Abstract
To evaluate functional and compositional properties of HDL in subjects from a kindred of genetic apoA-I deficiency, two homozygotes and six heterozygotes, with a nonsense mutation at APOA1 codon -2, Q[-2]X, were recruited together with age- and sex-matched healthy controls (n = 11). Homozygotes displayed undetectable plasma levels of apoA-I and reduced levels of HDL-cholesterol (HDL-C) and apoC-III (5.4% and 42.6% of controls, respectively). Heterozygotes displayed low HDL-C (21 ± 9 mg/dl), low apoA-I (79 ± 24 mg/dl), normal LDL-cholesterol (132 ± 25 mg/dl), and elevated TG (130 ± 45 mg/dl) levels. Cholesterol efflux capacity of ultracentrifugally isolated HDL subpopulations was reduced (up to −25%, P < 0.01, on a glycerophospholipid [GP] basis) in heterozygotes versus controls. Small, dense HDL3 and total HDL from heterozygotes exhibited diminished antioxidative activity (up to −48%, P < 0.001 on a total mass basis) versus controls. HDL subpopulations from both homozygotes and heterozygotes displayed altered chemical composition, with depletion in apoA-I, GP, and cholesteryl ester; enrichment in apoA-II, free cholesterol, and TG; and altered phosphosphingolipidome. The defective atheroprotective activities of HDL were correlated with altered lipid and apo composition. These data reveal that atheroprotective activities of HDL particles are impaired in homozygous and heterozygous apoA-I deficiency and are intimately related to marked alterations in protein and lipid composition.
Keywords: high density lipoprotein, apolipoprotein A-I, familial deficiency, genetics, HDL functionality, cellular cholesterol efflux, antioxidative activity, lipidomics
Severe forms of genetic HDL deficiency due to defects in apoA-I synthesis are characterized by very low HDL-cholesterol (HDL-C; <5 mg/dl) and undetectable plasma apoA-I concentrations (1). ApoA-I deficiency can occur either isolatedly or accompanied by deficiency of apoC-III/apoA-IV or apoC-III. In addition to low HDL-C and apo A-I levels, apoA-I/C-III/A-IV deficiency is characterized by low plasma TG levels due to lack of apoC-III, a lipolysis inhibitor, and malabsorption of fat-soluble vitamins consequent to lack of apoA-IV. Low plasma TG levels and planar xanthomas were described in apoA-I/C-III deficiency (1). Santos et al. (2) have previously characterized the clinical and laboratory profile of a consanguineous kindred consisting of two homozygous and eight heterozygous subjects with apoA-I deficiency caused by a nonsense autosomic dominant mutation, named Q[-2]X. Homozygous subjects presented cutaneous changes secondary to cholesterol accumulation, such as tuboeruptive or tendon xanthomas and corneal opacification as well as early onset of severe coronary heart disease; such anormalities appear to represent the consequence of deficient flux through the reverse cholesterol transport pathway. Analysis of HDL particles from homozygous subjects by two-dimensional gel electrophoresis showed undetectable apoA-I, decreased amounts of small α-3 migrating apoA-II particles, and only modestly decreased amounts of slow α-migrating apoA-IV- and apoE-containing HDL. In the heterozygotes, there was loss of large α-1 HDL particles (2).
Emerging data suggest that genetic factors regulate both circulating levels of HDL and its functionality (3–5). Growing evidence indicates that the atheroprotective effects of HDL are determined by qualitative factors such as the molecular composition of both the lipidome and proteome, in addition to HDL quantity (6, 7). Thus, mutations in various proteins involved in HDL metabolic pathways can potentially affect plasma levels, composition, structure, and function of HDL. It remains controversial, however, as to whether HDL function is altered in families with monogenic disorders involving low HDL-C (8, 9) Therefore, we evaluated the functional as well as compositional characteristics of ultracentrifugally isolated HDL particles in this well-characterized apoA-I-deficiency kindred. Two major metrics of HDL functionality, cellular cholesterol efflux capacity and antioxidative activity, were found to be impaired in apoA-I deficiency, in parallel to pronounced alterations in the composition of HDL particles.
MATERIALS AND METHODS
Subjects
Functional and compositional properties of HDL particles were evaluated in a Brazilian kindred with familial apoA-I deficiency resulting from a nonsense mutation at codon −2, Q[-2]X, in APOA1 gene (10) and associated with premature cardiovascular disease (2). As previously described (2), the kindred was composed of two male homozygotes, eight heterozygotes, and two unaffected subjects. Homozygotes presented markedly decreased HDL-C levels, undetectable plasma apoA-I, tuboeruptive and planar xanthomas, mild corneal arcus and opacification, and severe premature coronary artery disease. For the present study, two male homozygotes and six heterozygotes (five males and one female) were recruited.
The apoA-I-deficient subjects were compared with 11 age-matched healthy male normolipidemic controls recruited at the same center, Heart Institute (InCor) of the University of Sao Paulo Hospital, Sao Paulo, Brazil. All subjects had been off lipid-lowering medication for at least 2 months at the time of assessment.
Written informed consent was obtained from all subjects, and the project was approved by the Ethics Committee of the Heart Institute-InCor in accordance with local institutional guidelines conformed to the Declaration of Helsinki.
Blood samples
Blood samples were withdrawn from the cubital vein of each participant at the time of recruitment. Serum and EDTA plasma (final EDTA concentration; 1 mg/ml) were prepared from venous blood collected into sterile, evacuated tubes (Vacutainer). Plasma was immediately separated by low-speed centrifugation at 4°C; serum and plasma were each mixed with sucrose (final concentration, 0.6%) as a cryoprotectant for lipoproteins (11), aliquoted, and frozen at −80°C under nitrogen; each aliquot was thawed only once directly before analyses.
Standard biochemical parameters
Plasma levels of total cholesterol (TC), TG, and HDL-C were measured using commercially available enzymatic kits; LDL-cholesterol (LDL-C) was calculated using the Friedewald formula (12). Plasma apoA-I and apoB were quantitated by immunoturbidimetry (13). Systemic inflammation was assessed as the plasma level of high-sensitive C-reactive protein (hsCRP) measured by immunoassay (14).
Determination of endogenous plasma cholesteryl ester transfer from HDL to apoB-containing lipoproteins (cholesteryl ester transfer protein [CETP] activity)
Determination of endogenous plasma cholesteryl ester (CE) transfer from HDL to apoB-containing lipoproteins was performed by modification of the method of Guérin et al. (15) as previously described (16). CE transfer was determined after incubation of whole unlabeled individual plasmas (500 µl) at 37°C or 0°C for 3 h in the presence of trace amounts of radiolabeled normolipidemic HDL (<5% of the total HDL-CE mass in plasma). Iodoacetate was present at the final concentration of 1.5 mM to inhibit LCAT. Under these conditions, the level of endogenous HDL-CE in the assay does not impact the transfer of the radioactive label (17). The radioactive content of lipoprotein plasma was quantified by liquid scintillation spectrometry with a Trilux 1450 β counter (Perkin Elmer). CE transferred from HDL was calculated using the difference between the radioactivity counts at 37°C and 0°C and expressed as % radioactivity recovered in plasma.
Isolation of lipoproteins
Plasma lipoproteins were isolated from serum and plasma by single step, isopycnic nondenaturing density gradient ultracentrifugation in a Beckman SW41 Ti rotor at 40,000 rpm for 44 h in a Beckman XL70 ultracentrifuge at 15°C by a slight modification of the method of Chapman et al. (12) as previously described (18). After centrifugation, each gradient was fractionated in predefined volumes from the meniscus downward with an Eppendorf precision pipette into 11 fractions corresponding to VLDL + IDL (d < 1.019 g/ml), LDL (five subfractions: LDL1, d = 1.019–1.023 g/ml; LDL2, d = 1.023–1.029 g/ml ; LDL3, d = 1.029–1.039 g/ml; LDL4, d = 1.039–1.050 g/ml; and LDL5, d = 1.050–1.063 g/ml), and HDL (five subfractions: HDL2b, d = 1.063–1.091 g/ml; HDL2a, d = 1.091–1.110 g/ml; HDL3a, d = 1.110–1.133 g/ml; HDL3b, d = 1.133–1.156 g/ml; and HDL3c, d = 1.156–1.179 g/ml). The validity and reproducibility of this density gradient procedure, which facilitates fractionation of HDL particle subspecies in a nonoxidized, native state, have been extensively documented (12, 19). Lipoproteins were extensively dialyzed against PBS (pH 7.4) at 4°C in the dark, stored at 4°C, and used within 10 days.
Chemical analysis of lipoproteins
Total protein, TC, free cholesterol (FC), glycerophospholipid (GP), and TG contents of isolated lipoprotein subfractions were determined using commercially available assays (12, 13). CE was calculated by multiplying the difference between total and FC concentrations by 1.67 (12). Total lipoprotein mass was calculated as the sum of total protein, CE, FC, GP, and TG. ApoA-I, apoA-II, and apoE content in HDL was quantitated by immunoturbidimetry (Diasys, France).
Lipidome
Lipid standards.
1,2-Dipalmitoyl-sn-glycero-3-phosphocholine-N,N,N-trimethyl-d9 (PC 16:0/16:0 d9), 1-lauroyl-2-tridecanoyl-sn-glycero-3-phospho-(1’-myo-inositol) (PI 12:0/13:0), 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphoethanolamine (PE 12:0/13:0), 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (PG 12:0/13:0), 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phosphate (PA 12:0/13:0), 1-dodecanoyl-2-tridecanoyl-sn-glycero-3-phospho-l-serine (PS 12:0/13:0), 1-pentadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 15:0), and N-heptadecanoyl-d-erythro-sphingosine (Cer d18:1/17:0) were used as internal standards. 1-Palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 16:0), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC 18:0), 1,2-dimyristoyl-sn-glycero-3-phosphocholine (PC 14:0/14:0), 1-myristoyl-2-palmitoyl-sn-glycero-3-phosphocholine (PC 14:0/16:0), 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (PC 16:0/16:0), 1-palmitoyl-2-stearoyl-sn-glycero-3-phosphocholine (PC 16:0/18:0), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC 16:0/18:1), 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PC 16:0/18:2), 1,2-distearoyl-sn-glycero-3-phosphocholine (PC 18:0/18:0), 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine (PC 18:0/18:1), 1-stearoyl-2-linoleoyl-sn-glycero-3-phosphocholine (PC 18:0/18:2), 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (PC 18:0/20:4), 1-palmitoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PC 16:0/22:6), 1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine (PC 18:0/22:6), 1-stearoyl-2-hydroxy-sn-glycero-3-phosphoethanolamine (LPE 18:0), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (PE 18:0/18:0), 1-heptadecanoyl-2-(9Z-tetradecenoyl)-sn-glycero-3-phospho-(1’-myo-inositol) (PI 17:0/14:1), N-stearoyl-d-erythro-sphingosine (Cer d18:1/18:0), 1,2-distearoyl-sn-glycero-3-phosphate (PA 18:0/18:0), 1,2-distearoyl-sn-glycero-3-phospho-(1’-rac-glycerol) (PG 18:0/18:0), and 1-palmitoyl-2-linoleoyl-sn-glycero-3-phospho-l-serine (PS 16:0/18:2) were purchased from Avanti Polar Lipids (Alabaster, AL). LC/MS-grade solvents were used without further purification and obtained from Sigma-Aldrich (St. Louis, MO) or VWR (West Chester, PA).
Extraction.
HDL subpopulations were extracted according to a procedure adapted from Larijani et al. (20) Briefly, 30 µg of total GP mass determined using a commercially available assay was added to 4 ml of cold CHCl3/acidified CH3OH (5:2 v/v) containing 4 µg of PC d9 32:0, 100 ng of PI 25:0, 80 ng of PE 25:0, 80 ng of PA 25:0, 40 ng of PS 25:0, 20 ng of PG 25:0, and 20 ng of Cer 17:0. A blank (PBS) and a control (HDL2 obtained from a reference normolipidemic plasma) sample were extracted in parallel with each batch to ensure quality control; each sample was corrected for blank readings. K4EDTA (200 mM) solution was added (1:5 v/v), and the mixture was vortexed for 1 min and centrifuged at 3,600 g for 10 min at 4°C. The organic phase was transferred into 5 ml Chromacol glass tubes and dried under nitrogen. Lipids were reconstituted into 150 µl isopropanol-hexane-water (10:5:2 v/v), transferred into LC/MS amber vials with inserts, dried under nitrogen, and resuspended in 40 µl of isopropanol-hexane-water (10:5:2 v/v). Molecular lipid species were analyzed and quantitated by LC/MS/MS.
LC/MS analysis.
Seven principal GP subclasses [phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), phosphatidylinositol (PI), phosphatidylglycerol (PG), phosphatidylserine (PS), and phosphatidic acid (PA)] and two principal sphingolipid (SL) subclasses [sphingomyelin (SM) and ceramide (Cer)], which together comprise >160 individual molecular lipid species and account for >95% of total plasma GP and SM (21, 22), were assayed by LC/MS/MS. The lipid subclasses were divided into major (those whose content was >1% of total GP + SL, i.e., PC, SM, LPC, PE, and PI) and minor (those whose content was <1% of total GP + SL, i.e., PG, Cer, PS, and PA).
Lipids were quantified by LC-ESI/MS/MS using a QTrap 4000 mass spectrometer (AB Sciex, Framingham, MA) equipped with a turbo spray ion source (300°C) combined with an LC20AD HPLC system, a SIL-20AC autosampler (Shimadzu, Kyoto, Japan), and the Analyst 1.5 data acquisition system (AB Sciex).
Quantification of GPs and SLs was performed in positive-ion mode, except for PI species that were detected in negative-ion mode. Sample (4 µl) was injected onto a Symmetry Shield RP8 3.5 µm 2.1 × 50 mm reverse phase column (Waters Corporation, Milford, MA) using a gradient from 85:15 to 91:9 (v/v) methanol-water containing 5 mM ammonium formate and 0.1% formic acid at a flow rate of 0.1 ml/min for 30 min. Lipid species were detected using multiple reaction monitoring reflecting the headgroup fragmentation of each lipid class. PC, LPC, and SM species were detected as product ions of m/z 184; PE, PS, PG, and PA as neutral losses of respectively m/z 141, 185, 189, and 115; and PI molecular species as product ions of m/z −241. Air was used as nebulizing gas and N2 as collision gas. PE, PS, PG, PI, PA, and Cer species were monitored for 18 ms; PC, LPC, and SM species were monitored for 30 ms at a unit resolution (0.7 amu at half peak height).
Quantification.
Lipids were quantified using calibration curves specific for the nine individual lipid classes with up to 12 component fatty acid moieties. Twenty-three calibration curves were generated in nondiluted and 10-fold diluted matrices to correct for matrix-induced ion suppression effects. More abundant lipid species that displayed a nonlinear response in nondiluted extracts were quantified from a 10- or 100-fold diluted sample. An in-house-developed Excel Macro script (Microsoft Office 2010, Redmond, WA) was used to compile data from the three successive injections.
HDL enrichment in LPC
Total HDL fraction (1,500 µg GP of a mixture of HDL2b, 2a, 3a, 3b, and 3c subfractions at their equivalent plasma concentrations isolated from normolipidemic plasma by density gradient ultracentrifugation as described previously) was incubated with LPC suspensions overnight at 4°C under constant stirring. LPC (50 or 250 µg) was placed as a chloroformic solution in an empty tube, chloroform evaporated, and PBS (500 µl) added. The suspension was vigorously vortexed for 1 min and sonicated for 20 min at a maximal power in a Branson 2510 sonicator (Branson Ultrasonics, Danbury, CT) immediately before incubation with HDL. HDL from the same subject incubated in parallel with PBS in the absence of exogenous lipid was used as a control. Subsequently, lipid-enriched and control HDL subfractions were reisolated by density gradient ultracentrifugation as described previously to remove non-HDL-associated exogenous lipids, and cholesterol efflux capacity and antioxidative activity of HDL were measured as described subsequently.
In some experiments, LPC was incubated with whole plasma to obtain LPC-enriched HDL. LPC (2.5 or 5 mg) was placed as a chloroformic solution in an empty tube, chloroform evaporated, and plasma (3 ml) added. The suspension was gently mixed for 1 min and incubated overnight at 4°C under constant stirring. Plasma from the same subject was incubated in parallel in the absence of LPC as a control. LPC-enriched and control HDLs were isolated by density gradient ultracentrifugation as described previously.
Cholesterol efflux capacity of HDL
Cholesterol efflux capacity of HDL subpopulations (HDL2b, HDL2a, HDL3a, HDL3b, and HDL3c) and of total HDL were characterized in a human THP-1 monocytic cell system (ATCC) at 15 µg HDL-GP/ml for HDL subpopulations and at 30 µg HDL-GP/ml for total HDL. THP-1 cells differentiated into macrophage-like cells with PMA and loaded with acetylated LDL (acLDL) efflux cellular cholesterol predominantly via ABCA1 (23). Total HDL from each donor was prepared by mixing all five HDL subfractions at their equivalent plasma concentrations. HDL particles were compared on the basis of their GP concentrations because GP was shown to represent the key component determining cholesterol efflux capacity of HDL (24). Cholesterol efflux capacity of whole plasma samples (2.5%) was also assessed.
Assays of cellular cholesterol efflux were performed as previously described with minor modifications. In brief, THP-1 monocytes were cultured on 24-well tissue culture plates and grown in RPMI 1640 media with 10% FBS and differentiated into macrophage-like cells with 50 ng/ml PMA for 48 h. The cells were washed and loaded for 24 h with [3H]cholesterol-labeled acLDL (1 µCi/ml) in serum-free RPMI 1640 supplemented with 50 mM glucose, 2 mM glutamine, 0.2% BSA (RGGB), 100 µg/ml penicillin, and 100 µg/ml streptomycin to allow cell cholesterol pools to equilibrate. The labeling medium was removed, and human macrophages were then equilibrated in RGGB for an additional 16–24 h period. Cellular cholesterol efflux to 15 µg/ml HDL-GP for HDL subpopulations and to 30 µg/ml HDL-GP for total HDL was assayed in serum-free medium for a 4 h chase period. [3H]cholesterol-labeled cells were also incubated 4 h at 37°C in the presence of 40-fold diluted total plasma. Finally, culture media were harvested and cleared of cellular debris by a brief centrifugation. Cell radioactivity was determined by extraction in hexane-isopropanol (3:2 v/v), evaporation of the solvent, and liquid scintillation counting (Wallac Trilux 1450 Microbeta, Perkin Elmer). The percentage of cholesterol efflux was calculated as (medium cpm) / (medium cpm + cell cpm) × 100%. Specific cholesterol efflux was determined by subtracting nonspecific cholesterol efflux occurring in the absence of cholesterol acceptors.
Antioxidative activity of HDL
Antioxidative activities of serum-derived HDL3b and 3c subpopulations (final concentration of each, 10 mg total mass/dl), and of total HDL (final concentration, 40 mg total mass/dl), were assessed toward reference LDL isolated from one healthy normolipidemic control subject (14, 25, 26). Total HDL from each donor was prepared as described previously. HDL particles were compared on the basis of their total mass concentrations because both protein and lipid components were shown to contribute to the capacity of HDL to inhibit LDL oxidation (29, 28). Serum was used as a source of HDL for this assay to ensure intact paraxonase activity, which is inhibited by EDTA (29).
HDL subfractions were added to LDL directly before oxidation by an azo-initiator 2,2′-azo-bis-(2-amidinopropane) hydrochloride (AAPH; 1 mM) and accumulation of conjugated dienes was measured as the increment in absorbance at 234 nm (13, 14, 25, 26). Absorbance kinetics were corrected for the absorbance of AAPH itself run in parallel as a blank. The kinetics of diene accumulation revealed two characteristic phases, the lag and propagation phases. For each curve, the duration of each phase, average oxidation rates within each phase, and amount of dienes formed at the end of the propagation phase (maximal amount of dienes) were calculated (13, 14, 25, 26).
Statistical analysis
Distributions of all variables were analyzed for normality using the Kolmogorov-Smirnov test. Normally distributed variables are expressed as means ± SD unless otherwise indicated; non-Gaussian distributed variables are expressed as median (minimum, maximum). Between-group differences in normally distributed variables were analyzed using Student’s t-test. For non-Gaussian distributed variables, the Mann-Whitney U-test was used, or they were log transformed to ensure normality before statistical analysis. Differences in dichotomous variables were analyzed by Fisher’s exact test. Spearman’s correlation coefficients were calculated to evaluate relationships between variables.
RESULTS
Clinical and standard laboratory parameters
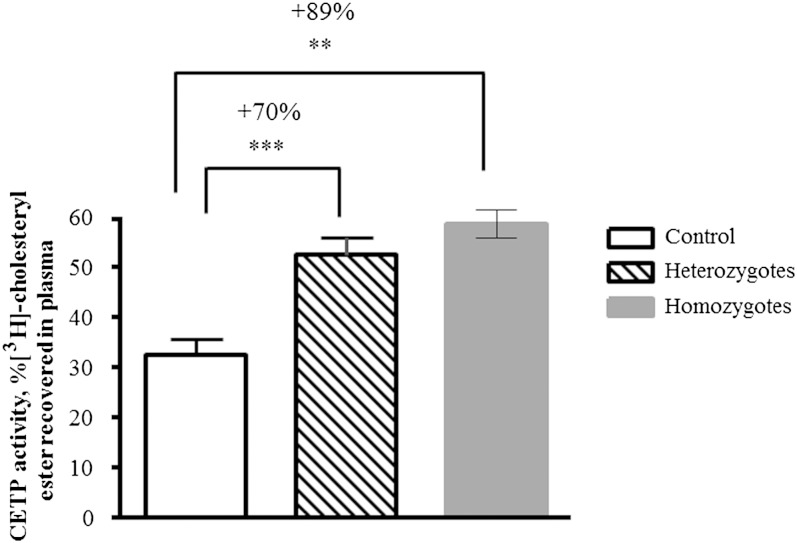
Table 1 shows clinical characteristics and biochemical parameters of controls as well as heterozygotes and homozygotes for apoA-I deficiency. Homozygotes displayed mean values of HDL-C, apoA-I, and apoC-III that were 5.4%, 0%, and 42.6% of normal plasma levels, respectively (all P < 0.05). Plasma lipid profiles of heterozygotes revealed characteristics of atherogenic dyslipidemia, with significantly reduced HDL-C (−62%, P < 0.001) and apoA-I (−40%, P < 0.001) levels and significantly elevated TG (+50%, P < 0.01), non-HDL-C (+28%, P < 0.05), and GP (+20%, P < 0.001) levels relative to normolipidemic controls, with normal fasting glucose plasma levels. No significant difference was observed in BMI between groups (Table 1). Consistent with these data, plasma CETP activity was significantly increased (+70%, P < 0.001) in heterozygotes versus controls (Fig. 1). Despite normal TG levels in homozygous subjects, they also revealed elevated plasma CETP activity (+89%, P < 0.01; Fig. 1). Furthermore, homozygotes but not heterozygotes displayed significantly increased plasma levels of hsCRP (15-fold, P < 0.01; Table 1), documenting a proinflammatory state. Importantly, homozygotes did not present with an acute illness and were relatively stable during blood collection. Indeed, plasma samples taken from these subjects at another occasion also revealed elevated levels of hsCRP, with a mean of 4.4 mg/l (range, 1.8 to 7.0 mg/l).
TABLE 1.
Clinical and biological characteristics of apoA-I-deficient subjects and age- and sex-matched controls
| Controls | Heterozygous Subjects (n = 6) | Homozygous Subjects (n = 2) | |
| Age, years | 54 ± 11 | 41 ± 2 | 41 (40, 42) |
| BMI, kg/m2 | 24.4 ± 3.1 | 26.1 ± 7.0 | 29.3 (32.2, 26.5) |
| Male/female | 11/0 | 5/1 | 2/0 |
| Plasma parameters | |||
| TC, mg/dl | 189 ± 24 | 191 ± 7 | 169 (142, 196) |
| LDL-C, mg/dl | 119 ± 26 | 132 ± 25 | 145 (120, 170) |
| HDL-C, mg/dl | 52 ± 11 | 21 ± 9a | 4 (3, 5)b |
| Non-HDL-C, mg/dl | 134 ± 28 | 171 ± 13c | 164 (137, 190) |
| TGs, mg/dl | 87 ± 27 | 130 ± 42c | 94 (86, 101) |
| GPs, mg/dl | 223 ± 29 | 267 ± 29a | 209 (202, 215) |
| ApoB100, mg/dl | 99 ± 23 | 114 ± 14 | 104 (81, 127) |
| ApoA-I, mg/dl | 132 ± 17 | 79 ± 24a | 0a |
| ApoC-III, mg/dl | 7.5 ± 2.0 | 6.0 ± 3.0 | 3.3 (3.1, 3.4)b |
| Fasting glucose, mg/dl | 91 ± 8 | 85 ± 10 | 83 (88, 78) |
| hsCRP, mg/l | 0.60 (0.20; 4.5) | 0.93 (0.37; 8.87) | 9.3 (4.7, 13.8)b |
Data obtained in two heterozygotes are shown as means (individual values).
P < 0.001; versus control subjects.
P < 0.01; versus control subjects.
P < 0.05; versus control subjects.
Fig. 1.
Plasma CETP activity in subjects with homozygous (n = 2) and heterozygous (n = 6) apoA-I deficiency and control subjects. [3H]CE-labeled HDL was incubated with unlabeled plasma for 3 h, and CETP activity was determined as radioactivity recovered in plasma. The number denotes % increase in CETP activity in apoA-I-deficient subjects versus control group; *** P < 0.001, ** P < 0.01 versus controls. Data obtained in two homozygotes are shown as means, and the range of individual values is represented as min–max.
Plasma levels and total lipid composition of HDL particles
Table 2 displays total mass, lipid composition, and protein content of HDL subpopulations in study subjects. Plasma concentrations of all HDL subpopulations were decreased in homozygotes with familial apoA-I deficiency relative to controls (up to −91%, P < 0.001), whereas in heterozygotes, solely plasma concentrations of large, light, cholesterol-rich HDL2b and 2a were significantly affected (up to −57% vs. controls, P < 0.01; Table 2).
TABLE 2.
Total mass and % lipid and protein composition (wt/wt) of HDL subpopulations from apoA-I-deficient patients and control subjects
| Group | HDL2b | HDL2a | HDL3a | HDL3b | HDL3c |
| Total mass (mg/dl) | |||||
| Control | 83.6 ± 33.2 | 94.5 ± 12.3 | 79.6 ± 14.6 | 35.3 ± 7.1 | 15.1 ± 3.4 |
| Heterozygotes | 36.3 ± 8.0b | 56.5 ± 19.0a | 66.7 ± 23.0 | 32.0 ± 12.0 | 12.8 ± 4.0 |
| Homozygotes | 23.0 (23.5, 22.6)b | 8.5 (8.1, 8.6)a | 8.2 (7.4, 9.0)a | 6.5 (5.8, 7.2)a | 3.1 (2.8, 3.4)a |
| CE, wt% | |||||
| Control | 28.5 ± 5.0 | 23.6 ± 3.7 | 22.2 ± 4.1 | 18.8 ± 3.2 | 16.6 ± 2.4 |
| Heterozygotes | 31.4 ± 2.6 | 20.1 ± 1.3c | 18.9 ± 0.9 | 17.3 ± 2.3 | 12.4 ± 3.0b |
| Homozygotes | 25.7 (24.0, 27.4) | 19.1 (18.3, 20.4) | 16.8 (17.0, 16.6) | 8.7 (8.9, 8.6)a | 13.9 (17.15, 10.8) |
| TG, wt% | |||||
| Control | 5.6 ± 2.3 | 3.8 ± 1.5 | 3.4 ± 1.3 | 3.1 ± 1.7 | 3.4 ± 1.9 |
| Heterozygotes | 7.2 ± 1.8 | 4.9 ± 1.0 | 4.7 ± 1.2c | 4.2 ± 1.8 | 3.1 ± 0.9 |
| Homozygotes | 7.2 (5.5, 8.9) | 6.5 (6.9, 6.0)c | 6.5 (8.2, 4.8)c | 3.0 (3.0, 3.0) | 2.9 (4.0, 1.7) |
| GP, wt% | |||||
| Control | 31.5 ± 5.1 | 29.0 ± 4.5 | 28.1 ± 3.5 | 26.1 ± 2.6 | 20.0 ± 2.6 |
| Heterozygotes | 21.9 ± 3.6b | 27.8 ± 2.7 | 25.7 ± 1.7 | 20.9 ± 1.9a | 15.3 ± 0.7a |
| Homozygotes | 24.1 (26.7, 21.6) | 25.0 (25.9, 24.3) | 20.5 (20.8, 20.0)c | 17.0 (17.4, 16.5)a | 12.3 (13.8, 12.0)a |
| FC, wt% | |||||
| Control | 6.5 ± 0.7 | 3.9 ± 0.5 | 2.9 ± 0.3 | 2.5 ± 0.3 | 2.0 ± 0.4 |
| Heterozygotes | 8.7 ± 1.1a | 3.7 ± 0.1 | 2.9 ± 0.2 | 2.7 ± 0.3 | 2.8 ± 0.7c |
| Homozygotes | 12.3 (13.9, 10.6)a | 8.3 (9.9, 6.7)a | 4.6 (5.4, 3.9)a | 5.9 (7.6, 4.3)a | 5.7 (5.3, 6.1)a |
| Total protein, wt% | |||||
| Control | 28.1 ± 4.6 | 39.5 ± 5.2 | 43.5 ± 4.5 | 49.1 ± 5.6 | 58.3 ± 4.8 |
| Heterozygotes | 30.8 ± 1.2 | 43.4 ± 2.4 | 47.7 ± 2.4 | 54.9 ± 3.0c | 66.4 ± 3.0b |
| Homozygotes | 30.6 (29.8, 31.5) | 40.9 (39.0, 42.8) | 51.5 (48.6, 54.6)c | 59.0 (63.1, 67.0)b | 64.4 (59.7, 69.3) |
Data obtained in two heterozygotes are shown as means (individual values).
P < 0.001; versus controls.
P < 0.01; versus controls.
P < 0.05; versus controls.
Total lipid composition of HDL was markedly altered in familial apoA-I deficiency. Neutral lipids of the hydrophobic HDL core were markedly affected in homozygotes. When expressed as wt%, CE content tended to be depleted in all HDL subpopulations, reaching significance in HDL3b (−54%, P < 0.001; Table 2). In parallel, homozygous HDL2a and HDL3a were significantly enriched in TG, when expressed as wt% (+71%, and +91%, P < 0.05, respectively; Table 2). As a result, the CE/TG ratio was significantly reduced in HDL3a and HDL3b (up to −63%, P < 0.05) and tended to be reduced in all other HDL subpopulations in homozygous subjects as compared with controls, consistent with enhanced CETP activity (Fig. 1).
Polar lipids of the HDL surface monolayer were also markedly influenced by the apoA-I deficiency in homozygotes. Indeed, decreases in wt% GP content in HDL3a, HDL3b, and HDL3c (up to −35%, P < 0.001; Table 2) were found in homozygous relative to controls; relevant difference in FC content was observed between the groups, with increased wt% FC content in all HDL subpopulations (up to 2.9-fold in HDL3c, P < 0.001). Interestingly, altered relative content of core versus surface lipids, manifested as a modestly reduced (CE + TG)/(GP + FC) ratio, was only observed in HDL3b particles (−32%, P < 0.05).
HDL subpopulations from heterozygous subjects equally displayed altered chemical composition, with enrichment in core TG (significantly in HDL3a; +48%, P < 0.05) and depletion in CE (significantly in HDL2a and HDL3c; up to −26%, P < 0.01). As a result, the CE/TG ratio was significantly reduced in HDL2a, HDL3a, and HDL3b (up to −46%, P < 0.05; Table 2) and tended to be reduced in other HDL subpopulations in heterozygous subjects as compared with controls, consistent with elevated CETP activity (Fig. 1).
As in homozygotes, polar lipids were also affected in heterozygotes, with depletion in wt% GP content in HDL2b, HDL3b, and HDL3c (up to −31%, P < 0.001; Table 2) and enrichment in wt% FC content in HDL2b and HDL3c (up to +42%, P < 0.001). Interestingly and in contrast to homozygotes, altered relative content of core versus surface lipids was manifested as an increased (CE + TG)/(GP + FC) ratio, as observed in HDL2b and HDL3b particles (up to +42%, P < 0.01).
Protein composition of HDL particles
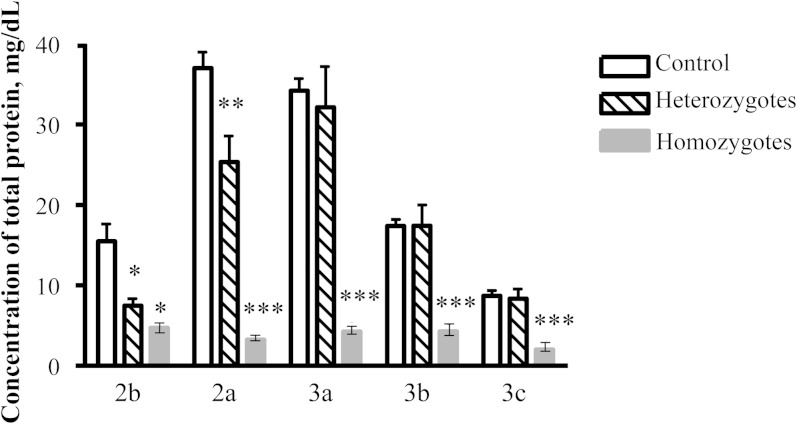
The total protein concentrations in all HDL particles (as mg/dl) were significantly reduced in homozygotes (Fig. 2), whereas in heterozygotes, significant reduction in this parameter was only observed in large, light HDL2 (up to −52%, P < 0.01; Fig. 2). By contrast, HDL content of total protein expressed as wt% was unchanged in large, light HDL2 and elevated in small, dense HDL3 in both homo- and heterozygotes (Table 2), indicating that protein components can compensate for the deficiency in surface lipid components in HDL from heterozygotes.
Fig. 2.
Total protein concentration in HDL subpopulations, expressed as mg/dl, in homozygous (n = 2) and heterozygous (n = 6) apoA-I-deficient subjects. Note that no apoA-I was detected in HDL from the homozygous subjects; ** P < 0.01, * P < 0.05 versus controls (n = 11). Data obtained in two homozygotes are shown as means, and the range of individual values is represented as min–max.
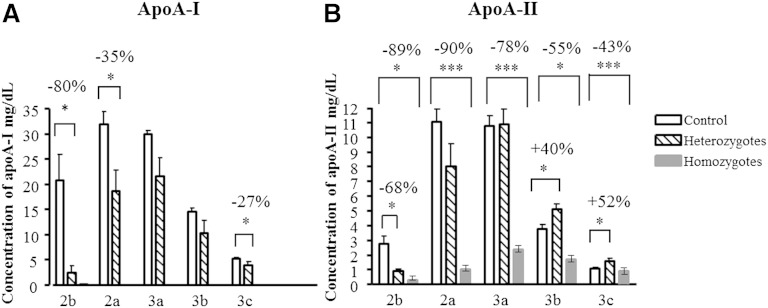
Expectedly, apoA-I, the major HDL apo in controls, was absent from HDL in homozygotes. Circulating levels of apoA-I were significantly diminished in HDL2b, 2a, and 3c in heterozygotes (up to −80%, P < 0.05; Fig. 3A). By contrast, apoA-II was detected in all HDL subpopulations obtained from homozygotes. Circulating levels of apoA-II were differentially affected in heterozygotes, with a reduction observed in HDL2b (−68%, P < 0.05; Fig. 3B) and an elevation found in HDL3b and 3c (up to +52%, P < 0.05; Fig. 3B).
Fig. 3.
ApoA-I (A) and apoA-II (B) concentrations in HDL subpopulations, expressed as mg/dl, in homozygous (n = 2) and heterozygous (n = 6) apoA-I-deficient subjects. Note that no apoA-I was detected in HDL from the homozygous subjects; ** P < 0.01, * P < 0.05 versus controls (n = 11). Data obtained in two homozygotes are shown as means, and the range of individual values is represented as min–max.
HDL content of apoA-I (as wt%) was significantly reduced in heterozygous HDL2b and 3b, whereas that of apoA-II was significantly elevated in both homo- and heterozygous HDL3b and 3c (data not shown).
ApoE only provided a minor contribution to the HDL proteome that did not exceed 2% of total protein and tended to be elevated in apoA-I-deficient subjects (data not shown).
As reported earlier (30), HDL2b from both apoA-I-deficient subjects and controls contained low amounts of Lp(a) (<5% of total HDL protein in control HDL2b; data not shown), reflecting the presence of Lp(a) whose hydrated density overlaps that of HDL2 (31). As a result of low plasma concentrations of HDL2b in apoA-I-deficient subjects, such contamination by Lp(a), however, contributed some 23% of total protein to this subfraction (data not shown).
Lipidome of HDL subpopulations
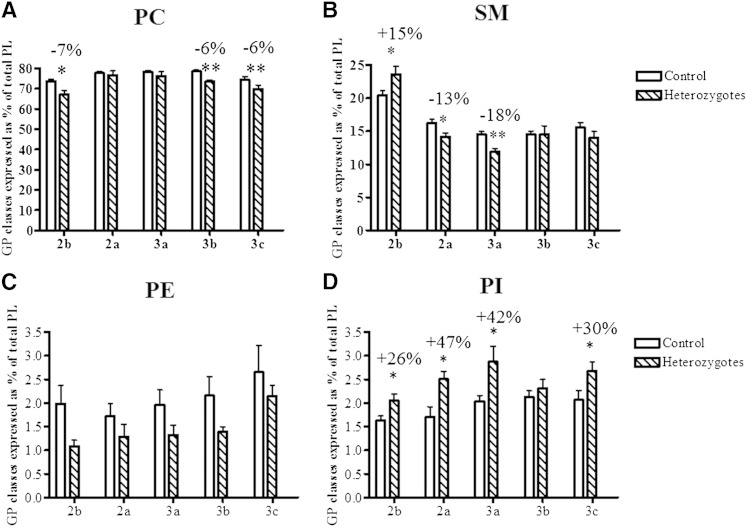
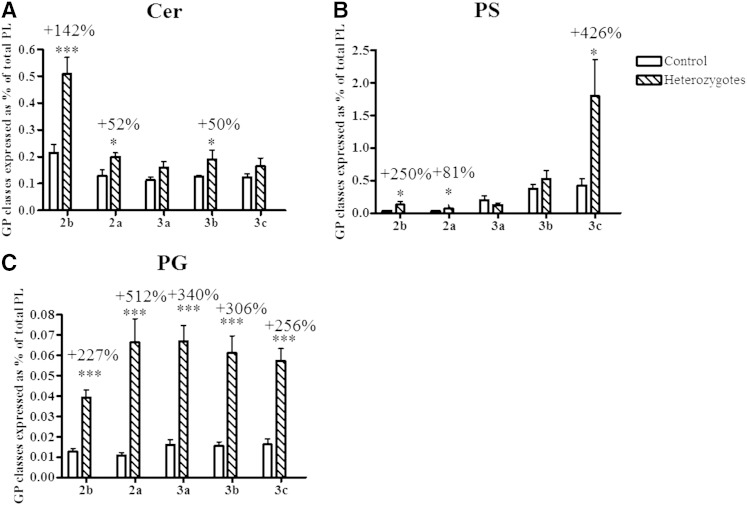
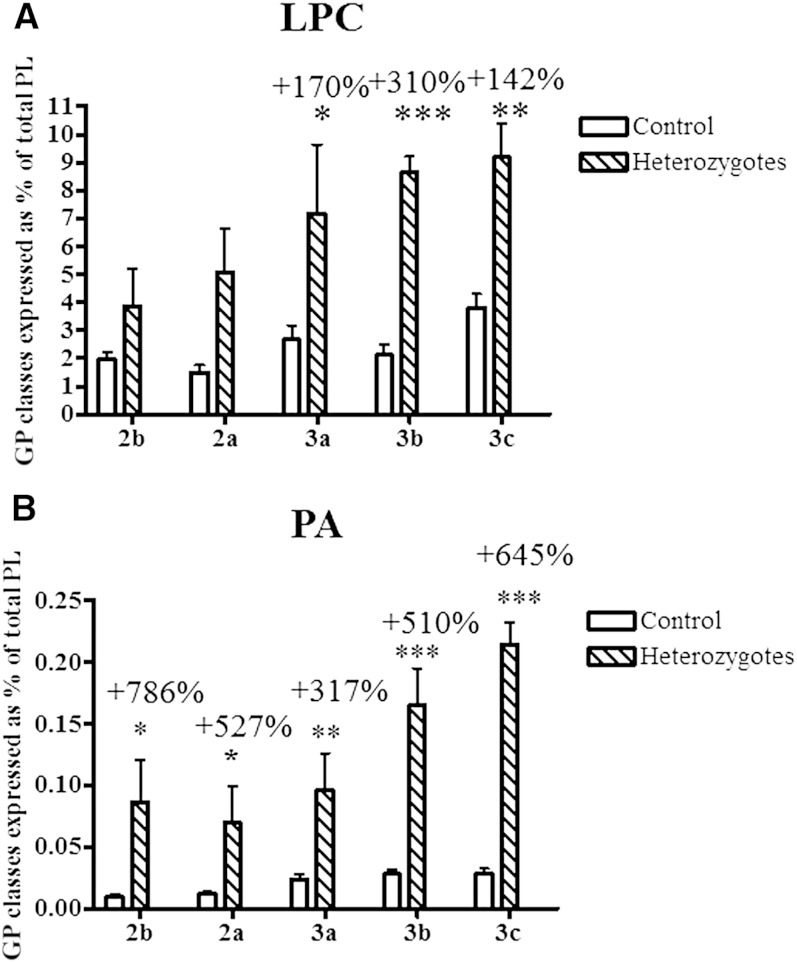
Nine GP and SL subclasses were measured in HDL particles from heterozygous subjects and controls, in the order of decreasing abundance, PC, SM, LPC, PE, PI, Cer, PS, PG, and PA. We were unable to assay the HDL lipidome in homozygotes due to the lack of samples as a result of extremely low HDL levels. When expressed as % of total GP + SL, a reduction (up to −7%, P < 0.05; Fig. 4A) in the content of PC, the most abundant GP subclass, was observed in HDL2b, HDL3b, and HDL3c of heterozygous subjects relative to control HDLs. The content of SM, another key structural GP, was also diminished in HDL2a and 3a in heterozygotes (up to −18%, P < 0.01; Fig. 4B) but was increased by +15% (P < 0.05) in HDL2b. All HDL subpopulations from heterozygotes displayed increased content of Cer species relative to controls; these differences reached significance in HDL2b, HDL2a, and HDL3b (up to +142%, P < 0.001; Fig. 5A). PS, a negatively charged minor GP, was increased in heterozygous HDL2b, HDL2a, and HDL3c (up to +426%, P < 0.001; Fig. 5B). Finally, all HDL subpopulations from heterozygotes were enriched in PG (up to +512%, P < 0.001; Fig. 5C). Interestingly, HDL % content of LPC, a primary product of PC hydrolysis, was markedly elevated in small, dense HDL3 from heterozygotes (up to +310% P < 0.001; Fig. 6A) versus corresponding control HDLs. All HDLs from heterozygous subjects were also markedly enriched in PA (up to +786%, P < 0.05; Fig. 6B), another product of GP hydrolysis and a key cellular signaling molecule. In addition, HDL % content of PI was elevated in all HDL subpopulations from heterozygotes (up to +47%, P < 0.05; Fig. 4C) versus controls, except in HDL3b. The reported differences in GP and SL subclasses were entirely consistent with those in their respective individual isobaric lipid species, notably PC 34:1, 34:2, and 36:2; SM 34:1 and 42:2; PE 36:2, 38:4, and 38:6; PI 36:2 and 38:4; Cer d18:1 22:0, d18:1 23:0, d18:1 24:0, and d18:1 24:1; PS 36:1, 36:2, and 38:4; PG 34:1, 34:2, 36:1, and 36:2; LPC 16:0, 18:0, and 18:2; and PA 34:1, 34:2, 36:2, and 36:3 (supplementary Fig. I).
Fig. 4.
Content of PC (A), SM (B), PE (C), and PI (D) in HDL subpopulations, expressed as % of total GP + SL, in heterozygous subjects with apoA-I deficiency versus controls; ** P < 0.01, * P < 0.05 versus controls.
Fig. 5.
Content of Cer (A), PS (B), and PG (C) in HDL subpopulations, expressed as % of total GP + SL (A, B), in heterozygous subjects with apoA-I deficiency versus controls; *** P < 0.001, ** P < 0.01, * P < 0.05 versus controls.
Fig. 6.
Content of LPC (A) and PA (B) in HDL subpopulations, expressed as % of total GP + SL, in heterozygous subjects with apoA-I deficiency versus controls; *** P < 0.001, ** P < 0.01, * P < 0.05 versus controls.
Cholesterol efflux capacity of HDL particles in THP-1 macrophages
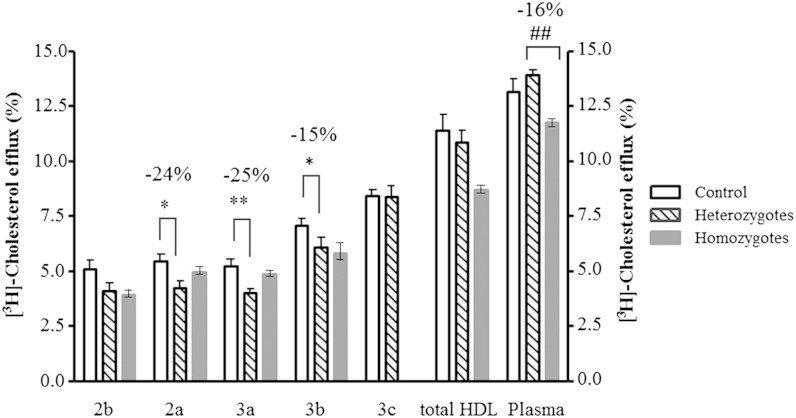
The capacity of all HDL subpopulations from homozygous subjects (except for HDL3c, which was not assessed as a result of the low available amounts) and of total HDL (assessed at 30 µg total GP/ml) to promote cholesterol efflux from lipid-laden THP-1 macrophages tended to be reduced relative to their counterparts from normolipidemic controls, with more pronounced differences found in HDL2b (−23%, P = 0.25), HDL3b (−18%, P = 0.12), and total HDL (−21%, P = 0.10) (Fig. 7). Interestingly, all HDL subpopulations from heterozygous subjects revealed similarly reduced intrinsic cholesterol efflux capacity relative to their counterparts from controls; these differences reached significance in HDL2a (−24%, P < 0.05), HDL3a (−25%, P < 0.01), and HDL3b (−15%, P < 0.05) (Fig. 7). By contrast, no difference was found in cholesterol efflux capacity of total HDL and whole plasma in heterozygous subjects relative to controls (Fig. 7). On the other hand, a reduction in the capacity of whole plasma and total HDL to promote cholesterol efflux was observed in homozygous subjects (−16%, P < 0.01, and −21%, P = 0.07, respectively, vs. heterozygotes; Fig. 7).
Fig. 7.
Cholesterol efflux capacity of HDL subpopulations (15 µg total GP/ml), of total HDL (30 µg GP/ml) and whole plasma (2.5%) from subjects with homozygous (n = 2) and heterozygous (n = 6) apoA-I-deficiency and control subjects (n = 10); ** P < 0.01, *P < 0.05 versus corresponding HDL type from controls; ## P < 0.01 versus heterozygous plasma. Data obtained in two homozygotes are shown as means, and the range of individual values is represented as min–max.
Antioxidative activity of HDL particles toward LDL oxidation
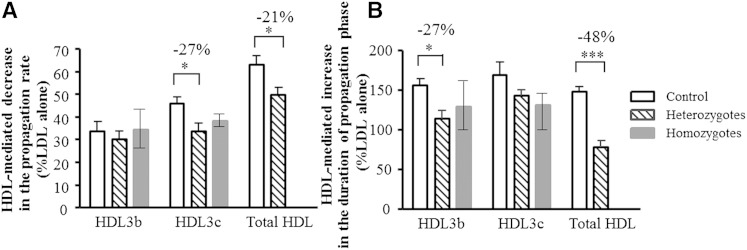
When HDL particles isolated from controls or subjects with familial apoA-I deficiency were added to reference LDL at physiological HDL/LDL ratios of about 2 to 6 mol/mol for HDL3b and 3c, and about 13 for total HDL, LDL oxidation was significantly delayed (Fig. 8). Unexpectedly, such antioxidative activity of HDL was markedly impaired in heterozygous patients but only tended to be reduced in homozygotes. The inhibitory effects of small, dense HDL3b and 3c, and of total HDL on LDL oxidation were significantly lower in heterozygous patients relative to their counterparts from controls (impairment in the HDL-mediated prolongation of the propagation phase of LDL oxidation of −27%, P < 0.05 and −48%, P < 0.001, in HDL3b and total HDL, respectively; Fig. 8B). Similarly, HDL-mediated decrease in the propagation rate of LDL oxidation was impaired in heterozygotes (HDL3c, −27%, P < 0.05; total HDL, −21%, P < 0.05) and tended to be impaired in homozygotes (HDL3c, −17%, P = 0.20; Fig. 8A).
Fig. 8.
Influence of small, dense HDL3b (10 mg total mass/dl) and HDL3c (10 mg total mass/dl) subpopulations and of total HDL (40 mg total mass/dl) on AAPH-induced oxidation of reference LDL (LDL, 10 mg TC/dl; AAPH, 1 mM) in subjects with homozygous (n = 2) and heterozygous (n = 6) apoA-I-deficiency and control subjects (n = 10). Effects of HDL particles on the oxidation rate in the propagation phase of LDL oxidation (A) and on the duration of this phase (B) are shown. LDL was oxidized in PBS at 37°C, and conjugated diene formation was measured by absorbance increment at 234 nm; *** P < 0.001, * P < 0.05 versus corresponding HDL type from controls. Data obtained in two homozygotes are shown as means, and the range of individual values is represented as min–max.
Correlations
Defective cholesterol efflux capacity and antioxidative activity of HDL were correlated with altered HDL composition in our study population. Cholesterol efflux from THP-1 cells to total HDL was positively correlated with HDL content of apoA-I and CE and negatively correlated with the content of FC, whereas the capacity of both small, dense HDL3c and total HDL to delay LDL oxidation was positively associated with HDL content of apoA-I, CE, and GP and negatively associated with the content of apoA-II and FC (data not shown).
Interestingly, cholesterol efflux to HDL3b, but not to other HDL particles, was positively correlated with plasma levels of HDL-C and apoA-I (r = 0.50 and 0.52, P < 0.05, respectively) and negatively correlated with plasma levels of hsCRP and TG (r = −0.55 and −0.60, P < 0.05, respectively). Similarly, antioxidative activity of both small, dense HDL3c and total HDL was positively associated with plasma levels of HDL-C and apoA-I (data not shown).
Cholesterol efflux to HDL3b was negatively correlated with HDL content of LPC (r = −0.77, P < 0.05). In addition, antioxidative activity of small, dense HDL3b and 3c was negatively correlated with HDL content of LPC (r = −0.82 and −0.93, P < 0.05, respectively), suggesting that LPC may contribute to deficient functionality of HDL in our study.
To directly evaluate the role of LPC for HDL function, total HDL isolated from control subjects was incubated in vitro in the presence of LPC. Subsequently, HDL subpopulations were reisolated by density gradient ultracentrifugation. In parallel, total HDL was incubated with PBS and equivalent HDL subpopulations reisolated as a control. Such in vitro HDL enrichment in LPC up to the level of 6.8% of total GP + SL (mean of two independent experiments), which closely corresponded to LPC content of apoA-I-deficient HDL (Fig. 6A), reduced cholesterol efflux capacity of small, dense HDL3 to 62 ± 14% of control incubations (n = 3, P < 0.05). In parallel, antioxidative activity of HDL3 was diminished by the LPC enrichment to 57% of control incubations (n = 2).
DISCUSSION
In this study, we report for the first time that atheroprotective activities of both ultracentrifugally isolated HDL subpopulations and total HDL are impaired in homozygous and heterozygous apoA-I deficiency involving a nonsense mutation at APOA1 codon −2. Two major atheroprotective activities of HDL evaluated in our study, notably cellular cholesterol efflux capacity from THP-1 macrophages and antioxidative activity toward LDL, were defective in apoA-I-deficient subjects. Importantly, the atheroprotective activities of HDL were assessed either on a unit GP basis (cholesterol efflux capacity) or on a unit particle mass basis (antioxidative activity), thereby demonstrating that the functional impairments observed herein represent an intrinsic defect of HDL particles independent of their circulating levels.
HDL subpopulations from apoA-I-deficient subjects displayed markedly altered chemical composition, with depletion in apoA-I, GP, and CE, and enrichment in apoA-II, FC, and TG. Furthermore, the phospho- and sphingolipidome of HDL was altered in heterozygotes. Depletion of HDL in apoA-I is an obvious consequence of the mutation at APOA1 codon -2, which results in reduced circulating levels of apoA-I in heterozygotes and complete absence of the protein in homozygotes. Depletion in GP may reflect reduction in concentrations of HDL particles secondary to the decrease in apoA-I; indeed, GP is the major structural component of HDL. Consistent with these data, the content of PC and SM, two major contributors to HDL-GP, was reduced in heterozygous subjects. In parallel, the content of LPC and PA, two products of PC hydrolysis, was elevated, potentially reflecting enhanced hydrolytic processing of HDL lipids.
Depletion of HDL in CE may result from reduced or absent LCAT activation as a consequence of low or absent A-I, which functions as an activator for LCAT. This pathway is consistent with the HDL enrichment in FC observed in apoA-I-deficient subjects. ApoA-II, the second major HDL apo, is known to be able to replace apoA-I as a structural component to form HDL particles. Elevated content of apoA-II observed in apoA-I-deficient HDL may therefore reflect a compensatory increase in circulating apoA-II-containing particles aimed to restore normal HDL metabolism. Finally, HDL enrichment in TG observed in heterozygotes most probably results from elevated CETP activity under conditions of moderate hypertriglyceridemia, while the source of TG elevation in homozygote HDL remains to be established.
Altered HDL lipid and apo composition, including depletion of apoA-I, GP, and CE as well as enrichment in apoA-II and FC, was correlated with defective atheroprotective activities of HDL, consistent with direct structure-function relationships. ApoA-I is the major HDL component underlying both cholesterol effluxing via ABCA1 and antioxidative properties of HDL toward free-radical-induced LDL oxidation. Decreased HDL content of apoA-I is therefore the most straightforward explanation for the impaired functional properties of HDL in apoA-I deficiency. Correlations of HDL functional metrics with other HDL components (GP, CE, FC, and apoA-II) might represent an epiphenomenon, reflecting positive associations of apoA-I-containing particles with GP and CE and their negative associations with FC and apoA-II as described previously. Alternatively, these components can directly modulate HDL functionality as exemplified by the beneficial role of GP for cellular cholesterol efflux (24). Further along this line, FC is capable of reducing antioxidative activity of HDL, acting via decrease in the surface lipid fluidity (27).
HDL functionality may also be deleteriously influenced by alterations in the phospho- and sphingolipidome. Indeed, both cholesterol efflux and antioxidative activity of HDL were negatively associated with the content of LPC. Consistent with these data, in vitro enrichment of HDL in LPC reduced both metrics of HDL functionality, attesting to LPC as a key component underlying impaired HDL functionality in familiar apoA-I deficiency.
Alterations in other lipids equally possess a potential to contribute to deficient HDL functionality in our study. Thus, elevated HDL content of PI, PS, and PG, three negatively charged GPs, suggests alterations in the surface HDL charge, an observation that may lead to impaired interaction of HDL with membrane proteins, including ABCA1. Furthermore, increased negative charges at the surface of an HDL particle would also be expected to affect and perhaps repel negative charged residues in apoA-I (32). On the other hand, increased content of Cer may deleteriously impact anti-inflammatory and antiapoptotic properties of HDL (33).
Another potential mechanism contributing to impaired HDL functionality in apoA-I deficiency is highlighted by the presence of chronic proinflammatory state in homozygotes, documented by significantly elevated (15-fold) plasma levels of hsCRP. Such chronic inflammation may aggravate antiatherogenic function of HDL, acting via displacement of functional HDL proteins by serum amyloid A, an acute-phase reagent, and via oxidative modifications of HDL components.
Interestingly, HDL particles isolated from subjects with homo- and heterozygous forms of apoA-I deficiency revealed specific differences in their functional properties, depending on the particle and assay type. Thus, HDL subpopulations from both homo- and heterozygous subjects displayed reduced intrinsic cholesterol efflux capacity relative to their counterparts from controls. By contrast, a significant reduction in the capacity of total HDL, and equally of the whole plasma, to promote cholesterol efflux was observed in homozygous subjects when compared with heterozygous subjects. These data suggest that collectively, HDL subpopulations can compensate for defective cholesterol efflux to individual HDL subpopulations in heterozygotes, indicative of a synergistic relationship. Such plausible interactions between different HDL particles may, however, become insufficient to maintain a normal level of cholesterol efflux in homozygotes, consistent with the more profound alterations in HDL composition in homo- relative to heterozygotes. It should be noted that similar between-group differences in the cholesterol efflux obtained with total HDL and whole plasma indicate that efflux to ultracentrifugally isolated total HDL correctly reflected efflux to whole plasma, thereby validating the relevance of the former metric. This conclusion is further supported by excellent correlation between cellular cholesterol efflux capacity of total HDL and apoB-depleted plasma samples obtained in a separate group of healthy normolipidemic controls (r = 0.98, P = 0.004, n = 5).
In addition, both small, dense HDL3b and 3c and total HDL from apoA-I-deficient subjects exhibited diminished intrinsic antioxidative activity relative to controls. In this functional assay, the defects observed in homo- and heterozygotes did not, however, differ markedly. The intriguing observation of the maintenance of intrinsic HDL antioxidative activity in homozygotes suggests that other than apoA-I, HDL components with potent antioxidative properties, such as apoA-IV, apoJ, or others, might accumulate in HDL in this metabolic state at the expense of apoA-I.
Subjects with homozygous apoA-I deficiency displayed strongly reduced plasma levels of apoA-I, HDL-C, and apoC-III. Whereas the decreases in apoA-I and HDL-C obviously result from the genetic apoA-I deficiency, the mechanism of apoC-III lowering can reflect reduction in the circulating HDL pool that normally acts as a reservoir for this protein. The concentrations of HDL-C and apoA-I were also significantly diminished in the heterozygotes but to a lesser extent. Importantly, cholesterol efflux to HDL3b as well as antioxidative activity of both small, dense HDL3c and total HDL were positively associated with plasma levels of HDL-C and apoA-I across the study population, thereby indicating that reduced plasma levels of HDL-C and apoA-I reflect defective functionality of HDL particles in familial apoA-I deficiency. It would, however, be premature to expand this conclusion to other forms of apoA-I deficiency, which result from different mutations, and particularly to other forms of low HDL-C dyslipidemia. Indeed, the small number of subjects, primarily of homozygotes, and the single apoA-I mutation investigated constitute the limitations of our study. The capacity of apoB-depleted serum to efflux cellular cholesterol was shown to better reflect cardiovascular risk as compared with measurements of circulating HDL-C in one study (34), an interesting finding that was not, however, fully confirmed by subsequent work (35). The association of in vitro metrics of cellular cholesterol efflux with cardiovascular events remains, therefore, controversial at this point. An important result of our present study is thus the demonstration that under conditions of severe apoA-I deficiency, plasma HDL-C and apoA-I may still serve as surrogate biomarkers of HDL function and potentially of cardiovascular risk.
In conclusion, our findings presented herein document defective intrinsic HDL functionality in homozygous and heterozygous apoA-I deficiency involving a nonsense mutation at APOA1 codon -2. Multiplied by low circulating HDL concentrations, such net deficiency in HDL function can be expected to contribute to accelerated atherogenesis observed in this clinical condition. As a corollary, increase in circulating levels of functional HDL, such as, for example, by infusion of recombinant HDL particles or delipidated HDL, may represent a promising therapeutic strategy to reduce cardiovascular risk in genetic apoA-I deficiency.
Footnotes
Abbreviations:
- AAPH
- 2,2′-azo-bis-(2-amidinopropane) hydrochloride
- CE
- cholesteryl ester
- Cer
- ceramide
- CETP
- cholesteryl ester transfer protein
- FC
- free cholesterol
- GP
- glycerophospholipid
- HDL-C
- HDL-cholesterol
- hsCRP
- high-sensitive C-reactive protein
- LDL-C
- LDL-cholesterol
- LPC
- lysophosphatidylcholine
- PA
- phosphatidic acid
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PG
- phosphatidylglycerol
- PI
- phosphatidylinositol
- PS
- phosphatidylserine
- SL
- sphingolipid
- SM
- sphingomyelin
- TC
- total cholesterol
These studies were supported by Coordination for the Improvement of Higher Level -or Education- Personnel (CAPES), São Paulo Research Foundation (FAPESP), and National Institute for Health and Medical Research (INSERM). R.D.S. has received honoraria consulting or speaker engagements from the following companies: Astra Zeneca, Amgen, Aegerion, Biolab, Boehringer Ingelheim, Bristol Myers Squibb, Genzyme, Unilever, Pfizer, Lilly, Novo Nordisk, Novartis, and Sanofi. A.K. has received honoraria from Astra Zeneca and Novo Nordisk and research grant funding from CSL. The authors acknowledge further support from the CODDIM Ile-de-France and ANR (CARINA Project). F.R. acknowledges financial support from the “Association pour la recherché sur Les Lipoproteines et l’Atherogenese” (ARLA, France).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure.
REFERENCES
- 1.Schaefer E. J., Santos R. D., Asztalos B. F. 2010. Marked HDL deficiency and premature coronary heart disease. Curr. Opin. Lipidol. 21: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos R. D., Schaefer E. J., Asztalos B. F., Polisecki E., Wang J., Hegele R. A., Martinez L. R., Miname M. H., Rochitte C. E., Da Luz P. L., et al. 2008. Characterization of high density lipoprotein particles in familial apolipoprotein A-I deficiency. J. Lipid Res. 49: 349–357. [DOI] [PubMed] [Google Scholar]
- 3.Iatan I., Palmyre A., Alrasheed S., Ruel I., Genest J. 2012. Genetics of cholesterol efflux. Curr. Atheroscler. Rep. 14: 235–246. [DOI] [PubMed] [Google Scholar]
- 4.Sorci-Thomas M. G., Thomas M. J. 2002. The effects of altered apolipoprotein A-I structure on plasma HDL concentration. Trends Cardiovasc. Med. 12: 121–128. [DOI] [PubMed] [Google Scholar]
- 5.von Eckardstein A. 2006. Differential diagnosis of familial high density lipoprotein deficiency syndromes. Atherosclerosis. 186: 231–239. [DOI] [PubMed] [Google Scholar]
- 6.Camont L., Lhomme M., Rached F., Le Goff W., Negre-Salvayre A., Salvayre R., Calzada C., Lagarde M., Chapman M. J., Kontush A. 2013. Small, dense high-density lipoprotein-3 particles are enriched in negatively charged phospholipids: relevance to cellular cholesterol efflux, antioxidative, antithrombotic, anti-inflammatory, and antiapoptotic functionalities. Arterioscler. Thromb. Vasc. Biol. 33: 2715–2723. [DOI] [PubMed] [Google Scholar]
- 7.Kontush A., Chapman M. J. 2006. Functionally defective HDL: a new therapeutic target at the crossroads of dyslipidemia, inflammation and atherosclerosis. Pharmacol. Rev. 58: 342–374. [DOI] [PubMed] [Google Scholar]
- 8.Kiss R. S., Kavaslar N., Okuhira K., Freeman M. W., Walter S., Milne R. W., McPherson R., Marcel Y. L. 2007. Genetic etiology of isolated low HDL syndrome: incidence and heterogeneity of efflux defects. Arterioscler. Thromb. Vasc. Biol. 27: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 9.Daniil G., Phedonos A. A., Holleboom A. G., Motazacker M. M., Argyri L., Kuivenhoven J. A., Chroni A. 2011. Characterization of antioxidant/anti-inflammatory properties and apoA-I-containing subpopulations of HDL from family subjects with monogenic low HDL disorders. Clin. Chim. Acta. 412: 1213–1220. [DOI] [PubMed] [Google Scholar]
- 10.Ng D. S., Leiter L. A., Vezina C., Connelly P. W., Hegele R. A. 1994. Apolipoprotein A-I Q[-2]X causing isolated apolipoprotein A-I deficiency in a family with analphalipoproteinemia. J. Clin. Invest. 93: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rumsey S. C., Galeano N. F., Arad Y., Deckelbaum R. J. 1992. Cryopreservation with sucrose maintains normal physical and biological properties of human plasma low density lipoproteins. J. Lipid Res. 33: 1551–1561. [PubMed] [Google Scholar]
- 12.Chapman M. J., Goldstein S., Lagrange D., Laplaud P. M. 1981. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J. Lipid Res. 22: 339–358. [PubMed] [Google Scholar]
- 13.Kontush A., Chantepie S., Chapman M. J. 2003. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler. Thromb. Vasc. Biol. 23: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 14.Hansel B., Giral P., Nobecourt E., Chantepie S., Bruckert E., Chapman M. J., Kontush A. 2004. Metabolic syndrome is associated with elevated oxidative stress and dysfunctional dense high-density lipoprotein particles displaying impaired antioxidative activity. J. Clin. Endocrinol. Metab. 89: 4963–4971. [DOI] [PubMed] [Google Scholar]
- 15.Guérin M., Dolphin P. J., Chapman M. J. 1994. New in-vitro method for the simultaneous evaluation of cholesteryl ester exchange and mass-transfer between HDL and apoB-containing lipoprotein subspecies - identification of preferential cholesteryl ester accepters in human plasma. Arterioscler. Thromb. 14: 199–206. [DOI] [PubMed] [Google Scholar]
- 16.Catalano G., Julia Z., Frisdal E., Vedie B., Fournier N., Le Goff W., Chapman M. J., Guerin M. 2009. Torcetrapib differentially modulates the biological activities of HDL2 and HDL3 particles in the reverse cholesterol transport pathway. Arterioscler. Thromb. Vasc. Biol. 29: 268–275. [DOI] [PubMed] [Google Scholar]
- 17.Lagrost L. 1994. Regulation of cholesteryl ester transfer protein (CETP) activity: review of in vitro and in vivo studies. Biochim. Biophys. Acta. 1215: 209–236. [DOI] [PubMed] [Google Scholar]
- 18.Guérin M., Bruckert E., Dolphin P. J., Turpin G., Chapman M. J. 1996. Fenofibrate reduces plasma cholesteryl ester transfer from HDL to VLDL and normalizes the atherogenic, dense LDL profile in combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 16: 763–772. [DOI] [PubMed] [Google Scholar]
- 19.Guerin M., Egger P., Soudant C., Le Goff W., van Tol A., Dupuis R., Chapman M. J. 2002. Dose-dependent action of atorvastatin in type IIB hyperlipidemia: preferential and progressive reduction of atherogenic apoB-containing lipoprotein subclasses (VLDL-2, IDL, small dense LDL) and stimulation of cellular cholesterol efflux. Atherosclerosis. 163: 287–296. [DOI] [PubMed] [Google Scholar]
- 20.Larijani B., Poccia D. L., Dickinson L. C. 2000. Phospholipid identification and quantification of membrane vesicle subfractions by 31P-1H two-dimensional nuclear magnetic resonance. Lipids. 35: 1289–1297. [DOI] [PubMed] [Google Scholar]
- 21.Quehenberger O., Dennis E. A. 2011. The human plasma lipidome. N. Engl. J. Med. 365: 1812–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quehenberger O., Armando A. M., Brown A. H., Milne S. B., Myers D. S., Merrill A. H., Bandyopadhyay S., Jones K. N., Kelly S., Shaner R. L., et al. 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 51: 3299–3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larrede S., Quinn C. M., Jessup W., Frisdal E., Olivier M., Hsieh V., Kim M-J., Van Eck M., Couvert P., Carrie A., et al. 2009. Stimulation of cholesterol efflux by LXR agonists in cholesterol-loaded human macrophages is ABCA1-dependent but ABCG1-independent. Arterioscler. Thromb. Vasc. Biol. 29: 1930–1936. [DOI] [PubMed] [Google Scholar]
- 24.Fournier N., Atger V., Cogny A., Vedie B., Giral P., Simon A., Moatti N., Paul J. L. 2001. Analysis of the relationship between triglyceridemia and HDL-phospholipid concentrations: consequences on the efflux capacity of serum in the Fu5AH system. Atherosclerosis. 157: 315–323. [DOI] [PubMed] [Google Scholar]
- 25.Nobécourt E., Jacqueminet S., Hansel B., Chantepie S., Grimaldi A., Chapman M. J., Kontush A. 2005. Defective antioxidative activity of small, dense HDL particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycemia. Diabetologia. 48: 529–538. [DOI] [PubMed] [Google Scholar]
- 26.Kontush A., de Faria E. C., Chantepie S., Chapman M. J. 2005. A normotriglyceridemic, low HDL-cholesterol phenotype is characterised by elevated oxidative stress and HDL particles with attenuated antioxidative activity. Atherosclerosis. 182: 277–285. [DOI] [PubMed] [Google Scholar]
- 27.Zerrad-Saadi A., Therond P., Chantepie S., Couturier M., Rye K-A., Chapman M. J., Kontush A. 2009. HDL3-mediated inactivation of LDL-associated phospholipid hydroperoxides is determined by the redox status of apolipoprotein A-I and HDL particle surface lipid rigidity: relevance to inflammation and atherogenesis. Arterioscler. Thromb. Vasc. Biol. 29: 2169–2175. [DOI] [PubMed] [Google Scholar]
- 28.Kontush A., Chapman M. J. 2010. Antiatherogenic function of HDL particle subpopulations: focus on antioxidative activities. Curr. Opin. Lipidol. 21: 312–318. [DOI] [PubMed] [Google Scholar]
- 29.Abbott C. A., Mackness M. I., Kumar S., Boulton A. J., Durrington P. N. 1995. Serum paraoxonase activity, concentration, and phenotype distribution in diabetes mellitus and its relationship to serum lipids and lipoproteins. Arterioscler. Thromb. Vasc. Biol. 15: 1812–1818. [DOI] [PubMed] [Google Scholar]
- 30.Davidson W. S., Silva R. A. G. D., Chantepie S., Lagor W. R., Chapman M. J., Kontush A. 2009. Proteomic analysis of defined HDL subpopulations reveals particle-specific protein clusters: relevance to antioxidative function. Arterioscler. Thromb. Vasc. Biol. 29: 870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campos E., McConathy W. J. 1986. Distribution of lipids and apolipoproteins in human plasma by vertical spin ultracentrifugation. Arch. Biochem. Biophys. 249: 455–463. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Sparks D. L., Marcel Y. L. 1996. Effect of the apolipoprotein A-I and surface lipid composition of reconstituted discoidal HDL on cholesterol efflux from cultured fibroblasts. Biochemistry. 35: 16510–16518. [DOI] [PubMed] [Google Scholar]
- 33.Taha T. A., Mullen T. D., Obeid L. M. 2006. A house divided: ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim. Biophys. Acta. 1758: 2027–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khera A. V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M. F., Jafri K., French B. C., Phillips J. A., Mucksavage M. L., Wilensky R. L., et al. 2011. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364: 127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X. M., Tang W. H., Mosior M. K., Huang Y., Wu Y., Matter W., Gao V., Schmitt D., Didonato J. A., Fisher E. A., et al. 2013. Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler. Thromb. Vasc. Biol. 33: 1696–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]