells and remained essentially unchanged upon MC activation (Fig. 2A). COX-1 (72 kDa) and HGPDS (25 kDa) displayed considerable protein levels in unstimulated cells with rather small changes over the time period analyzed after MC activation (up to 8 h) (Fig. 2B). In sharp contrast, the protein levels of COX-2 (70 kDa), which were undetectable in nonstimulated cells and up to 60 min in the stimulated cells, showed a transient surge with a peak at 120 min. Thus, the responses of the protein levels of ATGL, COX-1, COX-2, and HPGDS to immunological activation of the MCs agreed well with those of their respective transcript levels. It is important to note that, although the protein and transcript levels of ATGL remained stable throughout the MC activation period, this finding does not exclude the possibility that changes in its enzymatic activity had occurred. Thus, as shown in human adipose tissue, the protein and mRNA levels of ATGL do not necessarily correlate with its TG-hydrolyzing activity, which is primarily regulated by the LD-associated proteins of the PAT family (perilipins) and its activating/inhibitory cofactors comparative gene identification-58 (CGI-58)/G0S2 (20, 21).
Fig. 2.
Regulation of ATGL, COX-1, COX-2, and HPGDS protein levels in immunologically activated human MCs. IgE-dependent MC activation was performed as described in Fig. 1. MCs were harvested after the indicated periods of activation, and protein levels were analyzed by Western blotting. Fifty micrograms of total protein lysate per lane was separated by 10% SDS-PAGE under reducing conditions, blotted onto nitrocellulose membrane, and probed with specific antibodies against ATGL (A) and COX-1, COX-2, or HPGDS (B). GAPDH was used as a loading control.
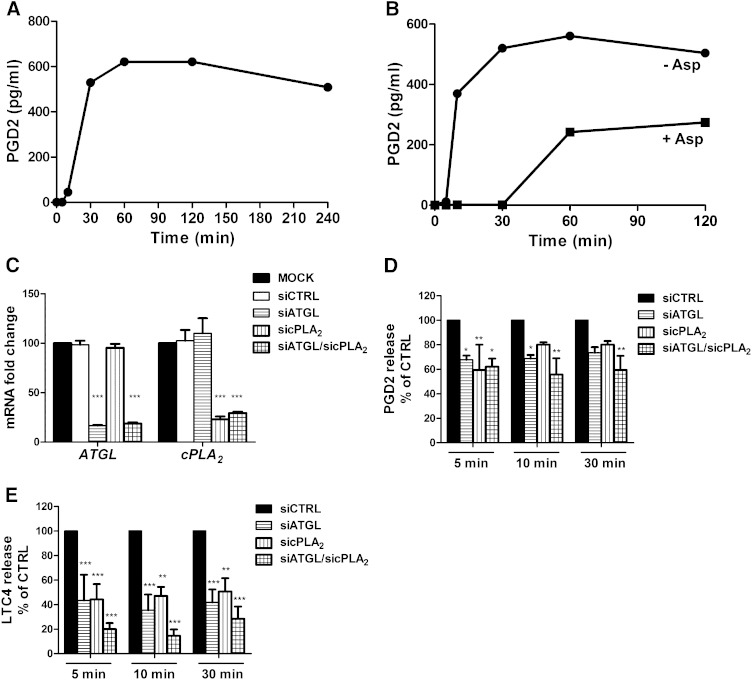
Next, we investigated the release of PGD2 upon activation of MCs. As shown in Fig. 3A, MC activation resulted in a rapid increase of the concentration of PGD2 in the incubation medium, which reached the maximal level 60 min after activation and thereafter slowly declined. We also incubated MCs with the COX inhibitor aspirin before their activation and measured the release of PGD2 by the activated MCs over 120 min in the absence of aspirin. As shown in Fig. 3B, during the initial 30 min, when only COX-1 (but not COX-2; see Fig. 1) was expressed by the cells, PGD2 secretion was fully inhibited and started to rise only after this period when the COX-2 mRNA levels also began to rise. Taken together, the data show that the immediate generation of PGD2 by activated human MCs solely depends on COX-1.
Fig. 3.
COX-1-dependent immediate release of eicosanoids is reduced in ATGL- and cPLA2-silenced human MCs. Human MCs were immunologically activated as described in Fig. 1 (A, B, D, E). A: Media were collected after the indicated periods of activation, and PGD2 concentrations (pg/ml) were measured with a PGD2mox enzyme immunoassay. Media of untreated MCs were used as a control (time 0). Representative data from one human donor are shown. B: The COX-1-mediated release of PGD2 was analyzed in MCs that had been incubated with or without aspirin (200 µM) for 3 h prior to MC activation. PGD2 concentrations (pg/ml) were measured at the indicated time points after MC activation, as described previously. C–E: Human MCs were transfected with siRNAs targeting ATGL (100 nM) and/or cPLA2 (25 nM) for 20 h at 37°C. Both mock-transfected cells and control siRNA-transfected cells were used as controls. C: ATGL and cPLA2 mRNA levels were analyzed by qPCR. For data normalization, GAPDH was used as an endogenous control. Transcript levels are shown as fold changes, representing the mean ± SEM of four donors. D, E: PGD2 and LTC4 concentrations (pg/ml) were measured from the collected media with a PGD2mox or LTC4 enzyme immunoassay, respectively. Data are shown as the percentage of control cells and are expressed as the mean ± SEM of data obtained from three and five different donors (for PGD2 and LTC4, respectively). *** P < 0.001, ** P < 0.01, * P < 0.05.
To test whether the action of ATGL is critical for eicosanoid biosynthesis in activated MCs, we silenced its expression using an siRNA-mediated approach. In addition, we also performed knockdown experiments targeting cPLA2. The ATGL and cPLA2 mRNA levels in siRNA-transfected MCs were examined by qPCR analysis (Fig. 3C). The siRNA treatment in the single knockdown experiments reduced ATGL expression by 83% and cPLA2 expression by 77%, while control siRNA did not show any effect. In the double knockdown, the mRNA expression levels of ATGL and cPLA2 were reduced by 81% and 71%, respectively (Fig. 3C). The release of the major MC eicosanoids, PGD2 and LTC4, was analyzed in the siRNA-silenced MCs at 5, 10, and 30 min after their activation. The siRNA control cells showed normal release responses of PGD2 and LTC4 to the IgE-mediated activation, which were similar to those shown in Fig. 3A. Thus, the release of PGD2 was maximally increased, on average, by 89-fold, and that of LTC4 by 79-fold. At all time points, the release of PGD2 was reduced in ATGL and cPLA2 single-silenced and in double-silenced MCs by 30% to 40% (Fig. 3D), while the release of LTC4 in the single-silenced MCs was reduced by ∼60% and in the double-silenced cells, on average, by 80% (Fig. 3E). Taken together, besides confirming the well-known function of cPLA2 as an enzyme capable of releasing AA from PLs for eicosanoid generation, these data reveal a novel role for ATGL in providing TG-derived AA as a substrate for eicosanoid production.
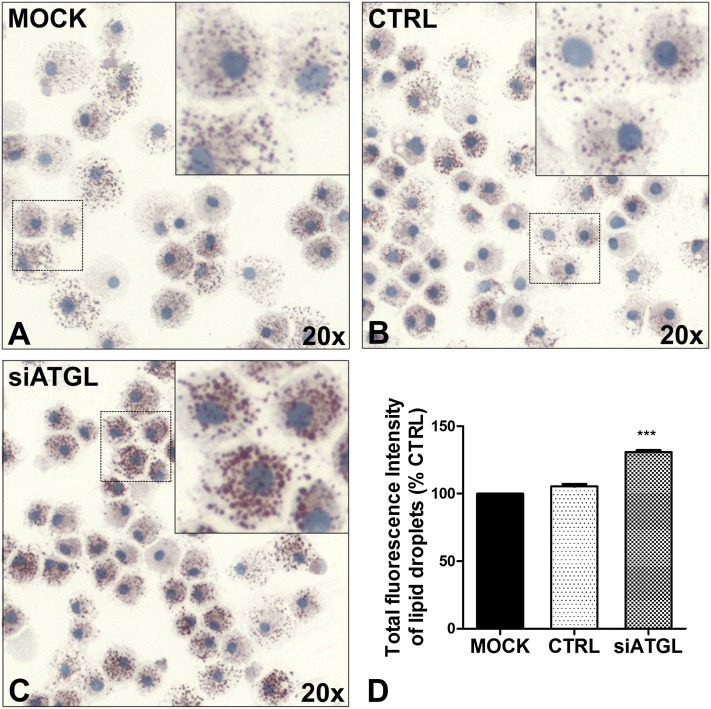
Based on the observation that ATGL silencing clearly impairs the availability of AA for the generation of PGD2 and LTC4 upon MC activation, we wanted to investigate its impact on TG hydrolysis. Therefore, neutral lipids of LDs were stained with Oil Red O (Fig. 4 A–C). Compared with mock-transfected and control siRNA-transfected MCs, silencing of ATGL showed a clear increase in number and size of LDs. To quantify the accumulation of neutral lipids, LDs were stained with the fluorescent dye Bodipy 493/503 and analyzed by flow cytometry (Fig. 4D). Reduced expression of ATGL resulted in a significant increase of mean fluorescence intensity of LDs compared with mock-transfected cells, reflecting intracellular accumulation of neutral lipids in their LDs. This finding is in good agreement with the results obtained by Oil Red O imaging of the LDs, which showed an increase in their number and size (Fig. 4 A–C). Because ATGL-silenced human MCs developed a neutral lipid-accumulating phenotype, ATGL appears to play a quantitatively important role in TG hydrolysis of LDs in these immune cells.
Fig. 4.
siRNA-mediated silencing of ATGL leads to accumulation of neutral lipids in cytoplasmic LDs of human MCs. Human MCs were transfected with siRNAs targeting ATGL, as described in Fig. 3. A–C: Cellular neutral LDs were visualized by Oil Red O/hematoxylin staining. Representative images of cells from two donors are shown (original magnification, 20×). Insets show magnified MCs from the respective panels. D: LDs were stained with Bodipy 493/503 and then quantified in 10 × 103 MCs by flow cytometry. The fluorescence intensities of the cells are shown and expressed as percentages of the fluorescence intensities of control cells (Mock). *** P < 0.001.
DISCUSSION
The current investigation into AA metabolism in MCs is based on our key observation that silencing of ATGL expression leads to a marked accumulation of neutral lipids in cytoplasmic LDs and, concomitantly, to a reduced production of eicosanoids by these cells. As human MCs can store substantial amounts of esterified AA within their large LD TG pool (7, 8), with a significant fraction of the TG molecules containing two or three arachidonyl chains (9), these stores could serve as a quantitatively major source of AA for eicosanoid biosynthesis. In adipocytes under basal conditions, ATGL mediates the initial step in TG hydrolysis by producing 1,3-DAG, while under hormonal stimulation of lipolysis the enzyme becomes activated by its coactivating factor CGI-58, and the selectivity of ATGL broadens to the sn-1 position leading to the production of 2,3-DAG as well (22). Therefore, because CGI-58 is also expressed in MCs (unpublished observation), MC activation could further enhance the TG deacylation in LDs by ATGL via hydrolysis of two arachidonyl chains from their AA-rich TG species. The conclusion that ATGL has a functional role in TG metabolism of MCs is supported by the observed intracellular accumulation of TGs in various tissues and peripheral blood leukocytes in patients with neutral lipid storage disease, which is caused by a loss-of-function mutation in ATGL (23–25). To our knowledge, however, no reports of eicosanoid production by the blood leukocytes of such patients are available.
Here, in focusing on the generation of PGD2 and LTC4 by activated MCs, we analyzed the expression of the major enzymes along the synthetic pathways of these two eicosanoids (11). No significant changes were observed in mRNA expression levels for the two major enzymes of the LTC4 pathway, ALOX5 and LTC4S, upon MC activation. The expression patterns of COX-1 and COX-2, the initializing enzymes of the PG pathway, found in resting and immunologically activated human MCs are in accordance with those observed in a murine MC cell line (MMC-34), in which IgE-dependent activation did not have any effect on COX-1 expression levels but strongly induced the expression of COX-2 (26). The COX-1 and COX-2 transcript and protein expression profiles in human MCs indeed showed that COX-1, as a constitutive enzyme, was expressed already prior to stimulation, while COX-2 expression was observed in the delayed phase of MC stimulation, suggesting that the generation of PGD2 during the immediate phase of eicosanoid release after MC stimulation was solely COX-1 dependent. This presumption was confirmed by incubating unstimulated MCs in the presence of aspirin to irreversibly inhibit COX-1, followed by stimulation of the cells in the absence of aspirin. Under these specific conditions, the release of PGD2 was fully abrogated during the first 30 min after MC activation, indicating that the MCs lacked sufficient activities of COX-1 or of the subsequently induced COX-2 (Fig. 3B) for the generation of detectable amounts of PGD2 within this early activation phase. Our data strongly suggest that the acute generation of PGD2 in activated human MCs is solely mediated by COX-1, emphasizing that under resting conditions, human MCs constitutively express sufficient amounts of COX-1 and HPGDS required for the immediate generation of PGD2 in response to stimuli that trigger eicosanoid release.
To gain further insight into the role of ATGL in eicosanoid metabolism in human MCs, we measured the acute phase release of PGD2 and LTC4 in ATGL and cPLA2 single-silenced and in double-silenced MCs after their activation. Importantly, the acute release of PGD2 by the ATGL and cPLA2 single-silenced MCs was clearly, but equally reduced compared with that by control siRNA (siCTRL)-silenced MCs (Fig. 3D). Surprisingly, the same effect was even more pronounced for the release of LTC4. Based on the current literature, ATGL is a single-compartment-acting enzyme that exerts its activity on TGs in LDs only, whereas cPLA2 is a multicompartment enzyme that acts on PLs in a multitude of cellular organelles (21, 27). Considering this fact, it is quite surprising that silencing ATGL in human MCs reduces the release of eicosanoids to the same extent as silencing cPLA2. This observation suggests that the potential contribution for eicosanoid production of AA present in the large TG pool of MC LDs can be maximally of the same magnitude as the amount of AA derived from various PL pools via the action of cPLA2.
The observed dominance of the TG-derived AA as a precursor of lipid mediators is a highly challenging finding because, historically, PLs of the cellular plasma membrane, and more recently also the PLs of various cellular compartments, have been described as the only source of AA for eicosanoid biosynthesis (28). In particular, cPLA2 has been considered to be the major enzyme mediating the release of AA from the sn-2 position of membrane PLs (6). Also, the formation of PGD2, LTC4, and leukotriene B4 (LTB4) in murine MCs is highly dependent on cPLA2, as demonstrated using bone marrow-derived MCs generated from cPLA2−/− mice (29). Although our studies support the hypothesis that, by providing AA for eicosanoid biosynthesis in human MCs, ATGL plays an important role as a TG hydrolase, it is not yet ascertained whether AA released from the TGs can be used directly or indirectly for eicosanoid generation. Direct utilization of TG-derived AA (i.e., bypassing the transfer of AA from TGs to PLs and its subsequent liberation by cPLA2) would enable an accelerated provision of the substrate for eicosanoid-forming enzymes, which would be important for the ultrarapid release of eicosanoids from MCs upon their activation. However, the results of the above-mentioned study (29) on eicosanoid formation in bone marrow-derived murine MCs generated from cPLA2-deficient animals point to an indirect pathway, at least in those cells.
It is becoming obvious that major functions of LDs in inflammatory cells differ fundamentally from the classical energy-providing role of LDs in adipocytes. Thus, LDs accumulate in various immune cells during inflammatory and infectious conditions (reviewed in Ref. 28), and, moreover, LDs have been identified as sites of eicosanoid biosynthesis in certain immune cells (30, 31). The early speculations that AA-containing TGs might also serve as a source of AA for the generation of lipid mediators (discussed in Refs. 7, 8, 28) have now been strengthened by a mechanistic revelation implicating that functional ATGL in activated human MCs is required for the generation of PGD2 and LTC4. The identification of the regulatory role of ATGL in eicosanoid production by MCs is an important step toward defining and specifying the unique role of LDs in inflammatory cells. The establishment of cultured human MCs as a well-defined cellular system for studies on the regulation of eicosanoid production during all phases of mediator release will facilitate our current studies aimed at directly quantifying the relative contributions of the substrate AA to eicosanoid production along the three candidate pathways (i.e., derived directly from TGs, indirectly from TGs, or directly from PLs; Fig. 5).
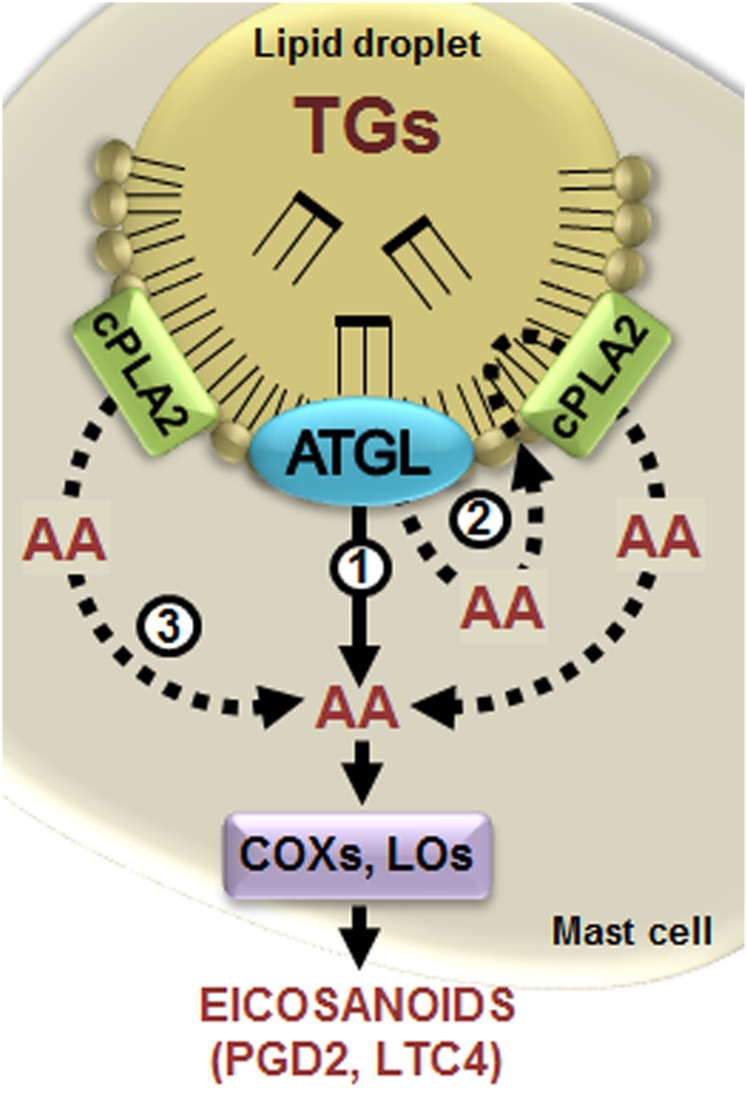
Fig. 5.
Schematic model for the proposed role of ATGL in providing triglyceride-derived AA for eicosanoid biosynthesis in human MCs. In human MCs, ATGL mediates the hydrolysis of AA from triglycerides (TGs) stored in intracellular LDs. The liberated AA may contribute to eicosanoid production via two different routes, either directly from TGs (1) or indirectly from TGs by transient incorporation into LD PLs and subsequent release by the LD-associated phospholipase cPLA2 (2). This enzyme can also provide AA directly from the PLs present in the LDs (3). Modulation of ATGL activity could alter the contribution of TG-derived AA to eicosanoid formation by enzymes of the PG pathway (the COXs) and the LT pathway (the LOs), thereby affecting the generation and release of eicosanoids from human MCs, as demonstrated in this study by knockdown of ATGL.
Acknowledgments
The authors thank Dr. Kati Öörni for carefully reading the manuscript and Mari Jokinen, Maija Atuegwu, and Jarmo Koponen for their excellent technical assistance.
Footnotes
Abbreviations:
- AA
- arachidonic acid
- ALOX5
- arachidonate 5-lipoxygenase
- ATGL
- adipose TG lipase
- CGI-58
- comparative gene identification-58
- COX
- cyclooxygenase
- cPLA2
- cytosolic phospholipase A2
- DAG
- diacylglycerol
- HPGDS
- hematopoietic prostaglandin D2 synthase
- LD
- lipid droplet
- LT
- leukotriene
- LTC4
- leukotriene C4
- LTC4S
- leukotriene C4 synthase
- MC
- mast cell
- PG
- prostaglandin
- PGD2
- prostaglandin D2
- PL
- phospholipid
- PNPLA2
- patatin-like phospholipase domain-containing protein A2
- qPCR
- quantitative PCR
The Wihuri Research Institute is maintained by the Jenny and Antti Wihuri Foundation. A. Dichlberger was supported by an Erwin Schrödinger Fellowship (J2994-B20) of the Austrian Science Fund (FWF). W. J. Schneider was a Senior Visiting Researcher of the Sigrid Juselius Foundation, Helsinki, Finland.
REFERENCES
- 1.Galli S. J., Tsai M. 2012. IgE and mast cells in allergic disease. Nat. Med. 18: 693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand P., Singh B., Jaggi A. S., Singh N. 2012. Mast cells: an expanding pathophysiological role from allergy to other disorders. Naunyn Schmiedebergs Arch. Pharmacol. 385: 657–670. [DOI] [PubMed] [Google Scholar]
- 3.Kovanen P. T. 2007. Mast cells: multipotent local effector cells in atherothrombosis. Immunol. Rev. 217: 105–122. [DOI] [PubMed] [Google Scholar]
- 4.Theoharides T. C., Kempuraj D., Tagen M., Conti P., Kalogeromitros D. 2007. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 217: 65–78. [DOI] [PubMed] [Google Scholar]
- 5.Boyce J. A. 2007. Mast cells and eicosanoid mediators: a system of reciprocal paracrine and autocrine regulation. Immunol. Rev. 217: 168–185. [DOI] [PubMed] [Google Scholar]
- 6.Murakami M., Taketomi Y., Miki Y., Sato H., Hirabayashi T., Yamamoto K. 2011. Recent progress in phospholipase A(2) research: from cells to animals to humans. Prog. Lipid Res. 50: 152–192. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak A. M., Dvorak H. F., Peters S. P., Shulman E. S., MacGlashan D. W., Jr, Pyne K., Harvey V. S., Galli S. J., Lichtenstein L. M. 1983. Lipid bodies: cytoplasmic organelles important to arachidonate metabolism in macrophages and mast cells. J. Immunol. 131: 2965–2976. [PubMed] [Google Scholar]
- 8.Triggiani M., Oriente A., Seeds M. C., Bass D. A., Marone G., Chilton F. H. 1995. Migration of human inflammatory cells into the lung results in the remodeling of arachidonic acid into a triglyceride pool. J. Exp. Med. 182: 1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dichlberger A., Schlager S., Lappalainen J., Kakela R., Hattula K., Butcher S. J., Schneider W. J., Kovanen P. T. 2011. Lipid body formation during maturation of human mast cells. J. Lipid Res. 52: 2198–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dichlberger A., Kovanen P. T., Schneider W. J. 2013. Mast cells: from lipid droplets to lipid mediators. Clin. Sci. (Lond.). 125: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funk C. D. 2001. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 294: 1871–1875. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak A. M. 2005. Mast cell secretory granules and lipid bodies contain the necessary machinery important for the in situ synthesis of proteins. Chem. Immunol. Allergy. 85: 252–315. [DOI] [PubMed] [Google Scholar]
- 13.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., et al. 2004. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 306: 1383–1386. [DOI] [PubMed] [Google Scholar]
- 14.Zechner R., Kienesberger P. C., Haemmerle G., Zimmermann R., Lass A. 2009. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J. Lipid Res. 50: 3–21. [DOI] [PubMed] [Google Scholar]
- 15.Chandak P. G., Radovic B., Aflaki E., Kolb D., Buchebner M., Frohlich E., Magnes C., Sinner F., Haemmerle G., Zechner R., et al. 2010. Efficient phagocytosis requires triacylglycerol hydrolysis by adipose triglyceride lipase. J. Biol. Chem. 285: 20192–20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Radovic B., Aflaki E., Kratky D. 2012. Adipose triglyceride lipase in immune response, inflammation, and atherosclerosis. Biol. Chem. 393: 1005–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maaninka K., Lappalainen J., Kovanen P. T. 2013. Human mast cells arise from a common circulating progenitor. J. Allergy Clin. Immunol. 132: 463–469.e3. [DOI] [PubMed] [Google Scholar]
- 18.Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 19.Dubois R. N., Abramson S. B., Crofford L., Gupta R. A., Simon L. S., Van De Putte L. B., Lipsky P. E. 1998. Cyclooxygenase in biology and disease. FASEB J. 12: 1063–1073. [PubMed] [Google Scholar]
- 20.Steinberg G. R., Kemp B. E., Watt M. J. 2007. Adipocyte triglyceride lipase expression in human obesity. Am. J. Physiol. Endocrinol. Metab. 293: E958–E964. [DOI] [PubMed] [Google Scholar]
- 21.Lass A., Zimmermann R., Oberer M., Zechner R. 2011. Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50: 14–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichmann T. O., Kumari M., Haas J. T., Farese R. V., Jr, Zimmermann R., Lass A., Zechner R. 2012. Studies on the substrate and stereo/regioselectivity of adipose triglyceride lipase, hormone-sensitive lipase, and diacylglycerol-O-acyltransferases. J. Biol. Chem. 287: 41446–41457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campagna F., Nanni L., Quagliarini F., Pennisi E., Michailidis C., Pierelli F., Bruno C., Casali C., DiMauro S., Arca M. 2008. Novel mutations in the adipose triglyceride lipase gene causing neutral lipid storage disease with myopathy. Biochem. Biophys. Res. Commun. 377: 843–846. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi K., Inoguchi T., Maeda Y., Nakashima N., Kuwano A., Eto E., Ueno N., Sasaki S., Sawada F., Fujii M., et al. 2008. The lack of the C-terminal domain of adipose triglyceride lipase causes neutral lipid storage disease through impaired interactions with lipid droplets. J. Clin. Endocrinol. Metab. 93: 2877–2884. [DOI] [PubMed] [Google Scholar]
- 25.Tavian D., Missaglia S., Redaelli C., Pennisi E. M., Invernici G., Wessalowski R., Maiwald R., Arca M., Coleman R. A. 2012. Contribution of novel ATGL missense mutations to the clinical phenotype of NLSD-M: a strikingly low amount of lipase activity may preserve cardiac function. Hum. Mol. Genet. 21: 5318–5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawata R., Reddy S. T., Wolner B., Herschman H. R. 1995. Prostaglandin synthase 1 and prostaglandin synthase 2 both participate in activation-induced prostaglandin D2 production in mast cells. J. Immunol. 155: 818–825. [PubMed] [Google Scholar]
- 27.Ghosh M., Tucker D. E., Burchett S. A., Leslie C. C. 2006. Properties of the Group IV phospholipase A2 family. Prog. Lipid Res. 45: 487–510. [DOI] [PubMed] [Google Scholar]
- 28.Bozza P. T., Bakker-Abreu I., Navarro-Xavier R. A., Bandeira-Melo C. 2011. Lipid body function in eicosanoid synthesis: an update. Prostaglandins Leukot. Essent. Fatty Acids. 85: 205–213. [DOI] [PubMed] [Google Scholar]
- 29.Fujishima H., Sanchez Mejia R. O., Bingham C. O., III, Lam B. K., Sapirstein A., Bonventre J. V., Austen K. F., Arm J. P. 1999. Cytosolic phospholipase A2 is essential for both the immediate and the delayed phases of eicosanoid generation in mouse bone marrow-derived mast cells. Proc. Natl. Acad. Sci. USA. 96: 4803–4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Araújo-Santos T., Prates D. B., Andrade B. B., Nascimento D. O., Clarêncio J., Entringer P. F., Carneiro A. B., Silva-Neto M. A., Miranda J. C., Brodskyn C. I., et al. 2010. Lutzomyia longipalpis saliva triggers lipid body formation and prostaglandin E(2) production in murine macrophages. PLoS Negl. Trop. Dis. 4: e873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bozza P. T., Yu W., Penrose J. F., Morgan E. S., Dvorak A. M., Weller P. F. 1997. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J. Exp. Med. 186: 909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]