Abstract
Plant sterols and stanols are structurally similar to cholesterol and when added to the diet they are able to reduce serum total- and LDL-cholesterol concentrations. They also lower serum triglyceride concentrations in humans, particularly under conditions of hypertriglyceridemia. The aim of this study was to unravel the mechanism by which plant sterols and stanols reduce serum triglyceride concentrations in high-fat diet (HFD) fed mice. Male C57BL/6J mice were fed HFD for 4 weeks. Subsequently, they received HFD, HFD supplemented with 3.1% plant sterol ester (PSE) or HFD supplemented with 3.1% plant stanol ester (PSA) for another three weeks. Both PSE and PSA feeding resulted in decreased plasma triglyceride concentrations compared with HFD, while plasma cholesterol levels were unchanged. Interestingly, hepatic cholesterol levels were decreased in the PSE/PSA groups compared with HFD and no differences were found in hepatic triglyceride levels between groups. To investigate the mechanism underlying the hypotriglyceridemic effects from PSE/PSA feeding, we measured chylomicron and VLDL secretion. PSE and PSA feeding resulted in reduced VLDL secretion, while no differences were found between groups in chylomicron secretion. In conclusion, our data indicate that plasma triglyceride-lowering resulting from PSE and PSA feeding is associated with decreased hepatic VLDL secretion.
Keywords: phytosterols, triglycerides, very low density lipoprotein, chylomicrons, liver
Plant sterols and their saturated forms, plant stanols, are nonnutritive compounds that lower serum total and LDL-cholesterol concentrations in normolipidemic and hypercholesterolemic subjects without affecting serum HDL-cholesterol levels (1). Plant sterols and plant stanols have a chemical structure that resembles cholesterol, and therefore it is generally believed that plant sterols and stanols reduce plasma cholesterol levels by competing with cholesterol for incorporation into mixed micelles in the small intestine (2–4). Consequently, a new cholesterol-lowering mechanism of plant sterols has been suggested, which involves increased direct intestinal cholesterol excretion by bypassing the “classical” hepato-biliary route (5).
Besides lowering serum LDL-cholesterol concentrations, it has been shown that, particularly in subjects with metabolic syndrome, plant sterols and stanols also lower serum triglyceride concentrations where the efficacy was associated to baseline triglyceride levels (6–9). Several mechanisms have been proposed to explain these effects in decreased triglyceride; e.g., increased LPL activity, changes in cholesteryl ester transfer protein activity, reduced hepatic VLDL production or increased de novo lipogenesis, and some related regulatory genes (such as Ppara) as a compensatory response (6, 7, 10).
Traditionally, fasting triglycerides were considered to be an independent risk factor for cardiovascular diseases (11). However, after correcting for several confounding factors, the association between fasting triglyceride levels and cardiovascular risk became very low or even absent (11). Because humans are mostly in the fed state, the level of nonfasting triglycerides may be more relevant; indeed, there is an increasing body of evidence showing nonfasting triglyceride levels as an independent cardiovascular risk factor (12–14).
The goal of this study was to unravel the mechanism by which plant sterols and plant stanols lower plasma triglyceride concentrations in high-fat diet (HFD) fed wild-type mice by evaluating changes in processes that result in triglyceride appearance in the circulation. Prior to the start of the plant sterol/stanol intervention, all mice received HFD for 4 weeks in order to induce hypertriglyceridemia. Our results are consistent with observations in humans that plant sterol and stanol ester consumption results in decreased plasma triglyceride levels, which is associated with and most likely due to decreased hepatic VLDL secretion.
Material and Methods
Animals
Male C57BL/6J mice of 3 months of age were purchased from Charles River Laboratories (L’Abresle, Brussels, Belgium). All animals were housed individually in a temperature- and light- controlled facility. In all experiments, mice received a dietary treatment of 7 weeks. The dietary treatment started by feeding mice HFD containing 40% fat derived from beef for 4 weeks. After this period, mice were split in three groups (n = 6) and received either HFD, HFD supplemented with 3.1% (wt/wt) plant sterol esters (PSE) or HFD supplemented with 3.1% (wt/wt) plant stanol esters (PSA) for 3 more weeks (Table 1). The reason that the HFD contains more olive oil, soybean oil, and linseed oil compared with the PSE and PSA diets is that plant sterols and stanols were not added at the expense of dietary fat because the plant sterols and stanols were esterified with a fatty acid and the exact amount of fat was added to the HFD to make sure that the three diets had a similar absolute fat content and similar fatty acid composition. Mice received food and water ad libitum. All experiments were approved by the Ethical Committee for Animal Experiments of the University of Groningen.
TABLE 1.
Composition of the diets
| HFD | PSE | PSA | |
| Composition (%) | |||
| Sucrose | 39.75 | 38.97 | 38.97 |
| Casein | 23.64 | 23.18 | 23.18 |
| Beef fat | 15.78 | 15.47 | 15.47 |
| Cellulose | 5.91 | 5.79 | 5.79 |
| Olive oil | 2.94 | 2.07 | 2.07 |
| Soybean oil | 2.27 | 2.07 | 2.07 |
| Corn starch | 2.59 | 2.54 | 2.54 |
| Vitamin mixa | 0.58 | 0.58 | 0.58 |
| Mineral mixb | 5.44 | 5.33 | 5.33 |
| Choline | 0.47 | 0.46 | 0.46 |
| DL Methionine | 0.24 | 0.23 | 0.23 |
| Cholesterol | 0.20 | 0.20 | 0.20 |
| Linseed oil | 0.19 | — | — |
| Plant sterol esters | — | 2.0 | — |
| Plant stanol esters | — | — | 2.0 |
Vitamin mix: vitamins premix, trace elements premix.
Mineral mix: calcium hydrogen phosphate, calcium carbonate, potassium chloride, potassium dihydrogen phosphate, magnesium sulfate heptahydrate, sodium chloride, magnesium oxide.
Experimental procedures
Biochemical parameters.
Body weight and food intake were determined at the start of the experiment and repeated weekly until the end of the experiment. Feces were collected during the last 3 days of the experiment. At the end of the dietary treatment, mice were euthanized by heart puncture under isofluorane anesthesia followed by cervical dislocation. Plasma was stored at −20°C until analyzed. The liver was removed, weighed, and snap-frozen in liquid nitrogen. The intestine was excised, flushed with (4°C) PBS containing 1x Complete Protease Inhibitor (Roche Diagnostics), and subsequently snap-frozen in liquid nitrogen. Both liver and intestine were stored at −80°C until biochemical analysis and RNA isolation. Hepatic lipids were extracted according to Bligh and Dyer (15). Plasma parameters were measured by using commercially available kits (Roche Diagnostics, Mannheim, Germany and DiaSys Diagnostic Systems, Holzheim, Germany).
Determination of VLDL secretion.
In a separate experiment, after the dietary treatment, overnight fasted mice (n = 6) were injected intraperitoneally with Poloxamer 407 (1 g/kg body weight) in saline as previously described (16). Blood samples were drawn by retro-orbital bleeding into heparinized tubes at 0, 30, 60, 120, and 240 min after injection under isofluorane anesthesia. Animals were euthanized by cervical dislocation. Blood samples were centrifuged (10 min, 4,000 × g, 4°C) to obtain plasma. TG production rates were determined as previously described (16). Nascent VLDL (d<1.006) was isolated from the final plasma sample of each animal from experiment 2 using an Optima TM LX tabletop ultracentrifuge (Beckman Instruments Inc., Palo Alto, CA) at 508.000 × g for 150 min. VLDL composition was determined by using commercial kits (for triglycerides and cholesterol: Roche Diagnostics, Mannheim, Germany and DiaSys Diagnostic Systems, Holzheim, Germany; for phospholipids: Wako Chemicals).
Determination of chylomicron secretion.
In a third experiment, after the dietary treatment, mice (n = 6) received an intragastric load of 200 μl olive oil after overnight fasting. Subsequently, blood samples were drawn by retro-orbital bleeding into heparinized tubes at 0, 30, 60, 120, and 240 min under isofluorane anesthesia. Animals were euthanized by cervical dislocation. Plasma was stored at −20°C until analysis of plasma triglycerides.
Plasma lipoprotein analysis.
Plasma lipoproteins were separated by fast protein liquid chromatography as described previously (17). Total cholesterol and triglyceride levels of the collected fractions were determined using a commercially available kit (Roche Diagnostics, Mannheim, Germany).
β-Hydroxybutyrate.
The concentration of β-hydroxybutyrate was determined using a commercial kit (Diasys Diagnostic Systems, Holzheim Germany) using a VITALAB Selectra E instrument (VWR ser.Nr.:0-2496).
RNA isolation.
RNA isolation, cDNA synthesis and real-time quantitative PCR were performed as described previously (18). PCR results of liver and intestine were normalized to 36b4 mRNA levels. Primer and probe sequences for the genes tested are listed in Supplemental Table I.
Statistics
Data are shown as means ± SD. Statistical analysis was performed by using Kruskal-Wallis H test followed by Conover posthoc comparisons using the Brightstat (19). Levels of significance were set at P < 0.05.
Results
Effects of plant sterol and plant stanol feeding on plasma and hepatic lipids and sterols
Prior to beginning the diets, each group was matched by body weight. PSE treatment resulted in an attenuated increase in body weight compared with HFD-fed mice. Similar results were found in the PSA group, although they did not reach significance (Table 2). Interestingly, the attenuated increase in body weight was parallel to significantly higher food intake (g/d) and a concomitant higher feces production (Table 2). The HFD contains slightly but negligibly more calories than the PSE and PSA diets (supplemental Table II), but this difference was compensated for in the experiment because the mice consumed more of the plant sterol/stanol supplemented diets (Table 2).
TABLE 2.
Basal parameters in C57BL/6J fed HFD, PSE, and PSA diets
| HFD | PSE | PSA | |
| Basal parameters | |||
| Body weight | |||
| Start weight | 28.4 ± 2.2 | 28.3 ± 0.9 | 27.9 ± 1.6 |
| Final weight | 30.7 ± 2.2 | 28.2 ± 1.2 | 29.1 ± 1.5 |
| Change from starta (%) | 13.2 ± 5.0 | 5.9 ± 4.2b | 10.7 ± 3.4 |
| Food intake (g/d) | 3.6 ± 0.5 | 5.2 ± 0.3b | 5.9 ± 0.3b |
| Calorie intake (kcal/day) | 16.8 ± 2.1 | 23.4 ± 1.6b | 26.7 ± 1.3b |
| Feces production (g/d) | 0.41 ± 0.04 | 0.68 ± 0.05b | 0.73 ± 0.07b |
| Plasma | |||
| TC (mmol/l) | 3.9 ± 0.3 | 3.8 ± 0.2 | 3.9 ± 0.2 |
| TG (mmol/l) | 0.96 ± 0.23 | 0.59 ± 0.03b | 0.65 ± 0.10b |
| NEFA (mmol/l) | 1.08 ± 0.10 | 0.89 ± 0.12b | 0.83 ± 0.14b |
| Liver | |||
| Liver (% BW) | 4.8 ± 0.2 | 4.7 ± 0.3 | 4.8 ± 0.4 |
| TG (nmol/mg) | 15.3 ± 2.4 | 16.6 ± 5.0 | 17.5 ± 5.5 |
| TC (nmol/mg) | 7.0 ± 0.6 | 4.8 ± 0.3b | 5.4 ± 1.2b |
| PL (nmol/mg) | 25.4 ± 2.6 | 25.8 ± 1.8 | 25.0 ± 1.6 |
Values are presented as means ± SD. n = 6 animals per group. TC, total cholesterol; PL, phospholipids.
Start feeding diets with or without supplementation of PSE or PSA.
P < 0.05 compared with HFD.
PSE and PSA treatments resulted in a 25% decrease in hepatic cholesterol content as compared with HFD, whereas hepatic triglyceride and phospholipid levels did not differ between the treatments (Table 2).
Although the liver TG content did not change after plant sterol or stanol ester consumption, plasma triglyceride levels decreased. Similarly, the PSE and PSA groups had the lowest concentrations in plasma nonesterified fatty acids (NEFAs) (Table 2). Plasma cholesterol concentrations were similar in the HFD-, PSE-, and PSA-fed mice.
Plant sterols and plant stanols supplemented to HFD results in decreased VLDL secretion in mice
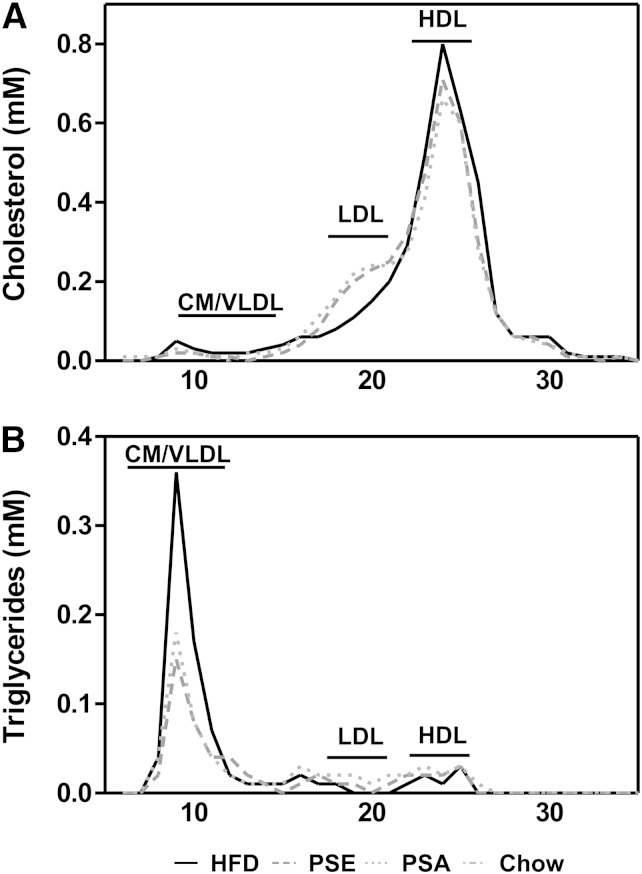
The reduction in plasma triglyceride levels was associated with a reduction in the VLDL/chylomicron fraction in the PSE- and PSA-fed mice (Fig. 1). To determine the rate of hepatic VLDL secretion, lipases were inactivated by intraperitoneal administration of poloxamer 407 and subsequent measurement of plasma triglycerides over a period of 4 h. PSE and PSA feeding resulted in a 12.0% and 15.1% (P < 0.05) lower VLDL-TG secretion compared with HFD fed mice, respectively (P < 0.01) (Fig. 2). Subsequently, the hepatic expression of the most important genes involved in VLDL secretion and assembly were measured. No differences between treatments were found in the expression of Ppara, Apob, and Mttp whereas hepatic Cd36 expression was decreased and Apoa4 expression was elevated in the PSE and PSA groups as compared with the HFD group (Table 3). Dgat2 and Hepatic lipase were unchanged after PSE and PSA feeding (Table 3). To investigate a potential anti-inflammatory effect of plant sterols/stanols, we also determined the expression of several hepatic pro-inflammatory genes (20). Interestingly, PSE/PSA treatments did not result in changes in the expression of any pro-inflammatory genes (Table 4) suggesting that the observed effect of PSE and PSA on hepatic VLDL production occurred independent of potential anti-inflammatory effects of these compounds.
Fig. 1.
FPLC profiles in C57BL/6J mice fed HFD, PSE, and PSA diets. A: Cholesterol concentrations. B: TG concentrations. Values are presented as a pool of 6 animals per group. CM, chylomicron.
Fig. 2.
Hepatic VLDL secretion in mice fed HFD, PSE, and PSA diets. A: Plasma TG concentrations in mice after an intraperitoneal administration of poloxamer 407 (1 g/kg) i.p. B: VLDL-TG production rate. Values are presented as means ± SD. n = 6 animals per group. *P < 0.05 compared with HFD.
TABLE 3.
mRNA expression of genes involved in fat absorption in the intestine and in the liver in C57BL/6J mice fed HFD, PSE, and PSA diets
| HFD | PSE | PSA | |
| Small intestine | |||
| Ppara (Pparα) | 1.0 ± 0.2 | 0.6 ± 0.2a | 0.7 ± 0.2a |
| Cd36 (Fat) | 1.0 ± 0.2 | 0.3 ± 0.2a | 0.5 ± 0.3a |
| Slc27a4 (Fatp4) | 1.0 ± 0.2 | 0.8 ± 0.1 | 0.8 ± 0.2 |
| Apob | 1.0 ± 0.4 | 0.6 ± 0.2 | 0.7 ± 0.2 |
| Mttp | 1.0 ± 0.1 | 0.8 ± 0.2a | 0.7 ± 0.1a |
| Apoa4 | 1.0 ± 0.2 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| Liver | |||
| Ppara (Pparα) | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 |
| Cd36 (Fat) | 1.0 ± 0.1 | 0.5 ± 0.1a | 0.6 ± 0.2a |
| Apob | 1.0 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.1 |
| Mttp | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.0 ± 0.2 |
| Apoa4 | 1.0 ± 0.3 | 2.7 ± 0.9a | 3.5 ± 1.2a |
| Dgat2 | 1.0 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.3 |
| Lipc (Hepatic Lipase) | 1.0 ± 0.1 | 1.0 ± 0.2 | 0.9 ± 0.1 |
Values are presented as means ± SD. n = 6 animals per group. Apob, apolipoprotein B 4; Mttp, microsomal triglyceride transfer protein; Apoa4, apolipoprotein AIV; Dgat2, diacylglycerol O-acyltransferase 2; Slc27a4, solute carrier family 27 (fatty acid transporter), member 4.
P < 0.05 compared with HFD.
TABLE 4.
Hepatic expression of inflammatory genes in C57BL/6J mice fed HFD, PSE and PSA diet
| HFD | PSE | PSA | |
| Tnf (Tnfa) | 1.0 ± 0.5 | 0.9 ± 0.3 | 0.5 ± 0.2 |
| Ilb | 1.0 ± 0.2 | 1.0 ± 0.4 | 1.0 ± 0.3 |
| Cd68 | 1.0 ± 0.2 | 1.2 ± 0.4 | 1.3 ± 0.3 |
| Ccl2 (Mcp1) | 1.0 ± 0.9 | 0.6 ± 0.2 | 0.7 ± 0.4 |
| Icam1 (cd54) | 1.0 ± 0.5 | 0.7 ± 0.2 | 0.7 ± 0.2 |
Values are presented as means ± SD. n = 6 animals per group. Tnf (Tnfa), tumor necrosis factor α; Ilb, interleukin-1β Cd68, cluster of differentiation 68; Ccl2 (Mcp1), monocyte chemotactic protein-1; Icam1, intracellular adhesion molecule.
Plant sterol and plant stanol feeding results in downregulation of Lxr target genes in the liver
Hepatic VLDL secretion and de novo lipogenesis have been reported to be regulated by several transcription factors including liver X receptor (LXR) (17, 21–23). To study whether PSE and PSA feeding may influence VLDL secretion via downregulation of LXR signaling, we determined expression levels of LXR target genes. As shown in Table 5, LXR target genes were indeed downregulated by PSE and PSA supplementation.
TABLE 5.
Hepatic expression of LXR target genes in C57BL/6J mice fed HFD, PSE, and PSA diets
| HFD | PSE | PSA | |
| Abca1 | 1.0 ± 0.2 | 0.8 ± 0.2 | 0.7 ± 0.1a |
| Abcg5 | 1.0 ± 0.1 | 0.5 ± 0.1a | 0.5 ± 0.0a |
| Abcg8 | 1.0 ± 0.2 | 1.5 ± 0.1a | 0.6 ± 0.1a |
| Lpl | 1.0 ± 0.2 | 0.5 ± 0.1a | 0.5 ± 0.1a |
| Srebf1(Srebp1c) | 1.0 ± 0.2 | 0.6 ± 0.1a | 0.7 ± 0.1 |
Values are presented as means ± SD. n = 6 animals per group. Abca1, ATP-binding cassette transporter 1; Abcg5, ATP-binding cassette transporter G5; Abcg8, ATP-binding cassette transporter G8; Lpl, lipoprotein lipase; srebf1, sterol regulatory element binding transcription factor 1.
P < 0.05 compared with HFD.
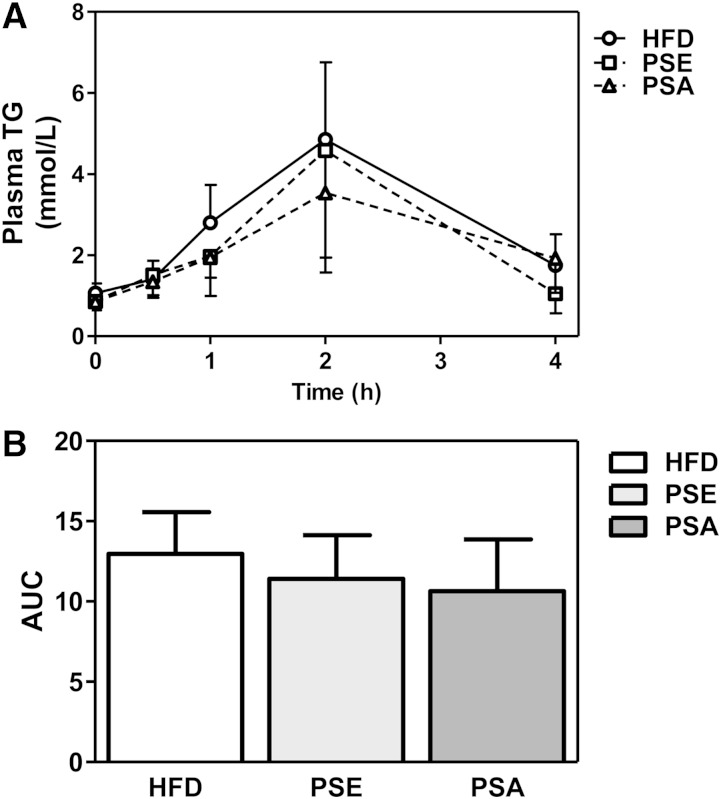
Plant sterols and plant stanols supplemented to HFD did not interfere in chylomicron secretion
To determine whether absorption of lipids was impaired after PSE and PSA feeding, we determined the rate of chylomicron production by administration of an oral fat bolus to the mice after overnight fasting. No differences were found in chylomicron secretion or in the postprandial excursion for serum TGs between the three different dietary conditions (Fig. 3). Next, we evaluated intestinal mRNA expression of Ppara (a nuclear receptor involved in lipid metabolism) and several genes known to be involved in intestinal fat absorption and chylomicron assembly. Supplementing plant sterols and stanols to HFD resulted in decreased expression of Ppara, Cd36, and Mttp in proximal small intestine compared with HFD-fed mice (Table 3).
Fig. 3.
Chylomicron formation in mice fed HFD, PSE, and PSA diets. A: Plasma TG concentrations in mice after an intragastric load (200 μl olive oil). B: Area under the curve (AUC). Values are presented as means ± SD. n = 6 animals per group. *P < 0.05 compared with HFD.
Plant sterols and stanols feeding changes expressions of β-oxidation related genes in liver
To investigate whether increased hepatic β-oxidation might explain the unchanged hepatic triglyceride levels while VLDL secretion is decreased, we measured hepatic gene expression of genes involved in β-oxidation and plasma levels of β-hydroxybutyrate.
PSE or PSA feeding resulted in a slight increase in Acacb (Acc2) and Cpt1a (both genes involved in mitochondrial β-oxidation) whereas Acox1 (a gene involved in peroxisomal β-oxidation) was decreased (Table 6). Concentrations of β-hydroxybutyrate, a ketone body, in plasma were unchanged after supplementation of plant sterols or stanols to HFD.
TABLE 6.
Hepatic expression of genes involved in β-oxidation and plasmic β-hydroxybutyrate in C57BL/6J mice fed HFD, PSE, and PSA diets
| HFD | PSE | PSA | |
| Liver | |||
| Acox1 (Aox) | 1.0 ± 0.1 | 0.7 ± 0.1a | 0.6 ± 0.3a |
| Acacb (Acc2) | 1.0 ± 0.4 | 1.5 ± 0.3a | 1.5 ± 0.2a |
| Cpt1a | 1.0 ± 0.2 | 1.1 ± 0.1 | 1.2 ± 0.1a |
| Plasma | |||
| BHB (mmol/l) | 0.33 ± 0.22 | 0.40 ± 0.08 | 0.22 ± 0.17 |
Values are presented as means ± SD. n = 6 animals per group. Acc2, acetyl-CoA carboxylase 2; Acox1, acyl-CoA oxidase 1, palmitoyl; BHB, β-hydroxybutyrate; Cpt1a, carnitine palmitoyltransferase 1a, liver.
P < 0.05 compared with HFD.
Discussion
Plant sterols and stanols are well known for their ability to reduce plasma cholesterol levels. In our study we found a lack of change in plasma cholesterol; however, this result was expected because in earlier studies using C57BL/6J mice, both our own group as well as others observed a lack of effect of plant sterol/stanol consumption on plasma cholesterol concentrations (10, 24). However, we did see a decrease of hepatic cholesterol concentrations, which indicates that plant sterols affect hepatic lipid and lipoprotein metabolism independent of changes in serum cholesterol concentrations.
Besides their cholesterol-lowering properties, plant sterols/stanols are also able to reduce triglyceride levels (8, 9). In our study, we observed that plant sterol and stanol feeding led to a decrease in plasma triglycerides whereas hepatic triglyceride levels remain unchanged. The latter is a surprising observation because previous results from Rideout et al. in C57BL/6J show a decrease in hepatic triglycerides after plant sterol feeding and similar results were found in ApoE −/− mice and ApoE3*-Leiden transgenic mice (10, 25, 26). The different diet composition used in these studies may explain the variation in hepatic triglyceride levels observed.
Because plant sterols and stanols are nonnutritional compounds and very poorly absorbed in the intestine, we first hypothesized that these compounds exert their primary effects at the intestinal level (27). Gene expression levels of CD36, involved in fatty acid absorption, were decreased after PSE and PSA treatment. However, this hypothesis could not be confirmed as chylomicron secretion was unaltered after plant sterol and stanol feeding.
In this study, we showed that the triglyceride-lowering properties of plant sterol/stanol feeding are associated with changes in VLDL secretion. After administration of poloxamer 407, we observed a significant decrease in VLDL-TG secretion in plasma in PSA- and PSE-fed mice Hepatic triglyceride levels have been described to determine for the number and size of VLDL-TG particles (28–30). However, in our study, hepatic triglyceride concentrations did not differ between the HFD- and PSE/PSA-fed mice. Similarly, several authors have reported a lack of association between hepatic triglyceride content and VLDL-TG production rate (31–33).
Because there is a potential link between hepatic inflammation and VLDL production, we investigated whether plant sterols/stanols could also act as anti-inflammatory agents, as has been previously suggested [for review see (20)], and whether this could explain the decrease in hepatic VLDL production. We did not find any differences in hepatic mRNA levels of several inflammatory markers. However, expression levels of these pro-inflammatory genes were rather low, suggesting that there was hardly any inflammation in the liver of C57BL/6J mice on a HFD for this duration. In addition, the HFD-fed animals showed a higher VLDL-TG production rate as compared with chow-fed animals (data not shown) without showing a clear hepatic inflammatory phenotype, which also suggests that these two entities are not directly linked. Therefore, it seems unlikely that the decrease in VLDL secretion after PSE/PSA treatment is due to changes in hepatic inflammation in these C57BL/6J mice.
Because VLDL secretion rates are decreased while hepatic triglyceride concentration remain unchanged, the decrease in plasma triglyceride concentrations could be due to increased β-oxidation. We observed a slight increase in the gene expression of Acacb (Acc2) and Cpt1a, which are involved in mitochondrial β-oxidation; in contrast, Acox1 gene expression, involved in peroxisomal β-oxidation, was decreased. Subsequently, plasma levels of β-hydroxybutyrate, a ketone body, were measured and no changes were observed. Taken together, our data on β-oxidation is not conclusive enough to say that β-oxidation is increased.
The question of how the hepatic VLDL secretion is decreased after PSE and PSA feeding could be answered by a decrease in hepatic LXR. LXRA (NR1H3) is a transcription factor involved in lipid metabolism and is an intracellular sensor for sterols (34). According to Basciano et al. (35) , activation of LXR leads to increased plasma triglycerides and VLDL. In our study, we observed opposite effects; i.e., decreased plasma TG and VLDL production in combination with downregulation of hepatic LXR target genes after supplementation of plant sterols and stanols to HFD. Subsequently, the expression of the LXR target gene Cd36 (Fat) in the liver, a transporter involved in fatty acid uptake, was examined (36). Koonen et al. (37) investigated the role of hepatic Cd36 and showed that increased Cd36 leads to increased fatty acid uptake, hepatic TG storage, and secretion via VLDL. After PSE and PSA feeding, hepatic TG remained unchanged and VLDL secretion was decreased, which could be explained by a decrease in fatty acid uptake by Cd36. Expression of the LXR target gene Apoa4 was increased after PSE and PSA feeding. Verhage et al. (38) observed that hepatic Apoa4 expression was associated with increased VLDL secretion and decreased hepatic TG content, which is in contradiction to our data. This contradiction could be explained by the observed steatosis in the study of Verhage et al. (38), which was not observed in our study. Studies from our group using LXR knockout mice indicated that disruption of LXRα induced a decrease in VLDL production while on the other hand, treatment with an LXR agonist increased VLDL production (17, 39). Our study is in line with these observations because we observed a decreased expression of hepatic LXR target genes together with decreased VLDL production. According to earlier studies by our group, plant sterols can activate LXR in vitro in Caco2 cells (40). In this study, however, we showed a decrease in the expression of hepatic LXR target genes. We hypothesize that the concentration of plant sterols in the liver is not high enough to activate hepatic LXR. This is also in line with data from our own group that LXR target gene expression was lower in liver samples from plant sterol-fed C57BL/6J mice (24) and the lack of hepatic LXR activation is therefore consistent.
In summary, similarly as observed in humans, plant sterol and stanol ester consumption resulted in decreased plasma triglyceride levels in HFD-fed mice. This decrease was associated with decreased hepatic VLDL secretion.
For many years, it has been accepted that chylomicrons and their remnants are the main carrier of TGs in the nonfasting state because these lipoproteins are increased after ingestion of a meal (41). Recently, it has been proven that the major triglyceride carriers in the postprandial state are VLDL and its remnants (42). Future studies investigating the association between TG metabolism and plant sterol/stanol intake should include a full kinetic characterization of VLDL, chylomicrons, and their remnants.
Supplementary Material
Acknowledgments
The authors would like to thank Vincent Bloks, Renze Boverhof, Angelika Jurdzinski, and Rick Havinga, University of Groningen, The Netherlands. Plant sterol and stanol esters were provided by RAISIO, Finland.
Footnotes
Abbreviations:
- HFD
- high-fat diet
- NEFA
- nonesterified fatty acid
- LXR
- liver X receptor
- PSA
- HFD supplemented with 3.1% plant stanol esters
- PSE
- HFD supplemented with 3.1% plant sterol esters
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three tables.
REFERENCES
- 1.Wu T., Fu J., Yang Y., Zhang L., Han J. 2009. The effects of phytosterols/stanols on blood lipid profiles: a systematic review with meta-analysis. Asia Pac. J. Clin. Nutr. 18: 179–186. [PubMed] [Google Scholar]
- 2.Brufau G., Canela M. A., Rafecas M. 2008. Phytosterols: physiologic and metabolic aspects related to cholesterol-lowering properties. Nutr. Res. 28: 217–225. [DOI] [PubMed] [Google Scholar]
- 3.Calpe-Berdiel L., Escola-Gil J. C., Blanco-Vaca F. 2009. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis. 203: 18–31. [DOI] [PubMed] [Google Scholar]
- 4.De Smet E., Mensink R. P., Plat J. 2012. Effects of plant sterols and stanols on intestinal cholesterol metabolism: suggested mechanisms from past to present. Mol. Nutr. Food Res. 56: 1058–1072. [DOI] [PubMed] [Google Scholar]
- 5.Brufau G., Kuipers F., Lin Y., Trautwein E. A., Groen A. K. 2011. A reappraisal of the mechanism by which plant sterols promote neutral sterol loss in mice. PLoS ONE. 6: e21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plat J., Brufau G., Dallinga-Thie G. M., Dasselaar M., Mensink R. P. 2009. A plant stanol yogurt drink alone or combined with a low-dose statin lowers serum triacylglycerol and non-HDL cholesterol in metabolic syndrome patients. J. Nutr. 139: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 7.Sialvera T. E., Pounis G. D., Koutelidakis A. E., Richter D. J., Yfanti G., Kapsokefalou M., Goumas G., Chiotinis N., Diamantopoulos E., Zampelas A. 2012. Phytosterols supplementation decreases plasma small and dense LDL levels in metabolic syndrome patients on a westernized type diet. Nutr. Metab. Cardiovasc. Dis. 22: 843–848. [DOI] [PubMed] [Google Scholar]
- 8.Naumann E., Plat J., Kester A. D., Mensink R. P. 2008. The baseline serum lipoprotein profile is related to plant stanol induced changes in serum lipoprotein cholesterol and triacylglycerol concentrations. J. Am. Coll. Nutr. 27: 117–126. [DOI] [PubMed] [Google Scholar]
- 9.Demonty I., Ras R. T., van der Knaap H. C., Meijer L., Zock P. L., Geleijnse J. M., Trautwein E. A. 2013. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 52: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rideout T. C., Harding S. V., Jones P. J. 2010. Consumption of plant sterols reduces plasma and hepatic triglycerides and modulates the expression of lipid regulatory genes and de novo lipogenesis in C57BL/6J mice. Mol. Nutr. Food Res. 54(Suppl 1): S7–S13. [DOI] [PubMed] [Google Scholar]
- 11.Emerging Risk Factors Collaboration, E. Di Angelantonio, N. Sarwar, P. Perry, S. Kaptoge, K. K. Ray, A. Thompson, A. M. Wood, S. Lewington, N. Sattar, C. J. Packard, R. Collins, S. G. Thompson, and J. Danesh. 2009. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 302: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima K., Nakano T., Tokita Y., Nagamine T., Inazu A., Kobayashi J., Mabuchi H., Stanhope K. L., Havel P. J., Okazaki M., et al. 2011. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin. Chim. Acta. 412: 1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson K. G., Walden C. M., Murray P., Smith A. M., Lovegrove J. A., Minihane A. M., Williams C. M. 2012. A sequential two meal challenge reveals abnormalities in postprandial TAG but not glucose in men with increasing numbers of metabolic syndrome components. Atherosclerosis. 220: 237–243. [DOI] [PubMed] [Google Scholar]
- 14.Nordestgaard B. G., Benn M., Schnohr P., Tybjaerg-Hansen A. 2007. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA. 298: 299–308. [DOI] [PubMed] [Google Scholar]
- 15.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 16.Gautier T., Tietge U. J., Boverhof R., Perton F. G., Le Guern N., Masson D., Rensen P. C., Havekes L. M., Lagrost L., Kuipers F. 2007. Hepatic lipid accumulation in apolipoprotein C–I-deficient mice is potentiated by cholesteryl ester transfer protein. J. Lipid Res. 48: 30–40. [DOI] [PubMed] [Google Scholar]
- 17.Grefhorst A., Elzinga B. M., Voshol P. J., Plosch T., Kok T., Bloks V. W., van der Sluijs F. H., Havekes L. M., Romijn J. A., Verkade H. J., et al. 2002. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 277: 34182–34190. [DOI] [PubMed] [Google Scholar]
- 18.Plosch T., Bloks V. W., Baller J. F., Havinga R., Verkade H. J., Jansen P. L., Kuipers F. 2002. Mdr P-glycoproteins are not essential for biliary excretion of the hydrophobic heme precursor protoporphyrin in a griseofulvin-induced mouse model of erythropoietic protoporphyria. Hepatology. 35: 299–306. [DOI] [PubMed] [Google Scholar]
- 19.Stricker D. 2008. BrightStat.com: free statistics online. Comput. Methods Programs Biomed. 92: 135–143. [DOI] [PubMed] [Google Scholar]
- 20.Othman R. A., Moghadasian M. H. 2011. Beyond cholesterol-lowering effects of plant sterols: clinical and experimental evidence of anti-inflammatory properties. Nutr. Rev. 69: 371–382. [DOI] [PubMed] [Google Scholar]
- 21.Grefhorst A., Parks E. J. 2009. Reduced insulin-mediated inhibition of VLDL secretion upon pharmacological activation of the liver X receptor in mice. J. Lipid Res. 50: 1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim D. H., Zhang T., Ringquist S., Dong H. H. 2011. Targeting FoxO1 for hypertriglyceridemia. Curr. Drug Targets. 12: 1245–1255. [DOI] [PubMed] [Google Scholar]
- 23.Rakhshandehroo M., Knoch B., Muller M., Kersten S. 2010. Peroxisome proliferator-activated receptor alpha target genes. PPAR Res. 2010 Epub. September 26, 2010. 10.1155/2010/612089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plösch T., Kruit J. K., Bloks V. W., Huijkman N. C., Havinga R., Duchateau G. S., Lin Y., Kuipers F. 2006. Reduction of cholesterol absorption by dietary plant sterols and stanols in mice is independent of the Abcg5/8 transporter. J. Nutr. 136: 2135–2140. [DOI] [PubMed] [Google Scholar]
- 25.Calpe-Berdiel L., Escola-Gil J. C., Ribas V., Navarro-Sastre A., Garces-Garces J., Blanco-Vaca F. 2005. Changes in intestinal and liver global gene expression in response to a phytosterol-enriched diet. Atherosclerosis. 181: 75–85. [DOI] [PubMed] [Google Scholar]
- 26.Volger O. L., van der Boom H., de Wit E. C., van Duyvenvoorde W., Hornstra G., Plat J., Havekes L. M., Mensink R. P., Princen H. M. 2001. Dietary plant stanol esters reduce VLDL cholesterol secretion and bile saturation in apolipoprotein E*3-Leiden transgenic mice. Arterioscler. Thromb. Vasc. Biol. 21: 1046–1052. [DOI] [PubMed] [Google Scholar]
- 27.Ostlund R. E., Jr 2002. Phytosterols in human nutrition. Annu. Rev. Nutr. 22: 533–549. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons G. F., Wiggins D., Brown A. M., Hebbachi A. M. 2004. Synthesis and function of hepatic very-low-density lipoprotein. Biochem. Soc. Trans. 32: 59–64. [DOI] [PubMed] [Google Scholar]
- 29.Adiels M., Taskinen M. R., Packard C., Caslake M. J., Soro-Paavonen A., Westerbacka J., Vehkavaara S., Hakkinen A., Olofsson S. O., Yki-Jarvinen H., et al. 2006. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 49: 755–765. [DOI] [PubMed] [Google Scholar]
- 30.Matikainen N., Taskinen M. R., Stennabb S., Lundbom N., Hakkarainen A., Vaaralahti K., Raivio T. 2012. Decrease in circulating fibroblast growth factor 21 after an oral fat load is related to postprandial triglyceride-rich lipoproteins and liver fat. Eur. J. Endocrinol. 166: 487–492. [DOI] [PubMed] [Google Scholar]
- 31.Wiegman C. H., Bandsma R. H., Ouwens M., van der Sluijs F. H., Havinga R., Boer T., Reijngoud D. J., Romijn J. A., Kuipers F. 2003. Hepatic VLDL production in ob/ob mice is not stimulated by massive de novo lipogenesis but is less sensitive to the suppressive effects of insulin. Diabetes. 52: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 32.Grefhorst A., Hoekstra J., Derks T. G., Ouwens D. M., Baller J. F., Havinga R., Havekes L. M., Romijn J. A., Kuipers F. 2005. Acute hepatic steatosis in mice by blocking beta-oxidation does not reduce insulin sensitivity of very-low-density lipoprotein production. Am. J. Physiol. Gastrointest. Liver Physiol. 289: G592–G598. [DOI] [PubMed] [Google Scholar]
- 33.van Diepen J. A., Vroegrijk I. O., Berbee J. F., Shoelson S. E., Romijn J. A., Havekes L. M., Rensen P. C., Voshol P. J. 2011. Aspirin reduces hypertriglyceridemia by lowering VLDL-triglyceride production in mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 301: E1099–E1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oosterveer M. H., Grefhorst A., Groen A. K., Kuipers F. 2010. The liver X receptor: control of cellular lipid homeostasis and beyond Implications for drug design. Prog. Lipid Res. 49: 343–352. [DOI] [PubMed] [Google Scholar]
- 35.Basciano H., Miller A., Baker C., Naples M., Adeli K. 2009. LXRalpha activation perturbs hepatic insulin signaling and stimulates production of apolipoprotein B-containing lipoproteins. Am. J. Physiol. Gastrointest. Liver Physiol. 297: G323–G332. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J., Febbraio M., Wada T., Zhai Y., Kuruba R., He J., Lee J. H., Khadem S., Ren S., Li S., et al. 2008. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology. 134: 556–567. [DOI] [PubMed] [Google Scholar]
- 37.Koonen D. P., Jacobs R. L., Febbraio M., Young M. E., Soltys C. L., Ong H., Vance D. E., Dyck J. R. 2007. Increased hepatic CD36 expression contributes to dyslipidemia associated with diet-induced obesity. Diabetes. 56: 2863–2871. [DOI] [PubMed] [Google Scholar]
- 38.VerHague M. A., Cheng D., Weinberg R. B., Shelness G. S. 2013. Apolipoprotein A-IV expression in mouse liver enhances triglyceride secretion and reduces hepatic lipid content by promoting very low density lipoprotein particle expansion. Arterioscler. Thromb. Vasc. Biol. 33: 2501–2508. [DOI] [PubMed] [Google Scholar]
- 39.van der Veen J. N., Havinga R., Bloks V. W., Groen A. K., Kuipers F. 2007. Cholesterol feeding strongly reduces hepatic VLDL-triglyceride production in mice lacking the liver X receptor alpha. J. Lipid Res. 48: 337–347. [DOI] [PubMed] [Google Scholar]
- 40.Plat J., Nichols J. A., Mensink R. P. 2005. Plant sterols and stanols: effects on mixed micellar composition and LXR (target gene) activation. J. Lipid Res. 46: 2468–2476. [DOI] [PubMed] [Google Scholar]
- 41.Zilversmit D. B. 1979. Atherogenesis: a postprandial phenomenon. Circulation. 60: 473–485. [DOI] [PubMed] [Google Scholar]
- 42.Nakajima K., Nakano T., Tokita Y., Nagamine T., Yatsuzuka S., Shimomura Y., Tanaka A., Sumino H., Nara M., Machida T., et al. 2012. The characteristics of remnant lipoproteins in the fasting and postprandial plasma. Clin. Chim. Acta. 413: 1077–1086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.