Abstract
Many of the beneficial and adverse effects of niacin are mediated via a G protein receptor, G protein-coupled receptor 109A/hydroxycarboxylic acid 2 receptor (GPR109A/HCA2), which is highly expressed in adipose tissue and macrophages. Here we demonstrate that immune activation increases GPR109A/HCA2 expression. Lipopolysaccharide (LPS), TNF, and interleukin (IL) 1 increase GPR109A/HCA2 expression 3- to 5-fold in adipose tissue. LPS also increased GPR109A/HCA2 mRNA levels 5.6-fold in spleen, a tissue rich in macrophages. In peritoneal macrophages and RAW cells, LPS increased GPR109A/HCA2 mRNA levels 20- to 80-fold. Zymosan, lipoteichoic acid, and polyinosine-polycytidylic acid, other Toll-like receptor activators, and TNF and IL-1 also increased GPR109A/HCA2 in macrophages. Inhibition of the myeloid differentiation factor 88 or TIR-domain-containing adaptor protein inducing IFNβ pathways both resulted in partial inhibition of LPS stimulation of GPR109A/HCA2, suggesting that LPS signals an increase in GPR109A/HCA2 expression by both pathways. Additionally, inhibition of NF-κB reduced the ability of LPS to increase GPR109A/HCA2 expression by ∼50% suggesting that both NF-κB and non-NF-κB pathways mediate the LPS effect. Finally, preventing the LPS-induced increase in GPR109A/HCA2 resulted in an increase in TG accumulation and the expression of enzymes that catalyze TG synthesis. These studies demonstrate that inflammation stimulates GPR109A/HCA2 and there are multiple intracellular signaling pathways that mediate this effect. The increase in GPR109A/HCA2 that accompanies macrophage activation inhibits the TG accumulation stimulated by macrophage activation.
Keywords: cytokines, G proteins, diacylglycerol transferase, lipopolysaccharide, triglycerides, G protein-coupled receptor 109A, hydroxycarboxylic acid 2 receptor
In the 1950s, Altschul and colleagues demonstrated that high doses of niacin reduced plasma cholesterol levels, and niacin became the first drug available for treating hypercholesterolemia (1). Numerous studies have since shown that niacin decreases plasma TG, VLDL, LDL, and Lipoprotein (a) levels, while increasing HDL (1, 2).
The mechanisms by which niacin alters plasma lipid levels are not fully understood (1–3). One of the most rapid effects of niacin is to decrease plasma free fatty acid levels, and it has been hypothesized that the decrease in plasma free fatty acids reduces the delivery of fatty acids to the liver resulting in a reduction in hepatic TG synthesis leading to a decrease in VLDL production and secretion (1, 2). This action could contribute to the niacin-induced decrease in plasma TGs, VLDL, LDL, and Lp(a) (1, 2). The decrease in plasma free fatty acid levels induced by niacin is due to inhibition of lipolysis in adipose tissue mediated by a decrease in cyclic AMP levels (1, 2, 4–6). Cyclic AMP is well known to enhance lipolysis by activating protein kinase A, which phosphorylates hormone-sensitive lipase and perilipin, leading to increased TG breakdown (7, 8). Specific binding sites for nicotinic acid are present on adipose tissue, and data suggest that niacin binds to a Gi-coupled cell surface receptor (9, 10).
A major side effect of niacin therapy is cutaneous flushing, which is a troublesome side effect that frequently leads to the cessation of niacin treatment (11). The flushing has been shown to be due to increased prostaglandin D production by skin macrophages (Langerhans cells) and prostaglandin E2 production by keratinocytes leading to cutaneous vasodilation (11–14). Specific binding sites for nicotinic acid are also present in macrophage membranes, and niacin has been shown to activate phospholipase A2 leading to the increased production of arachidonic acid and the increased synthesis of prostaglandins (11, 12, 14). These observations and other data suggest that increased prostaglandin production induced by niacin may be mediated by G-coupled cell surface receptors.
In 2003, three groups identified a G protein-coupled receptor [G protein-coupled receptor 109A/hydroxycarboxylic acid 2 receptor (GPR109A/HCA2), HM74A in humans, protein upregulated in macrophages by IFN-gamma in mice] that binds nicotinic acid and is expressed predominantly in adipose tissue and immune cells, including macrophages (6, 15, 16). This receptor couples to G proteins of the Gi family and binds not only niacin but other related compounds that have similar effects (e.g., acipimox) (17, 18). In mice deficient in GPR109A/HCA2, niacin does not decrease plasma free fatty acid or TG levels, suggesting that the niacin-induced inhibition of adipose tissue lipolysis is mediated by GPR109A/HCA2 (6, 17). Similarly, studies have also demonstrated that the increase in prostaglandin production and the cutaneous flushing induced by niacin is also dependent on GPR109A/HCA2 in macrophages and keratinocytes (12–14, 17). Thus, many of both the beneficial and the adverse effects of niacin require GPR109A/HCA2.
The factors that regulate the expression of GPR109A/HCA2 have not been rigorously studied. Schaub et al. (19) have reported that activation of macrophages with IFNγ increases GPR109A/HCA2 expression, while Wanders et al.(20) and Zandi-Nejad et al. (21) have recently shown that LPS increases GPR109A/HCA2 expression in RAW 264.7 macrophages. Finally, both lipopolysaccharide (LPS) and TNFα have been shown to upregulate GPR109A/HCA2 in adipocytes (20, 22). In the present study, we have defined the effects of other cytokines and Toll-like receptor (TLR)-activating stimuli on GPR109A/HCA2 expression in fat cells and macrophages, determined the pathways leading to increased expression, and shown that inhibiting the increase in GPR109A/HCA2 expression or activity leads to increased lipid storage suggesting that the increase in GPR109A/HCA2 acts as a feedback inhibitor.
MATERIALS AND METHODS
Materials
LPS from Escherichia coli strain O55:B5 was purchased from Difco (Detroit, MI) and diluted in sterile normal saline to the desired concentration. DMEM and Intralipid were obtained from Fisher Scientific (Pittsburgh, PA). FBS was purchased from Hyclone (Logan, UT). Human serum albumin (HSA) was obtained from Bayer (Elkhart, IN). Tri Reagent, concanavalin A (Con A), thalidomide, and mepenzolate bromide were from Sigma (St. Louis, MO). Zymosan, lipoteichoic acid (LTA), polyinosine-polycytidylic acid (poly I:C), and BX795 were from InvivoGen (San Diego, CA). Parthenolide (PTN) was obtained from EMD Chemicals (Philadelphia, PA). Mouse TNFα, interleukin (IL) 1β, and IL-6 were purchased from R and D Systems (Minneapolis, MN). Acetylated low density lipoprotein (AcLDL) was from Intracel (Frederick, MD).
Animal experiments
Female C57BL/6 mice (8–12 weeks of age, ∼20 g) were obtained from Charles River Laboratories (Wilmington, MA). The animals were maintained in a normal-light-cycle room and were fed Purina mouse chow (Ralston Purina, St. Louis, MO) and water ad libitum. Animals were injected with either saline or LPS (5 mg/kg body weight ip), and food was removed from both control and treated animals following injection. At the indicated time points, mice were rapidly euthanized with an overdose of isoflurane, and the spleen and adipose tissue from the peri-uterine/-urinary bladder area were removed and snap frozen in liquid nitrogen, placed in storage tubes in dry-ice bath until the end of experiment, and then stored at −80°C until RNA extraction. All studies involving animals were conducted in conformity with the Public Health Service Policy on humane care and use of laboratory animals. All experimental protocols were approved by the Animal Studies Subcommittee of the San Francisco Veterans Affairs Medical Center.
Cell culture
Murine 3T3-L1 cells (ATCC, Manassas, VA) were grown to confluence and differentiated to adipocytes as described (23). Briefly, preadipocytes were cultured in DMEM and 10% FBS. When cells became confluent, cells were differentiated by treatment with 1.0 µg/ml insulin, 0.5 mM methylisobutylxanthine, and 1 µM dexamethasone in DMEM containing 10% FBS for 2 days. Cells were then maintained in DMEM supplemented with 10% FBS. Experiments were performed 10–12 days postdifferentiation. Cells were treated for 24 h with LPS (100 ng/ml), TNFα (10 ng/ml), or IL-1β (10 ng/ml). The doses of LPS and cytokines used in these experiments are similar to those previously shown to induce metabolic alterations in 3T3-L1 adipocytes and other cells (23).
RAW 264.7 cells, a murine macrophage cell line, were from ATCC. Cells were grown in DMEM supplemented with 10% FBS and incubated at 37°C in 5% CO2. When confluent, cells were washed with serum-free medium once and then treated in medium supplemented with 2.5% HSA for indicated times (4–24 h) prior to RNA isolation. For studies with immune stimulators, cells were treated with LPS at 100 ng/ml, zymosan at 500 μg/ml, LTA at 1 μg/ml, or poly I:C at 50 μg/ml for16 h. For lipid loading, cells were coincubated with LPS at 100 ng/ml and AcLDL at 100 μg/ml or Intralipid at 150 μg/ml for 16 h. For treatment with cytokines, cells were treated with TNFα, IL-1β, or IL-6 at 10 ng/ml for 16 h. For inhibitor studies, cells were preincubated with thalidomide at 500 μg/ml, PTN at 20 μM, BX795 at 10 μM, or MPN at 100 μM for 1 h before addition of LPS (100 ng/ml) for 16 h.
Mouse peritoneal macrophage culture
Peritoneal macrophages were harvested from C57BL/6 mice 3 days after the intraperitoneal injection of 40 μg of Con A in 0.5 ml of PBS and then cultured as described previously by Tang et al. (24). Cells were plated in 12-well plates in DMEM containing 10% FBS and 20% L-cell culture medium and allowed to adhere to wells for 1 h. Cells were washed with serum-free medium and then treated in DMEM supplemented with 2.5% HSA with LPS (100 ng/ml) for 16 h.
RNA isolation and quantitative PCR (qPCR)
Total RNA was isolated from tissues and cells using Tri Reagent. First-strand cDNA was synthesized from 1 μg of total RNA with the iScriptTM cDNA Synthesis Kit (Bio-Rad, Hercules, CA). The real-time PCR contained 20 ng of reverse transcribed total RNA, 450 nM forward and reverse primers, and 10 μl of 2 × LightCycler 480 SYBR Green I Master in a final volume of 20 μl in 96-well plates using MyiQTM Real-time PCR System (Bio-Rad). Quantification was performed by the comparative Ct method with 36B4 used for normalization. The following primers were used: GPR109A/HCA2, forward: 5′-TCCAAGTCTCCAAAGGTGGT-3′, reverse: 5′-TGTTTCTCTCCAGCACTGAGTT-3′ TNF, forward: 5′-CTACTCCCAGGTCTCTTCAA-3′, reverse: 5′-GCAGAGAGGAGGTTGACTTTC-3′ glycerol 3-phosphate acyltransferase 3 (GPAT3), forward: 5′-GGAGGATGAAGTGACCCAGA-3′, reverse: 5′- CCAGTTTTTGAGGCTGCTGT-3′ diacylglycerol transferase 2 (DGAT2), forward: 5′- AGTGGCAATGCTATCATCACGT-5′, reverse: 5′-AAGGAATAAGTGGGAACCAGATCA-3′ and 36B4, forward: 5′-GCGACCTGGAAGTCCAACTAC-3′, reverse: 5′-ATCTGCTGCATCTGCTTGG-3′.
Immunocytochemistry
RAW cells were seeded on chamber glass slides and grown overnight prior to treatment with 100 ng/ml LPS for 16 h. After fixation with 2% formaldehyde, cells were quenched with 100 mM glycine and then permeabilized in 0.5% saponin in PBS. Cells were then blocked for 1 h with 10% goat serum and 0.1% saponin in PBS. Slides were then incubated overnight at 4°C with rabbit anti-GPR109A/HCA2 at 1:100 (Novus Biologicals, Littleton, CO), followed by incubation for 1 h with Alexa 488 goat anti-rabbit IgG at 1:1000 (Invitrogen). All staining was observed with a Zeiss LSM510 Meta confocal microscope.
siRNA transfection
RAW cells were seeded at 1 × 106 cells per well into 6-well plates and grown overnight. The cells were transfected with GPR109A/HCA2 siRNA (ON-TARGETplus SMARTpool purchased from Dharmacon) or nontargeted control using TransIT-TKO Transfection Reagent (Mirus Bio LLC) following the manufacturer’s recommended protocol. After 24 h of transfection, cells were treated with LPS and harvested for RNA isolation or TG assay.
TG accumulation measurement
Following 24 h treatment, cells were washed twice with PBS and scraped into 200 μl PBS. The cell suspensions were sonicated, and TG levels were assayed using a commercially available enzymatic assay (Sigma Aldrich, St. Louis, MO). The TG accumulation was normalized to protein concentration for each sample.
Statistics
Data are presented as mean ± SEM. The Student’s t-test was used for comparisons between groups. A P value <0.05 was considered significant. When multiple samples were compared, one-way ANOVA was used to determine statistical significance.
RESULTS AND DISCUSSION
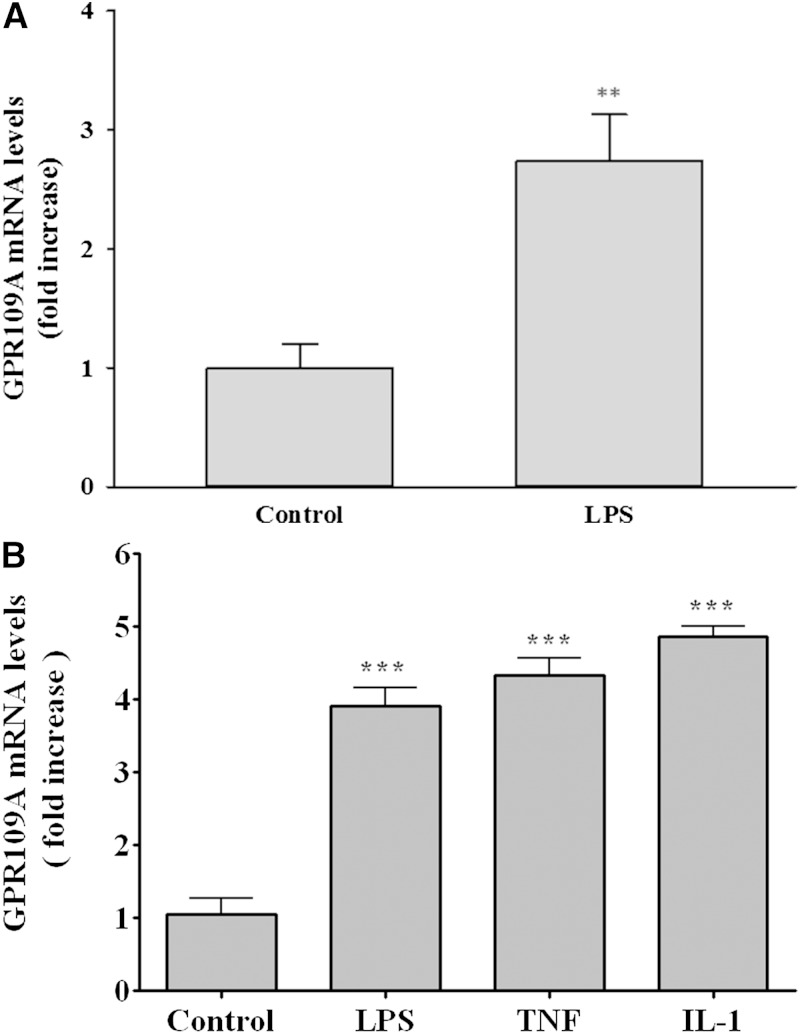
Because the expression of GPR109A/HCA2 is very abundant in adipose tissue, we first examined the effect of LPS administration, a model of gram-negative infection, on GPR109A/HCA2 expression in mouse adipose tissue. As shown in Fig. 1A, LPS administration increased GPR109A/HCA2 mRNA levels ∼2.7-fold. Similarly, treatment of 3T3-L1 adipocytes with LPS or cytokines (TNFα and IL-1β) also resulted in an ∼4- to 5-fold increase in GPR109A/HCA2 mRNA levels (Fig. 1B). Thus, inflammatory stimuli produce an increase in GPR109A/HCA2 expression in adipocytes. These results confirm and extend the in vitro studies that previously demonstrated that LPS and TNF increase GPR109A/HCA2 expression in 3T3-L1 adipocytes (20, 21).
Fig. 1.
Effect of LPS or cytokines on GPR109A/HCA2 mRNA levels in adipose. A: Mice were injected intraperitoneally with LPS (5 mg/kg), and the animals were euthanized at 16 h after LPS administration. Total RNA was isolated from adipose tissue. B: 3T3-L1 adipocytes were treated for 24 h with LPS (100 ng/ml), TNFα (10 ng/ml), or IL-1β (10 ng/ml), and total RNA was isolated. GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3–4 per group. ** P < 0.01, *** P < 0.001.
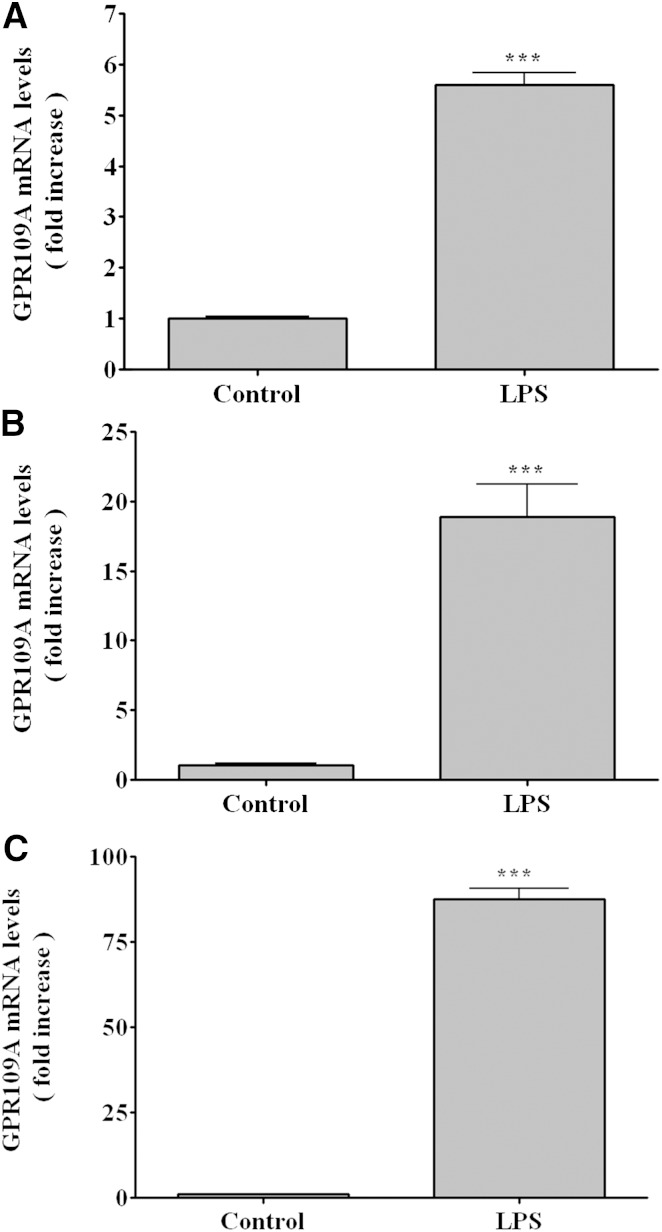
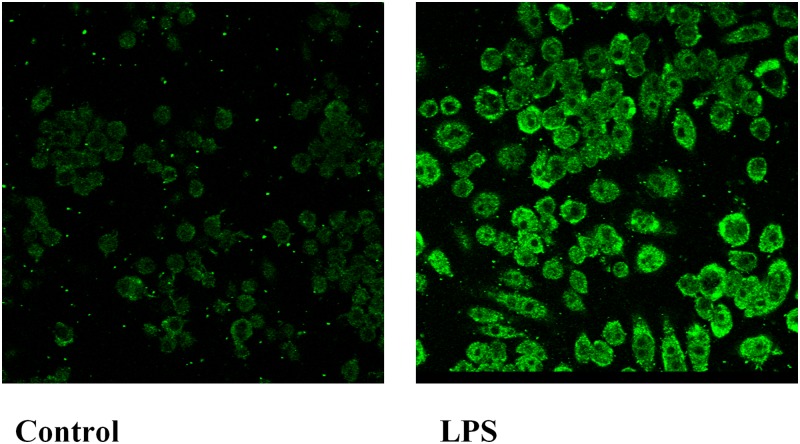
Macrophages are another site in which GPR109A/HCA2 is abundantly expressed. Therefore, we next determined the effect of LPS administration on GPR109A/HCA2 expression in the spleen, a tissue rich in macrophages. As shown in Fig. 2A, LPS administration increased GPR109A/HCA2 mRNA levels ∼5.6-fold in spleen. The effect of LPS treatment on isolated mouse peritoneal macrophages is shown in Fig. 2B. LPS treatment increased GPR109A/HCA2 mRNA ∼19-fold in mouse peritoneal macrophages in vitro. Moreover, LPS increased GPR109A/HCA2 mRNA levels ∼30- to 80-fold in cultured RAW cells, a murine macrophage cell line (Fig. 2C). Additionally, as shown in Fig. 3, LPS treatment increased GPR109A/HCA2 protein in macrophages as assessed by immunostaining. The increased GPR109A/HCA2 was seen in the plasma membrane accompanied by staining throughout the cytoplasm in macrophages.
Fig. 2.
Effect of LPS on GPR109A/HCA2 mRNA levels in immune tissue and cells. A: Mice were injected intraperitoneally with LPS (5 mg/kg), and the animals were euthanized at 16 h after LPS administration. Total RNA was isolated from spleen. B: Peritoneal macrophages were harvested from C57BL/6 mice, treated for 16 h with LPS (100 ng/ml), and total RNA was isolated. C: RAW cells were treated for 16 h with LPS (100 ng/ml), and total RNA was isolated. GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3–4 per group. *** P < 0.001.
Fig. 3.
Immunofluorescence analysis for GPR109A/HCA2 in RAW cells. Cells were treated with LPS at 100 ng/ml in serum-free medium for 16 h. Immunostaining was performed as described in Materials and Methods. Fluorescent GPR109A/HCA2 staining was visualized by confocal microscopy with a 40× oil immersion objective lens. All images were acquired with identical settings.
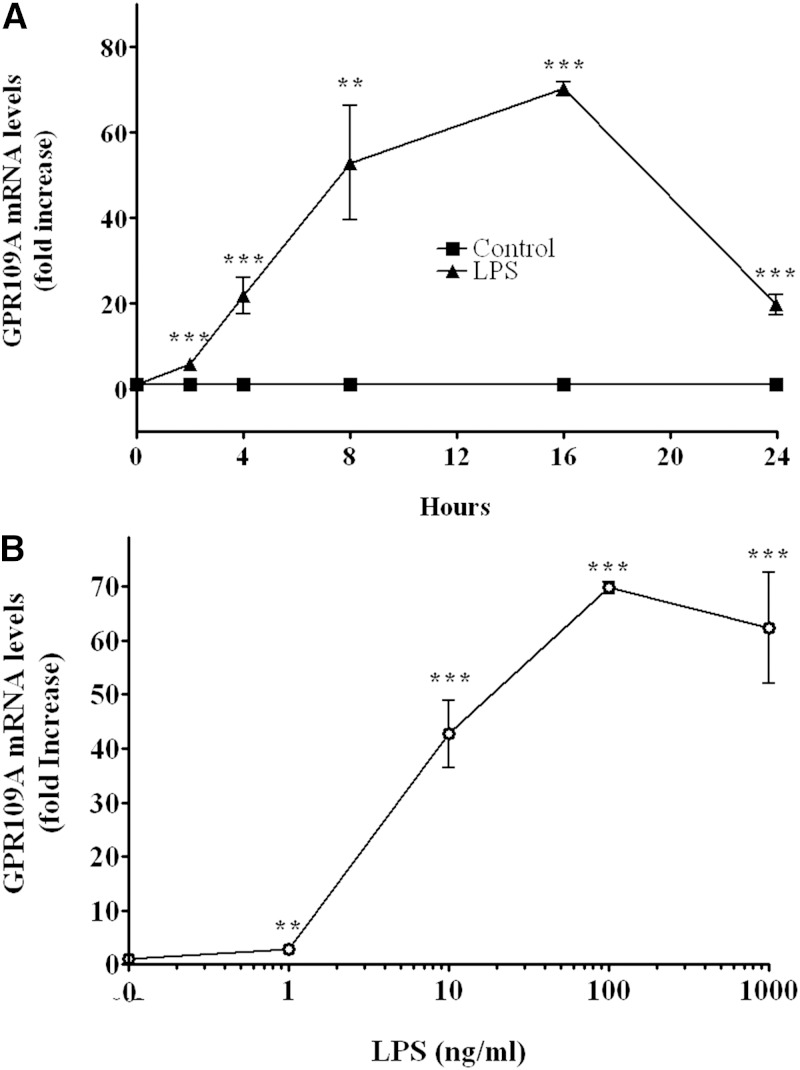
Given the marked increase in expression in RAW cells, we next carried out time course and dose response studies in RAW cells. As shown in Fig. 4A, the increase in GPR109A/HCA2 mRNA occurs rapidly (∼20-fold increase by 4 h), reaches a peak at 16 h (∼60-fold increase), and is sustained for at least 24 h. The ability of LPS to stimulate GPR109A/HCA2 expression is a relatively sensitive response with the half-maximal response occurring at ∼5 ng/ml, an ∼40-fold increase seen at 10 ng/ml, and an ∼65-fold increase seen at 100 ng/ml (Fig. 4B).
Fig. 4.
LPS induction of GPR109A/HCA2 mRNA levels in RAW cells. A: RAW cells were treated with LPS (100 ng/ml) for indicated times. B: RAW cells were treated for 16 h with indicated concentrations of LPS. Total RNA was isolated, and GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3 per group. ** P < 0.01, *** P < 0.001.
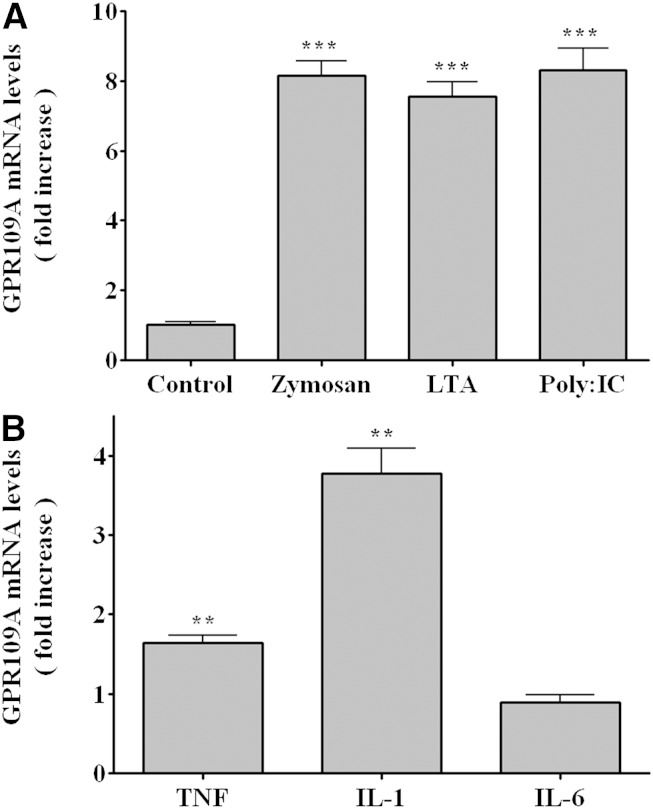
We next examined the effect of other immune stimulators on GPR109A/HCA2 expression in RAW cells. As shown in Fig. 5A, zymosan, a model of fungal infections; LTA, a model of gram-positive bacterial infections; and poly I:C, a model of viral infections, all increased GPR109A/HCA2 mRNA levels ∼8-fold. These increases are considerably smaller than the increase observed with LPS treatment. Additionally, treatment of RAW cells with the cytokines TNF and IL-1 had a smaller effect on GPR109A/HCA2 expression (Fig. 5B). The effect was specific as there was little effect of IL-6.
Fig. 5.
Effect of immune stimulators on GPR109A/HCA2 mRNA levels in RAW cells. A: RAW cells were treated with zymosan (500 μg/ml), LTA (1 μg/ml), or poly I:C (50 μg/ml) for 16 h. B: RAW cells were treated with TNFα, IL-1β, or IL-6 at10 ng/ml for 16 h. Total RNA was isolated, and GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3 per group. ** P < 0.01, *** P < 0.001.
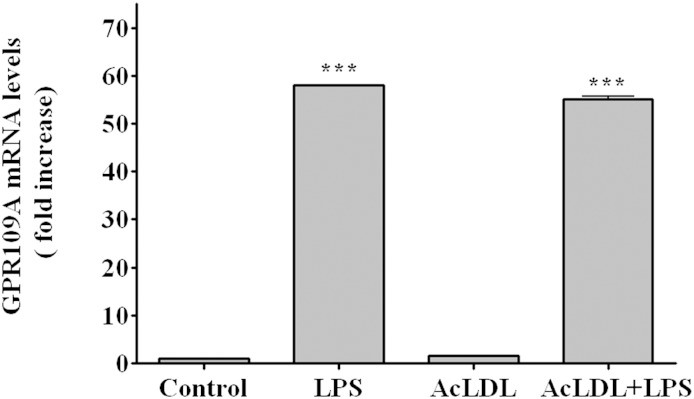
Macrophages in atherosclerotic lesions contain increased quantities of cholesteryl esters. To mimic this pathophysiological state, we incubated RAW cell macrophages with AcLDL, which leads to a marked increase in cholesteryl ester levels. As shown in Fig. 6, under these conditions AcLDL had little effect on GPR109A/HCA2 expression while LPS treatment still resulted in a marked increase in GPR109A/HCA2 expression, similar to cells not treated with AcLDL, indicating that the LPS stimulation of GPR109A/HCA2 expression occurs even in cholesteryl ester-loaded macrophages.
Fig. 6.
Effect of LPS on GPR109A/HCA2 mRNA in AcLDL-loaded RAW cells. RAW cells were coincubated with AcLDL (100 μg/ml) and LPS (100 ng/ml) for 16 h. Total RNA was isolated, and GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3 per group. *** P < 0.001.
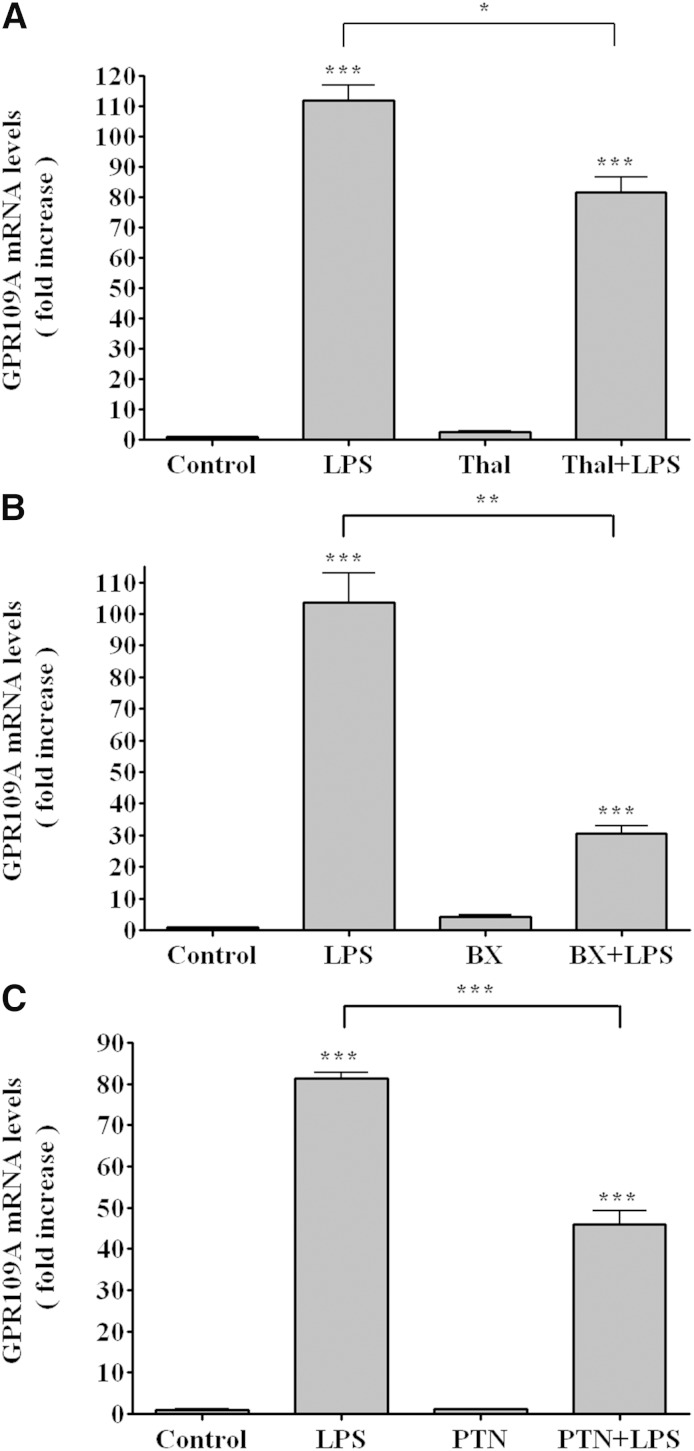
LPS signals via TLR4, which has two major intracellular pathways that regulate gene expression (25). The myeloid differentiation factor 88 (MyD88) pathway increases the activity of nuclear factor κB (NF-κB) and activator protein-1 while the TIR-domain-containing adaptor protein inducing IFNβ (TRIF) pathway increases the activity of NF-κB and interferon regulatory factor 3 (25). We therefore used a number of specific inhibitors to determine the signaling pathways responsible for the LPS-induced increase in GPR109A/HCA2 expression. All of these inhibitors, as expected, decreased the ability of LPS to increase the expression of TNFα (data not shown). As shown in Fig. 7A, thalidomide, an inhibitor of MyD88 (26), partially inhibited the ability of LPS to stimulate GPR109A/HCA2 expression suggesting that the LPS effect on GPR109A/HCA2 expression is not solely dependent on the MyD88 pathway. BX795, which inhibits the TRIF pathway (27), also resulted in a greater, but still partial inhibition of the ability of LPS to increase GPR109A/HCA2 (Fig. 7B), again suggesting that the LPS-induced increase in GPR109A/HCA2 expression is not solely dependent on the TRIF pathway. These results suggest that LPS activation of TLR4 signals an increase in GPR109A/HCA2 expression by both the MyD88 and TRIF pathways. PTN, which blocks NF-κB (28), reduced the ability of LPS to increase GPR109A/HCA2 expression by ∼50% (Fig. 7C), suggesting that both NF-κB pathways and non-NF-κB pathways are important for the LPS effect on GPR109A/HCA2. It is well recognized that LPS induces cytokines, such as TNF, by multiple pathways, and the increase in these cytokines is not fully blocked by inhibiting one pathway. Similarly, we find that inhibitors of a single pathway do not block the increase in GPR109A/HCA2, suggesting that multiple intracellular signaling pathways mediate the LPS-induced stimulation of GPR109A/HCA2 expression.
Fig. 7.
Effect of inhibitors on GPR109A/HCA2 mRNA levels in RAW cells. Cells were preincubated for 1 h with thalidomide (500 μg/ml) (A), BX795 (10 μM) (B), or PTN (20 μM) (C) and LPS (100 ng/ml) for 16 h. Total RNA was isolated, and GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3 per group. * P < 0.05, ** P < 0.01, *** P < 0.001.
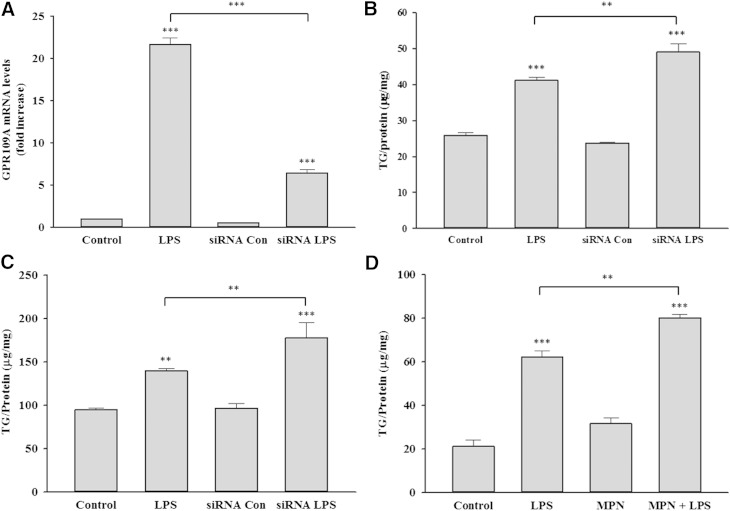
Previous studies by our and other laboratories have shown that the activation of macrophages with LPS and other TLR activators increases macrophage TG accumulation (29, 30). We therefore next examined the effect of preventing the increase in GPR109A/HCA2 activity on the ability of LPS to increase TG accumulation. As shown in Fig. 8A, using siRNA that targets GPR109A/HCA2, we were able to blunt the LPS-induced increase in GPR109A/HCA2. Moreover, as shown in Fig. 8B, this resulted in a small but statistically significant increase in the ability of LPS activation to increase TG accumulation in the macrophages. A similar increase in TG accumulation with LPS stimulation was observed when an identical experiment was carried out in the presence of intralipid, which enhances macrophage TG accumulation (Fig. 8C). Finally, as shown in Fig. 8D, inhibiting GPR109A/HCA2 activity with MPN also resulted in an increase in TG accumulation with LPS treatment.
Fig. 8.
Effect of inhibition of GPR109A/HCA2 on TG accumulation in RAW cells. Cells were transiently transfected with murine GPR109A/HCA2 siRNA or negative control siRNA, followed by treatment with LPS. A: Cells were treated with LPS for 16 h, total RNA was isolated, and GPR109A/HCA2 mRNA levels were quantified by qPCR performed as described in Materials and Methods. Data are expressed as fold increase over controls. B: Cells were treated with LPS for 24 h, and TG levels were measured as described in Materials and Methods. Data are presented as μg TG per mg protein. C: Cells were coincubated with Intralipid and LPS for 24 h, and TG levels were measured. Data are presented as μg TG per mg protein. D: Cells were coincubated with MPN and LPS for 24 h, and TG levels were measured. Data are presented as μg TG per mg protein. The data are presented as the mean ± SEM. N = 3 per group. ** P < 0.01, *** P < 0.001.
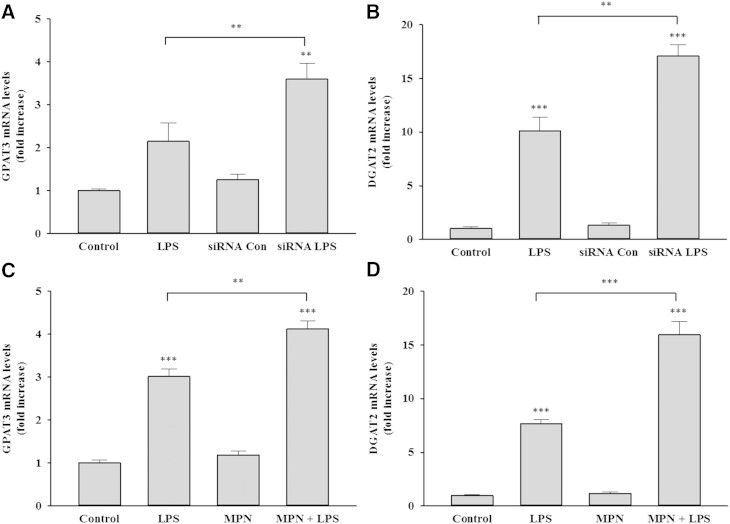
Moreover, previous studies have shown that LPS stimulates the incorporation of fatty acids into TGs, which is associated with the increased expression of GPAT3 and DGAT2, two key enzymes in the synthesis of TGs (29). As seen in prior studies, this increase in LPS-induced TG accumulation is associated with an increase in GPAT3 and DGAT2 expression (Fig. 9). Moreover, knocking down GPR109A/HCA2 expression with siRNA or inhibiting GPR109A/HCA2 activity with MPN resulted in LPS treatment increasing the expression of both GPAT3 and DGAT2 to a greater extent than seen with LPS treatment alone (Fig. 9). These results indicate that the increase in GPR109A/HCA2 expression that occurs during macrophage activation negatively regulates the increase in expression of GPAT3 and DGAT2 limiting the enhanced accumulation of TG that occurs during macrophage activation.
Fig. 9.
Effect of inhibition of GPR109A/HCA2 on gene expression in RAW cells. Cells were transiently transfected with murine GPR109A/HCA2 siRNA or negative control siRNA, followed by treatment with LPS. A: Cells were treated with LPS for 16 h, total RNA was isolated, and GPAT3 mRNA levels were quantified by qPCR performed as described in Materials and Methods. B: Cells were treated with LPS for 16 h, total RNA was isolated, and DGAT2 mRNA levels were quantified by qPCR. Cells were coincubated with MPN and LPS for 16 h, total RNA was isolated, and GPAT3 mRNA levels (C) or DGAT2 mRNA levels (D) were quantified by qPCR performed as described in Materials and Methods. Data are expressed as fold increase over controls. The data are presented as the mean ± SEM. Data are expressed as fold increase over controls. N = 3 per group. ** P < 0.01, *** P < 0.001.
The precise mechanism by which activation of GPR109A/HCA2 regulates the expression of either GPAT3 or DGAT2 is unknown. Studies by other investigators have also shown that niacin acting via GPR109A/HCA2 can directly affect macrophage lipid metabolism. Specifically, niacin has been shown to suppress the increase in LDL uptake that is induced by LPS treatment in wild-type macrophages but not in macrophages deficient in GPR109A/HCA2 (21). Additionally, niacin acting via GPR109A/HCA2 has been shown to stimulate cholesterol efflux by increasing the expression of ABCA1, ABCG1, and CD36 (31–33). These results coupled with our results suggest that activation of GPR109A/HCA2 by niacin, endogenous ligand, or enhanced basal activity decreases the ability of macrophages to accumulate lipid.
It should be noted that despite the beneficial effects of the activation of GPR109A/HCA2 by niacin on macrophage lipid accumulation, two recent randomized studies have failed to demonstrate that niacin therapy when added to statins reduces cardiovascular disease events (34–36). This absence of beneficial effect of niacin in combination with statin therapy contrasts with an earlier study demonstrating that niacin monotherapy reduces cardiovascular disease (37). The explanation for the failure of niacin in combination with a statin to reduce cardiovascular disease is unknown. Of note, statin treatment did not attenuate the increase in GPR109A/HCA2 in macrophages induced by LPS treatment (data not shown).
In conclusion, the present study demonstrates that multiple infectious and inflammatory stimuli stimulate GPR109A/HCA2 expression in adipose tissue and in macrophages. The increase in GPR109A/HCA2 that accompanies macrophage activation decreases the TG accumulation stimulated by macrophage activation as demonstrated by inhibiting the effects of GPR109A/HCA2.
Footnotes
Abbreviations:
- AcLDL
- acetylated low density lipoprotein
- DGAT2
- diacylglycerol transferase 2
- GPAT3
- glycerol 3-phosphate acyltransferase 3
- GPR109A
- G protein-coupled receptor 109A
- HCA2
- hydroxycarboxylic acid 2 receptor
- HSA
- human serum albumin
- IL
- interleukin
- LPS
- lipopolysaccharide
- LTA
- lipoteichoic acid
- MyD88
- myeloid differentiation factor 88
- NF-κB
- nuclear factor κB
- poly I:C
- polyinosine-polycytidylic acid
- PTN
- parthenolide
- qPCR
- quantitative PCR
- TLR
- Toll-like receptor
- TRIF
- TIR-domain-containing adaptor protein inducing IFNβ
This work was supported by grants from the Research Service of the Department of Veterans Affairs and Merck, by National Institutes of Health Grants 5 RO1 AR049932 and 2 RO1 HD29706, and by the Albert L. and Janet A. Schultz Supporting Foundation.
REFERENCES
- 1.Carlson L. A. 2005. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 258: 94–114. [DOI] [PubMed] [Google Scholar]
- 2.Guyton J. R. 2007. Niacin in cardiovascular prevention: mechanisms, efficacy, and safety. Curr. Opin. Lipidol. 18: 415–420. [DOI] [PubMed] [Google Scholar]
- 3.Kamanna V. S., Kashyap M. L. 2008. Mechanism of action of niacin. Am. J. Cardiol. 101: 20B–26B. [DOI] [PubMed] [Google Scholar]
- 4.Butcher R. W., Baird C. E., Sutherland E. W. 1968. Effects of lipolytic and antilipolytic substances on adenosine 3′,5′-monophosphate levels in isolated fat cells. J. Biol. Chem. 243: 1705–1712. [PubMed] [Google Scholar]
- 5.Carlson L. A. 1963. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med. Scand. 173: 719–722. [DOI] [PubMed] [Google Scholar]
- 6.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. 2003. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9: 352–355. [DOI] [PubMed] [Google Scholar]
- 7.Holm C. 2003. Molecular mechanisms regulating hormone-sensitive lipase and lipolysis. Biochem. Soc. Trans. 31: 1120–1124. [DOI] [PubMed] [Google Scholar]
- 8.McKnight G. S., Cummings D. E., Amieux P. S., Sikorski M. A., Brandon E. P., Planas J. V., Motamed K., Idzerda R. L. 1998. Cyclic AMP, PKA, and the physiological regulation of adiposity. Recent Prog. Horm. Res. 53: 139–159; discussion 160–131. [PubMed] [Google Scholar]
- 9.Lorenzen A., Stannek C., Burmeister A., Kalvinsh I., Schwabe U. 2002. G protein-coupled receptor for nicotinic acid in mouse macrophages. Biochem. Pharmacol. 64: 645–648. [DOI] [PubMed] [Google Scholar]
- 10.Lorenzen A., Stannek C., Lang H., Andrianov V., Kalvinsh I., Schwabe U. 2001. Characterization of a G protein-coupled receptor for nicotinic acid. Mol. Pharmacol. 59: 349–357. [DOI] [PubMed] [Google Scholar]
- 11.Davidson M. H. 2008. Niacin use and cutaneous flushing: mechanisms and strategies for prevention. Am. J. Cardiol. 101: 14B–19B. [DOI] [PubMed] [Google Scholar]
- 12.Benyó Z., Gille A., Kero J., Csiky M., Suchánková M. C., Nüsing R. M., Moers A., Pfeffer K., Offermanns S. 2005. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J. Clin. Invest. 115: 3634–3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson J., Gille A., Zwykiel S., Lukasova M., Clausen B. E., Ahmed K., Tunaru S., Wirth A., Offermanns S. 2010. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 120: 2910–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pike N. B. 2005. Flushing out the role of GPR109A (HM74A) in the clinical efficacy of nicotinic acid. J. Clin. Invest. 115: 3400–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., Furuichi K. 2003. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 303: 364–369. [DOI] [PubMed] [Google Scholar]
- 16.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., et al. 2003. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278: 9869–9874. [DOI] [PubMed] [Google Scholar]
- 17.Offermanns S. 2006. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol. Sci. 27: 384–390. [DOI] [PubMed] [Google Scholar]
- 18.Soudijn W., van Wijngaarden I., Ijzerman A. P. 2007. Nicotinic acid receptor subtypes and their ligands. Med. Res. Rev. 27: 417–433. [DOI] [PubMed] [Google Scholar]
- 19.Schaub A., Futterer A., Pfeffer K. 2001. PUMA-G, an IFN-gamma-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur. J. Immunol. 31: 3714–3725. [DOI] [PubMed] [Google Scholar]
- 20.Wanders D., Graff E. C., Judd R. L. 2012. Effects of high fat diet on GPR109A and GPR81 gene expression. Biochem. Biophys. Res. Commun. 425: 278–283. [DOI] [PubMed] [Google Scholar]
- 21.Zandi-Nejad K., Takakura A., Jurewicz M., Chandraker A. K., Offermanns S., Mount D., Abdi R. 2013. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J. 27: 4366–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Digby J. E., McNeill E., Dyar O. J., Lam V., Greaves D. R., Choudhury R. P. 2010. Anti-inflammatory effects of nicotinic acid in adipocytes demonstrated by suppression of fractalkine, RANTES, and MCP-1 and upregulation of adiponectin. Atherosclerosis. 209: 89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. 1986. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc. Natl. Acad. Sci. USA. 83: 8313–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang W., Walsh A., Tabas I. 1999. Macrophage-targeted CTP:phosphocholine cytidylyltransferase (1–314) transgenic mice. Biochim. Biophys. Acta. 1437: 301–316. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T., Akira S. 2006. TLR signaling. Cell Death Differ. 13: 816–825. [DOI] [PubMed] [Google Scholar]
- 26.Noman A. S., Koide N., Hassan F., Khuda I. I-E., Dagvadorj J., Tumurkhuu G., Islam S., Naiki Y., Yoshida T., Yokochi T. 2009. Thalidomide inhibits lipopolysaccharide-induced tumor necrosis factor-alpha production via down-regulation of MyD88 expression. Innate Immun. 15: 33–41. [DOI] [PubMed] [Google Scholar]
- 27.Clark K., Plater L., Peggie M., Cohen P. 2009. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J. Biol. Chem. 284: 14136–14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hehner S. P., Heinrich M., Bork P. M., Vogt M., Ratter F., Lehmann V., Schulze-Osthoff K., Droge W., Schmitz M. L. 1998. Sesquiterpene lactones specifically inhibit activation of NF-kappa B by preventing the degradation of I kappa B-alpha and I kappa B-beta. J. Biol. Chem. 273: 1288–1297. [DOI] [PubMed] [Google Scholar]
- 29.Feingold K. R., Shigenaga J. K., Kazemi M. R., McDonald C. M., Patzek S. M., Cross A. S., Moser A., Grunfeld C. 2012. Mechanisms of triglyceride accumulation in activated macrophages. J. Leukoc. Biol. 92: 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolaou G., Erridge C. 2010. Toll-like receptor-dependent lipid body formation in macrophage foam cell formation. Curr. Opin. Lipidol. 21: 427–433. [DOI] [PubMed] [Google Scholar]
- 31.Gaidarov I., Chen X., Anthony T., Maciejewski-Lenoir D., Liaw C., Unett D. J. 2013. Differential tissue and ligand-dependent signaling of GPR109A receptor: implications for anti-atherosclerotic therapeutic potential. Cell. Signal. 25: 2003–2016. [DOI] [PubMed] [Google Scholar]
- 32.Lukasova M., Malaval C., Gille A., Kero J., Offermanns S. 2011. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121: 1163–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rubic T., Trottmann M., Lorenz R. L. 2004. Stimulation of CD36 and the key effector of reverse cholesterol transport ATP-binding cassette A1 in monocytoid cells by niacin. Biochem. Pharmacol. 67: 411–419. [DOI] [PubMed] [Google Scholar]
- 34.AIM-HIGH Investigators. 2011. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 35. HPS2-THRIVE Collaborative Group. 2013. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34: 1279–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. HPS2-THRIVE Collaborative Group. 2014. Effects of extended-release niacin with laropiprant in high-risk patients. N. Engl. J. Med. 371: 203–212. [DOI] [PubMed] [Google Scholar]
- 37.Canner P. L., Berge K. G., Wenger N. K., Stamler J., Friedman L., Prineas R. J., Friedewald W. 1986. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8: 1245–1255. [DOI] [PubMed] [Google Scholar]