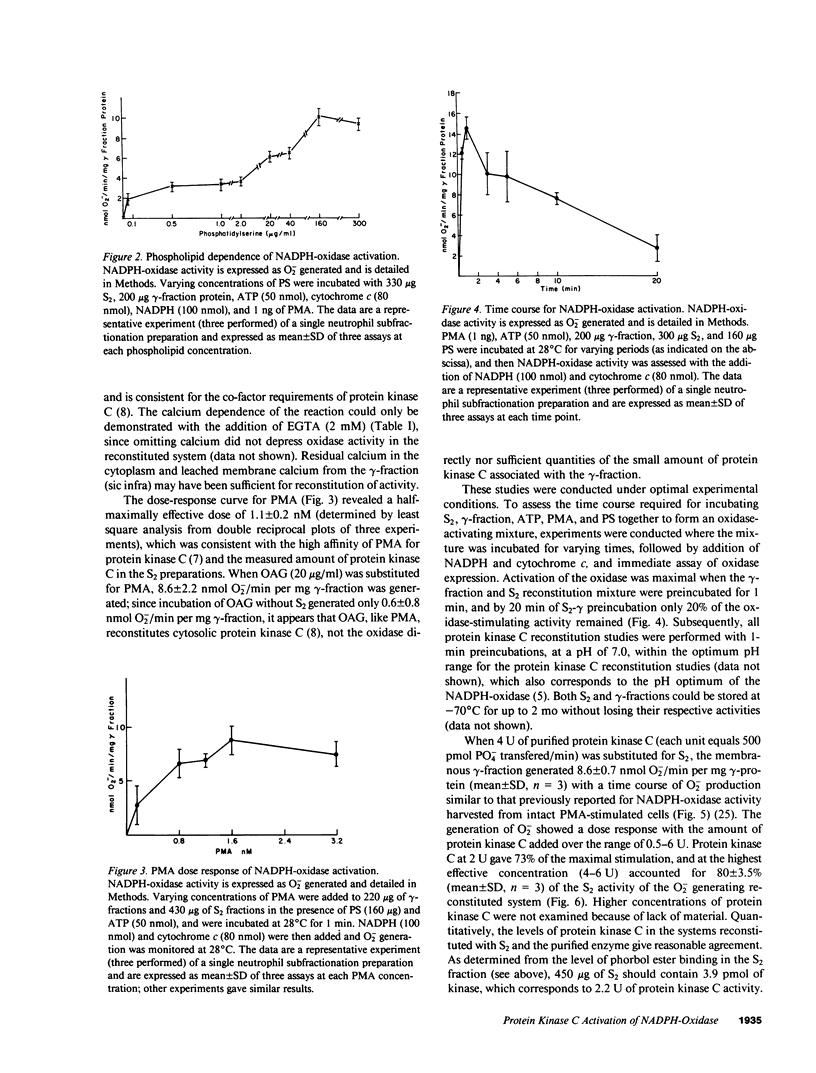
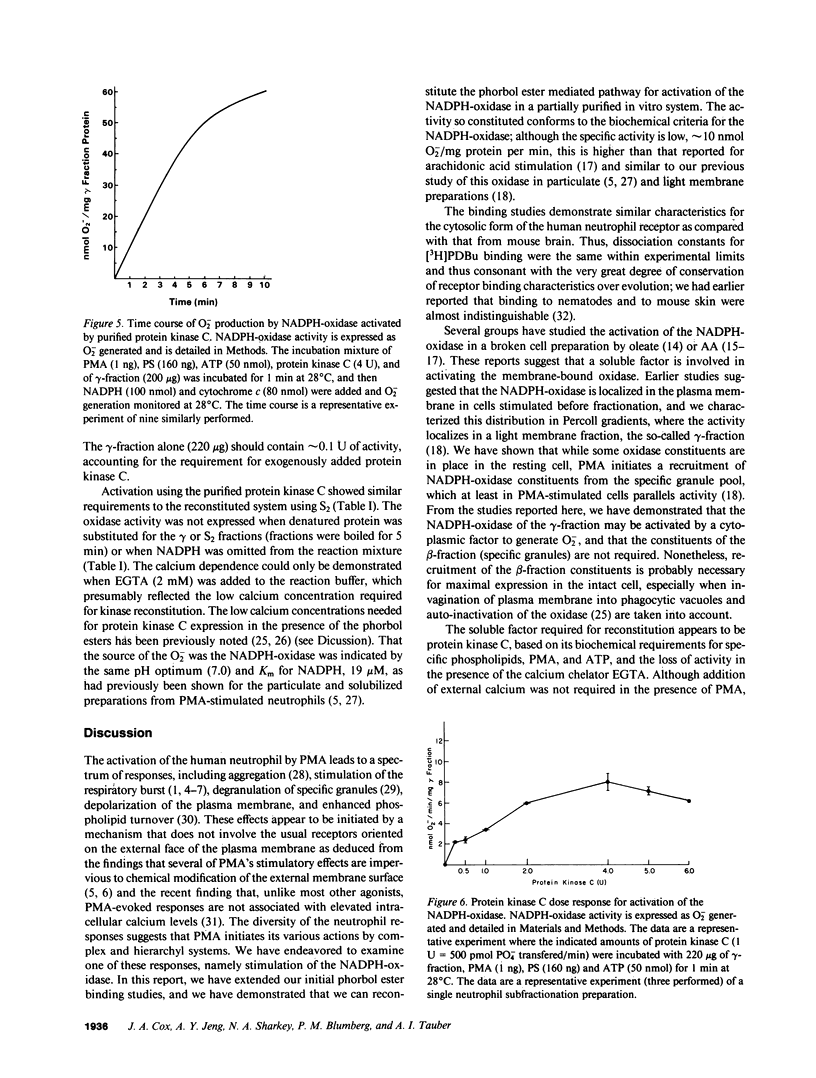
Abstract
A variety of phagocytosable and soluble agonists stimulate the human neutrophil respiratory burst enzyme, NADPH-oxidase, an activity required for normal microbicidal function. Of these agonists, the phorbol esters, which stimulate diverse systems by their ability to substitute for diacylglycerol to activate protein kinase C (the major phorbol ester receptor), have now been shown to directly stimulate NADPH-oxidase through this same receptor. Almost 90% of the specific receptors for phorbol 12,13-dibutyrate (PDBu) were found in the cytosol upon subcellular fractionation. The dissociation constant for [3H]PDBu was 1.2 nM. No significant difference was found in the distribution of the receptor between subcellular fractions from resting as compared with phorbol 12-myristate 13-acetate (PMA)-stimulated neutrophils. On the basis of these binding studies, we were able to establish a reconstituted system in which PMA activated dormant NADPH-oxidase in a light membrane fraction when cytosol, NADPH, phosphatidylserine, or phosphatidylinositol and ATP were added. The calcium chelator, EGTA, inhibited the activation, which suggested a requirement for calcium at low concentrations. The half-maximally effective PMA dose was 1.1 nM, as predicted from the receptor content in these preparations. Reconstitution of oxidase activity was rapid, peaking within 1 min of incubation. Purified protein kinase C was able to substitute for the cytosol fraction, and accounted for 80% of the cytosol activity. These studies demonstrate that phorbol esters stimulate the neutrophil respiratory burst through activation of cytosolic protein kinase C, which in turn activates either a regulatory constituent or the NADPH-oxidase directly in the plasma membrane to generate an active O-2-generating system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. C., Babior B. M. Endogenous protein phosphorylation by resting and activated human neutrophils. Blood. 1983 Feb;61(2):333–340. [PubMed] [Google Scholar]
- Arcoleo J. P., Weinstein I. B. Activation of protein kinase C by tumor promoting phorbol esters, teleocidin and aplysiatoxin in the absence of added calcium. Carcinogenesis. 1985 Feb;6(2):213–217. doi: 10.1093/carcin/6.2.213. [DOI] [PubMed] [Google Scholar]
- Badwey J. A., Curnutte J. T., Karnovsky M. L. cis-Polyunsaturated fatty acids induce high levels of superoxide production by human neutrophils. J Biol Chem. 1981 Dec 25;256(24):12640–12643. [PubMed] [Google Scholar]
- Blumberg P. M., Delclos K. B., Jaken S. Tissue and species specificity for phorbol ester receptors. Basic Life Sci. 1983;24:201–229. doi: 10.1007/978-1-4684-4400-1_11. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Jaken S., König B., Sharkey N. A., Leach K. L., Jeng A. Y., Yeh E. Mechanism of action of the phorbol ester tumor promoters: specific receptors for lipophilic ligands. Biochem Pharmacol. 1984 Mar 15;33(6):933–940. doi: 10.1016/0006-2952(84)90448-9. [DOI] [PubMed] [Google Scholar]
- Borregaard N., Tauber A. I. Subcellular localization of the human neutrophil NADPH oxidase. b-Cytochrome and associated flavoprotein. J Biol Chem. 1984 Jan 10;259(1):47–52. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bromberg Y., Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cell Immunol. 1984 Oct 1;88(1):213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. J Clin Invest. 1985 May;75(5):1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Gabig T. G., Babior B. M. The O2(-) -forming oxidase responsible for the respiratory burst in human neutrophils. Properties of the solubilized enzyme. J Biol Chem. 1979 Sep 25;254(18):9070–9074. [PubMed] [Google Scholar]
- Helfman D. M., Appelbaum B. D., Vogler W. R., Kuo J. F. Phospholipid-sensitive Ca2+-dependent protein kinase and its substrates in human neutrophils. Biochem Biophys Res Commun. 1983 Mar 29;111(3):847–853. doi: 10.1016/0006-291x(83)91376-1. [DOI] [PubMed] [Google Scholar]
- Heyneman R. A., Vercauteren R. E. Activation of a NADPH oxidase from horse polymorphonuclear leukocytes in a cell-free system. J Leukoc Biol. 1984 Dec;36(6):751–759. doi: 10.1002/jlb.36.6.751. [DOI] [PubMed] [Google Scholar]
- Iwasa Y., Hosey M. M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J Biol Chem. 1984 Jan 10;259(1):534–540. [PubMed] [Google Scholar]
- Kikkawa U., Takai Y., Minakuchi R., Inohara S., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase from rat brain. Subcellular distribution, purification, and properties. J Biol Chem. 1982 Nov 25;257(22):13341–13348. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B., Cooper H. L., Sando J. J. Decrease in cytosolic calcium/phospholipid-dependent protein kinase activity following phorbol ester treatment of EL4 thymoma cells. J Biol Chem. 1982 Nov 25;257(22):13193–13196. [PubMed] [Google Scholar]
- Kraft A. S., Anderson W. B. Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature. 1983 Feb 17;301(5901):621–623. doi: 10.1038/301621a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., James M. L., Blumberg P. M. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., O'Callaghan A. M., Goetzl E. J., Tauber A. I. Characteristics of the cofactor requirements for the superoxide-generating NADPH oxidase of human polymorphonuclear leukocytes. Biochemistry. 1981 Mar 17;20(6):1468–1476. doi: 10.1021/bi00509a010. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Clayton C. C., Snyderman R. A potential second messenger role for unsaturated fatty acids: activation of Ca2+-dependent protein kinase. Science. 1984 May 11;224(4649):622–625. doi: 10.1126/science.6231726. [DOI] [PubMed] [Google Scholar]
- McPhail L. C., Shirley P. S., Clayton C. C., Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system. Evidence for a soluble cofactor. J Clin Invest. 1985 May;75(5):1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Dechatelet L. R., McCall C. E., Bass D. A. Neutrophil aggregation: evidence for a different mechanism of action by phorbol myristate acetate. Proc Soc Exp Biol Med. 1980 Nov;165(2):225–232. doi: 10.3181/00379727-165-40962. [DOI] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Repine J. E., White J. G., Clawson C. C., Holmes B. M. The influence of phorbol myristate acetate on oxygen consumption by polymorphonuclear leukocytes. J Lab Clin Med. 1974 Jun;83(6):911–920. [PubMed] [Google Scholar]
- Smith G. P., Peters T. J. The release of granule components from human polymorphonuclear leukocytes in response to both phagocytic and chemical stimuli. Biochim Biophys Acta. 1982 Nov 24;719(2):304–308. doi: 10.1016/0304-4165(82)90103-9. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Tauber A. I., Brettler D. B., Kennington E. A., Blumberg P. M. Relation of human neutrophil phorbol ester receptor occupancy and NADPH-oxidase activity. Blood. 1982 Aug;60(2):333–339. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Inhibition of complement-mediated functions of human neutrophils by impermeant stilbene disulfonic acids. J Immunol. 1981 May;126(5):1786–1789. [PubMed] [Google Scholar]
- Tauber A. I., Goetzl E. J. Structural and catalytic properties of the solubilized superoxide-generating activity of human polymorphonuclear leukocytes. Solubilization, stabilization in solution, and partial characterization. Biochemistry. 1979 Dec 11;18(25):5576–5584. doi: 10.1021/bi00592a009. [DOI] [PubMed] [Google Scholar]
- Tauber A. I., Simons E. R. Dissociation of human neutrophil membrane depolarization, respiratory burst stimulation and phospholipid metabolism by quinacrine. FEBS Lett. 1983 May 30;156(1):161–164. doi: 10.1016/0014-5793(83)80269-5. [DOI] [PubMed] [Google Scholar]
- White J. R., Huang C. K., Hill J. M., Jr, Naccache P. H., Becker E. L., Sha'afi R. I. Effect of phorbol 12-myristate 13-acetate and its analogue 4 alpha-phorbol 12,13-didecanoate on protein phosphorylation and lysosomal enzyme release in rabbit neutrophils. J Biol Chem. 1984 Jul 10;259(13):8605–8611. [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]
- Wrenn R. W., Katoh N., Wise B. C., Kuo J. F. Stimulation by phosphatidylserine and calmodulin of calcium-dependent phosphorylation of endogenous proteins from cerebral cortex. J Biol Chem. 1980 Dec 25;255(24):12042–12046. [PubMed] [Google Scholar]


