Abstract
Herein, we characterize a generally applicable transformation of fatty acid epoxides by lipoxygenase (LOX) enzymes that results in the formation of a five-membered endoperoxide ring in the end product. We demonstrated this transformation using soybean LOX-1 in the metabolism of 15,16-epoxy-α-linolenic acid, and murine platelet-type 12-LOX and human 15-LOX-1 in the metabolism of 14,15-epoxyeicosatrienoic acid (14,15-EET). A detailed examination of the transformation of the two enantiomers of 15,16-epoxy-α-linolenic acid by soybean LOX-1 revealed that the expected primary product, a 13S-hydroperoxy-15,16-epoxide, underwent a nonenzymatic transformation in buffer into a new derivative that was purified by HPLC and identified by UV, LC-MS, and 1H-NMR as a 13,15-endoperoxy-16-hydroxy-octadeca-9,11-dienoic acid. The configuration of the endoperoxide (cis or trans side chains) depended on the steric relationship of the new hydroperoxy moiety to the enantiomeric configuration of the fatty acid epoxide. The reaction mechanism involves intramolecular nucleophilic substitution (SNi) between the hydroperoxy (nucleophile) and epoxy group (electrophile). Equivalent transformations were documented in metabolism of the enantiomers of 14,15-EET by the two mammalian LOX enzymes, 15-LOX-1 and platelet-type 12-LOX. We conclude that this type of transformation could occur naturally with the co-occurrence of LOX and cytochrome P450 or peroxygenase enzymes, and it could also contribute to the complexity of products formed in the autoxidation reactions of polyunsaturated fatty acids.
Keywords: fatty acid/physical chemistry, lipids/chemistry, nuclear magnetic resonance, fatty acid epoxide, monocyclic peroxide, epoxyeicosatrienoic acid, lipoxygenase, cytochrome P450
The oxygenation reactions of polyunsaturated fatty acids include several examples in which different enzymes collaborate in synthesis of the end product. The cyclooxygenases are paired with cytochrome P450s in the transformation of arachidonic acid to prostaglandin (PG) endoperoxides and on to thromboxane A2 and prostacyclin (1, 2). A P450 and cyclooxygenase participate in the opposite order in the metabolism of P450-derived 8,9-epoxyeicosatrienoic acid (8,9-EET) by cyclooxygenase, giving epoxy-hydroxy products (3). The plant pathway of jasmonic acid synthesis involves initial oxygenation by a 13S-lipoxygenase (LOX) followed by transformation to an allene oxide by the P450, CYP74 (2, 4). Other examples include the cooperation of LOX and peroxygenase enzymes in the formation of epoxy and hydroxy fatty acids (5–7). The present work describes a new type of interaction, not so far described in nature, yet with the potential to produce fatty acid hydroxy-endoperoxides. Further transformations could readily form triols or other derivatives.
The impetus for the present work arose from further studies of fatty acid epoxidation by catalase-related enzymes primed with an oxygen donor, analogous to an earlier report of arachidonate and 8-HETE epoxidation by catalase-related allene oxide synthase (8). One example involved enzymatic epoxidation of 13S-hydroxy-α-linolenic acid on the 15,16-double bond. To assign the configuration of the new 15,16-epoxide group, we proposed a scheme using soybean LOX to introduce a 13S-hydroperoxide into the individual 15R,16S and 15S,16R enantiomers of 15,16-epoxy-linolenic acid. Following reduction to the 13S-hydroxy derivatives, this would give authentic standards to compare with the original product. While the desired primary products were recovered from the LOX-catalyzed transformation, it was obvious that additional conjugated diene-containing derivatives were equally abundant in the extracts. This report describes the characterization of these novel products and the mechanism of their synthesis. Subsequent work with mammalian LOX enzymes and an arachidonic acid epoxide (14,15-EET) demonstrate the generality of these reactions.
MATERIALS AND METHODS
Materials
α-Linolenic acid and arachidonic acid were purchased from Nu-Check Prep. Meta-chloroperoxybenzoic acid (mCPBA), stannous chloride, and soybean (Glycine max) lipoxidase type V were from Sigma-Aldrich. Human 15-LOX-1 was prepared exactly as described by expression of the recombinant enzyme in Escherichia coli (9), and a baculovirus construct of the murine platelet-type 12-LOX was expressed in Sf9 cells as described (10).
Preparation of epoxy fatty acids
Preparation of α-linolenic acid 15,16-epoxide enantiomers.
α-Linolenic acid (25 mg) was epoxidized using mCPBA, the 15,16-epoxide purified by reversed phase (RP)-HPLC then straight phase (SP)-HPLC (11) and the enantiomers resolved using a Chiralpak AD column (12) and designated as AD1 and AD2 in order of elution: these were identified as the 15S,16R- and 15R,16S-epoxides, respectively, by CD analysis of the methyl ester O-benzoyl derivatives as described in detail for related linoleate epoxides (13).
Preparation of 14,15-EET enantiomers.
Arachidonic acid (100 mg) in 2 ml dichloromethane was incubated with a 1.5-fold molar excess of mCPBA at room temperature for 30 min. The formed products were taken to dryness and dissolved in methanol prior to HPLC analysis. The arachidonate monoepoxides are well-precedented to elute in RP-HPLC in the order 14,15-, 11,12-, 8,9-, and 5,6-EET (14); 14,15-EET was isolated by RP-HPLC using methanol/water/glacial acetic acid (HAc) 85:15:0.01 (v/v/v) on a Waters Symmetry C18 column (4.6 × 250 mm; 5 μ). The enantiomers of 14,15-EET were resolved using a Chiralcel OJ column (4.6 × 250 mm; 5 μ) and a solvent of hexane/methanol/HAc (100:0.5:0.1, v/v/v, run at 2 ml/min), eluting in the order 14S,15R as peak 1 and 14R,15S as peak 2 as reported (15).
Incubations with soybean LOX-1
The individual enantiomers of 15,16-epoxy-linolenate were reacted at room temperature with soybean LOX-1 (76 nM) in 0.5 ml 0.1 M K2HPO4 (pH 8.7) buffer in a quartz cuvette and the change in the absorbance recorded by repetitive scanning (200–300 nm) using a Lambda-35 UV/Vis spectrometer (Perkin-Elmer). Addition of enzyme led to the appearance of a conjugated diene chromophore (ε ∼25,000 M−1 cm−1) over the course of 10 min, with a faster reaction rate for the AD2 enantiomer (15R,16S-epoxy). To reduce hydroperoxide products in situ, incubations were conducted in the presence of 0.5 mM SnCl2. All the formed products were acidified with 1 M KH2PO4 and 1 N HCl down to pH 5 and extracted on 1 cc HLB Oasis cartridges and eluted with methanol prior to HPLC analyses. To isolate sufficient products for NMR analyses, the incubations were scaled up to 20 ml 0.1 M K2HPO4 buffer containing 152 nM soybean LOX-1. The methyl ester derivatives of isolated products were prepared using diazomethane.
Incubations with platelet 12-LOX and 15-LOX-1
The individual enantiomers of 14,15-EET were incubated with platelet 12-LOX (0.42 μM) in 0.5 ml 50 mM Tris pH 7.5 buffer containing 150 mM NaCl and the changes in the absorbance recorded by repetitive scanning (200–300 nm) using a Lambda-35 UV/Vis spectrometer (Perkin-Elmer). Addition of enzyme led to the appearance of a conjugated diene chromophore over the course of 5 min (ε ∼25,000 M−1 cm−1), with similar reaction rates for both enantiomers of 14,15-EET. To reduce hydroperoxide products in situ, incubations with platelet 12-LOX were conducted in the presence of 0.5 mM SnCl2. All the formed products were extracted on 1 cc HLB Oasis cartridges as previously described. Subsequent incubations with human 15-LOX-1 were carried out in a similar manner. The methyl esters of products formed by platelet 12-LOX and human 15-LOX-1 were prepared using diazomethane. For the NMR analyses, the incubations with platelet 12-LOX were scaled up to 10 ml buffer and use of 0.4 μM enzyme. The methyl ester derivatives of the human 15-LOX-1 products were reacted with 1.5 equivalents of TPP in methanol for 30 min at room temperature for 10 min prior to HPLC and GC-MS analyses.
HPLC analyses
The formed products were chromatographed on SP-HPLC (Hex/IPA/HAc, 100:3:0.1, by volume for free acids and Hex/IPA, 100:2 for methyl esters) using a Thomson silica column (4.6 × 250 mm, 5 μ). A flow rate of 1 ml/min was used and UV signals at 205, 220, 235, and 270 nm recorded using an Agilent 1100 series diode array detector. To prepare sufficient products for 1H-NMR, about 0.5 mg of fatty acids were injected for the collection. Each collected product was dissolved in methanol and kept at −20°C until further analysis.
LC- and GC-MS analysis
LC-MS analysis of soybean LOX-1-catalyzed products.
The products formed from the enantiomers of 15,16-epoxy-linolenate on incubation with soybean LOX-1 and LOX-1/SnCl2 were eluted on RP-HPLC (CH3CN/H2O/HAc, 45:55:0.01, by volume; 0.4 ml/min) using a Kinetex C18 column (3 × 100 mm, 2.6 μ) and molecular weights were established from the M-H anions measured using a negative ion electrospray LTQ1 ion trap LC-MS instrument.
GC-MS analysis of human 15-LOX-1-catalyzed products.
The methyl esters of hydroperoxy-EETs were treated with triphenylphosphine (TPP) to prepare the corresponding hydroxy-EETs and repurified by SP-HPLC. The methyl esters of hydroxy-EETs dissolved in 200 μl ethyl acetate were hydrogenated using H2 with ∼1 mg 5% palladium on alumina for 3 min at room temperature. The catalyst was removed by extraction with water and centrifugation, and the organic phase containing the saturated hydroxy-C20 fatty acids was transferred for further derivatization. GC-MS analyses of the methyl ester trimethylsilyl derivatives of the reduced and hydrogenated hydroperoxy-EETs was carried out in the positive ion electron impact mode (70 eV) using a ThermoFinnigan DSQ mass spectrometer. The initial temperature was set for 180°C and then increased to 300°C at 20°C/min and then held at 300°C for 4 min.
NMR analysis
1H-NMR and 1H,1H COSY NMR spectra were recorded on a Bruker 600 MHz spectrometer at 298 K. The parts/million values are reported relative to residual nondeuterated benzene (C6D6; δ = 7.15 ppm). All spectra were analyzed on Bruker TopSpin 3.0 software. In addition to NMR analyses, 3D models of the formed products were created using ACD-Labs software (supplementary data).
RESULTS
Reaction with soybean LOX-1 with the 15,16-epoxides of α-linolenic acid
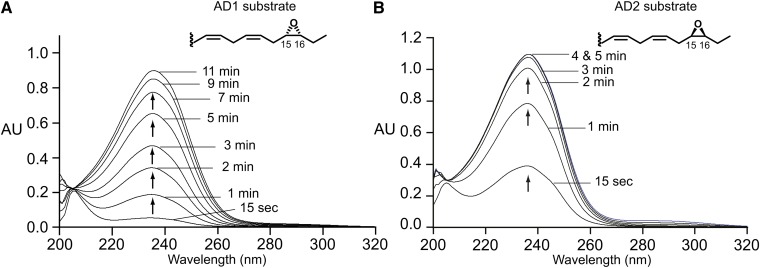
The 15,16-epoxide enantiomers of α-linolenic acid were prepared as outlined in the Materials and Methods and, for convenience here, are designated as AD1 (15S,16R) and AD2 (15R,16S), in order of their elution from the Chiralpak AD column. The 15,16-epoxides retain a 9cis,12cis pentadiene in the structure and are potential substrates for soybean LOX-1. Figure 1A, B illustrates transformation of the two mirror image 15,16-epoxides with the LOX-1 enzyme as observed by UV spectroscopy. Both enantiomers give rise to a typical conjugated diene chromophore, with a much slower reaction occurring with AD1, the 15S,16R isomer, (compare Fig. 2A, B); under the conditions used here, AD1 has an initial turnover of 2 s−1 and AD2 of 13 s−1, in the order of 1 and 5%, respectively, of the rates with α-linolenic acid itself (16).
Fig. 1.
Repetitive UV scans of the formation of products from the α-linolenate 15,16-epoxides AD1 (A) and AD2 (B) by soybean LOX-1. Turnover numbers are presented in the text.
Fig. 2.
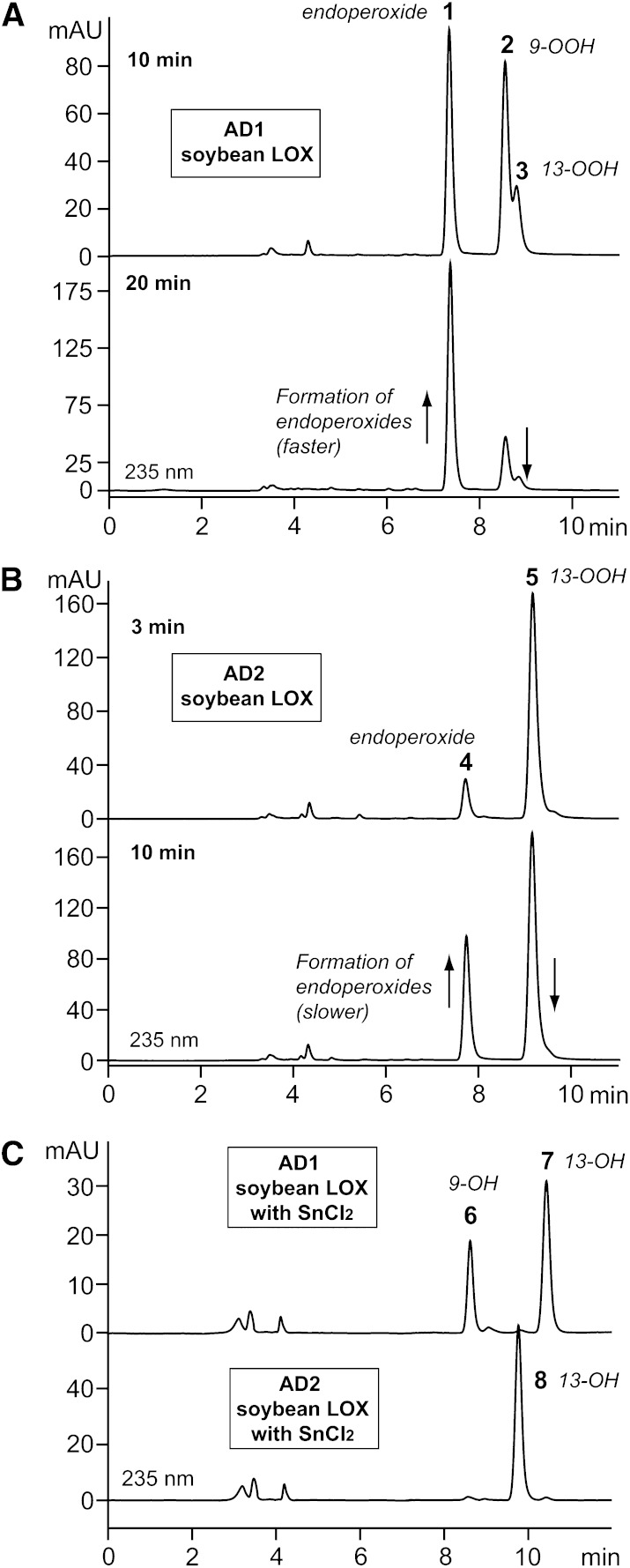
SP-HPLC analyses of products (analyzed as methyl esters) from 15,16-epoxides AD1 (A) and AD2 (B) by soybean LOX-1, and from identical incubations with soybean LOX-1 carried out in the presence of SnCl2 (C). The top chromatogram in (A) is from a 10 min incubation with enzyme and the lower chromatogram is from a 20 min incubation. In (B), the incubation times were 3 min and 10 min. In (C), the hydroxy products 6, 7, and 8 are analogs of hydroperoxy derivatives 2, 3, and 4, respectively. Samples were run on a Thomson 5 μ silica column (250 × 4.6 mm) with a solvent of hexane/IPA (100:2 by volume) at a flow rate of 1 ml/min with UV detection at 235 nm. The structures of products 1–5 are shown in Scheme 1.
Subsequent SP-HPLC analyses revealed a more complex pattern of products than initially expected, with three products recovered from a 10 min reaction of AD1 (Fig. 2A, top chromatogram) and two products from AD2 (Fig. 2B, top). All these products showed UV absorbance at 235 nm and contained a conjugated diene chromophore (supplementary Fig. I). The product pattern was time dependent, such that upon 20 min incubation in the reaction buffer with soybean LOX-1, there was an increase in the relative abundance of the earliest-eluting product (Fig. 2A, B, lower chromatograms, products 1 and 4).
To help understand the basis for the appearance of multiple products containing a conjugated diene chromophore, the incubations with soybean LOX-1 were repeated in the presence of stannous chloride (SnCl2) that will reduce hydroperoxides to the hydroxy derivative in situ. This simplified the product pattern such that only two conjugated dienes appeared from AD1 and only one product from AD2 (Fig. 2C). It appeared that the SnCl2-catalyzed reduction of the hydroperoxy products of the soybean LOX-1 reaction prevented the time-dependent transformation that gives rise to the earliest-eluting products (products 1 and 4 in Fig. 2A, B).
Structural analysis of soybean LOX-1 products 1–8
LC-MS analyses (negative ion mode, supplementary Fig. II) showed that all of the products formed in the initial incubations with soybean LOX-1 (products 1–5) have an M-1 ion at m/z 325, corresponding to the addition of molecular oxygen to the 15,16-epoxy-linolenate. All the SnCl2-reduced products each have an M-1 ion at m/z 309, corresponding to retention of one of the oxygens from O2.
Fragmentation of the peroxide products provided the first indications of the presence of an endoperoxide moiety in the structures of products 1 and 4. Whereas the other peroxide products showed only a prominent M-1 ion at m/z 325 in the negative ion mass spectrum, products 1 and 4 contained an equally prominent ion at m/z 237, interpreted as loss of C15-C18 (88 amu) and compatible with the presence of an oxygen attached to C15 in addition to the oxygen at C16.
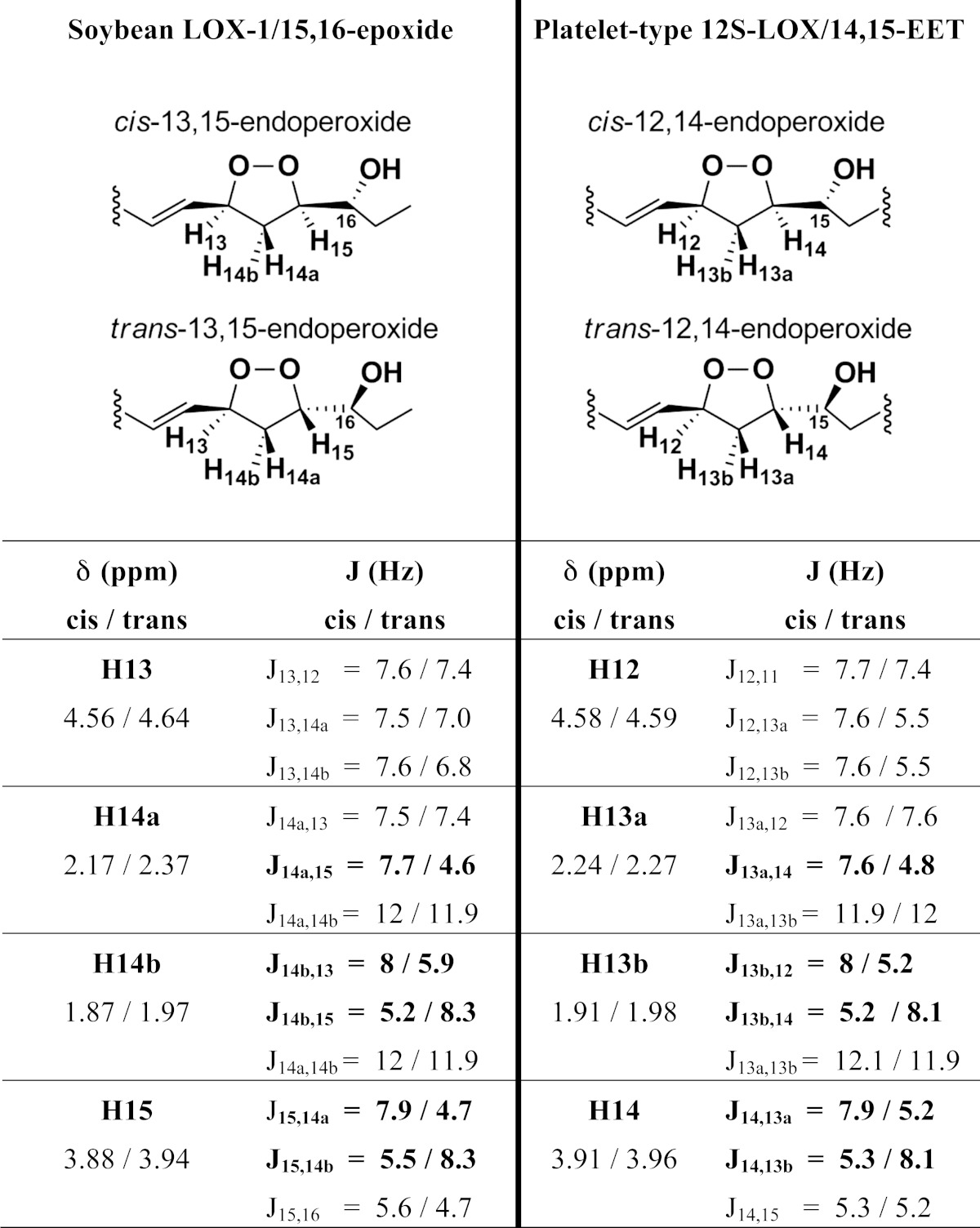
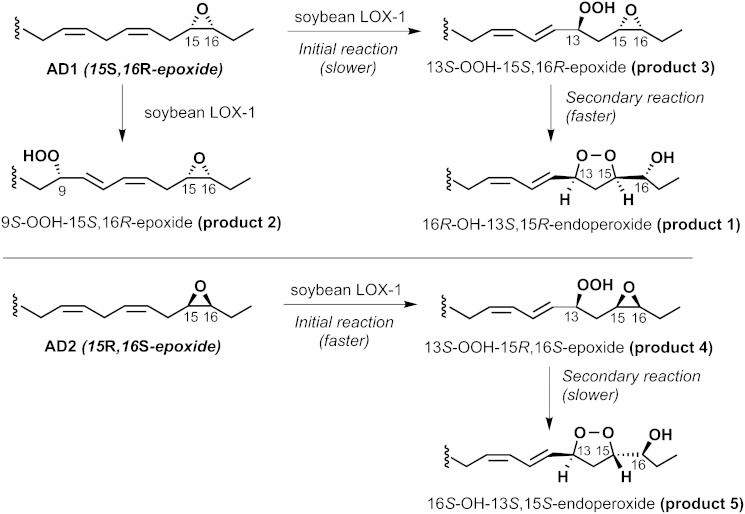
All the products described so far were prepared in sufficient amounts for NMR analysis, which revealed their covalent structures, and based on literature precedents, the relative stereochemistry of the carbon chain substituents of the endoperoxides in products 1 and 4 (17, 18) (Table 1). The NMR spectra and COSY analyses are given in the supplementary data, pages 10–18. Scheme 1 shows the structures of the peroxide products 1–5 and the deduced pathway to the endoperoxides (products 1 and 4). The AD1 enantiomer (15S,16R-epoxy) reacts relatively slowly with soybean LOX-1 and is converted to three products: one is the 13S-hydroperoxide (product 3), which is expected, and this is further transformed to the endoperoxide (product 1); in addition, the slowly-reacting AD1 enantiomer also gives the 9-hydroperoxide (product 2) as an additional product. The faster reacting AD2 enantiomer only gives the 13S-hydroperoxide (product 5) and the corresponding endoperoxide (product 4). The SnCl2 reduced products are the corresponding 13-hydroxy-15,16-epoxy derivatives (products 7 and 8), plus the 9-hydroxy-15,16-epoxide (product 6) formed from AD1.
TABLE 1.
1H-NMR chemical shifts and coupling constants of cis- and trans-endoperoxides formed in the soybean LOX-1-catalyzed and platelet-type 12S-LOX-catalyzed metabolism with 15,16-epoxy-α-linolenic acid and 14,15-EET, respectively
Characteristically different values of cis and trans are shown in bold. δ, chemical shifts; J, coupling constants.
Scheme 1.
Products formed from α-linoleate 15,16-epoxides AD1 and AD2 by soybean LOX-1.
Non-enzymatic formation of the endoperoxides
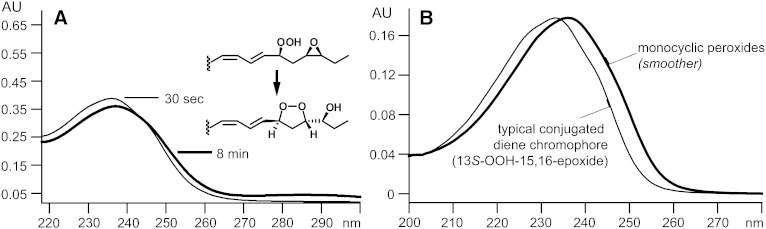
The 13-hydroperoxy-15,16-epoxides (products 2 and 5) were stable in organic solvents and could be purified with conventional HPLC methods at room temperature. Reincubation of the purified hydroperoxy-epoxides in the original pH 8.5 buffer in the absence of enzyme led to a perceptible change in the UV profile (Fig. 3A); this is attributed to their nonenzymatic transformation to the corresponding endoperoxides, which have a higher lambda max (239 nm, versus 237 nm of the hydroperoxy derivatives) (Fig. 3B). This transformation in buffer alone matches the time-dependent changes noted earlier upon extending the 10 min incubations to 20 min (Fig. 2A, B, upper and lower chromatograms). Formation of the endoperoxides in the absence of enzyme was confirmed by extraction and HPLC-UV analysis of the products (data not shown).
Fig. 3.
The nonenzymic formation of endoperoxides from 15,16-epoxide AD2 (A). Formation of endoperoxides from other hydroperoxy-epoxides can be detected in a similar manner due to the characteristic UV spectra of endoperoxides (B).
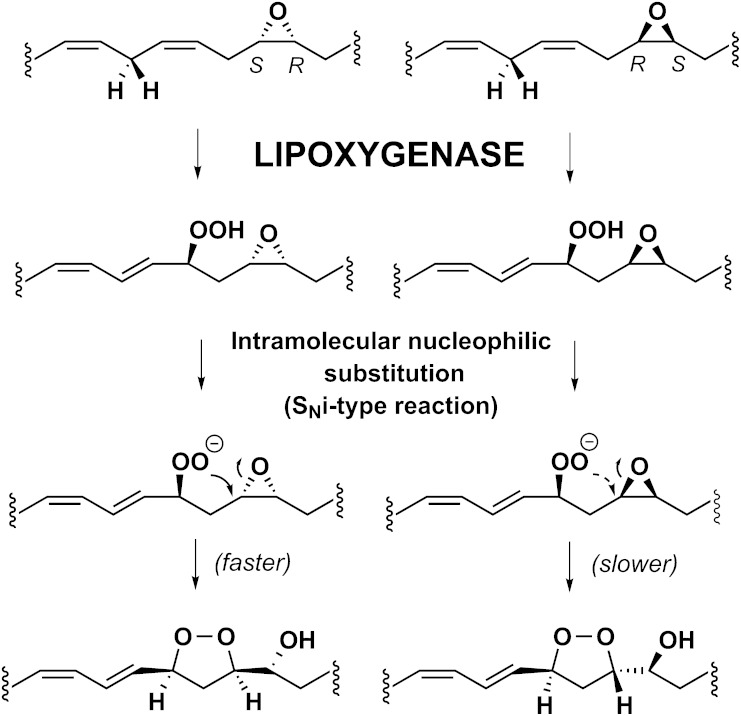
There is a noticeable difference in the rate of endoperoxide formation from the 13S-hydroperoxy derivatives of the linolenic acid AD1 and AD2 15,16-epoxides. Whereas AD1 reacts more slowly with soybean LOX-1 (Fig. 1A, B), its product (13S-hydroperoxy-15S,16R-epoxy) shows the faster nonenzymic transformation to the endoperoxide. This is understandable in that the newly formed hydroperoxy moiety is better positioned for intramolecular nucleophilic substitution (SNi) attack on the 15,16-epoxide (as explained in the Discussion).
Testing the generality of the endoperoxide formation
To test the generality of the endoperoxide formation from fatty acid hydroperoxy-epoxides, we used two mammalian LOX enzymes and the enantiomers of 14,15-EET as substrate. Reactions of the platelet-type 12-LOX with 14,15-EET are analogous to the linolenic acid transformations described above. On the other hand, the primary oxygenation activity of 15-LOX-1 is not possible using 14,15-EET as substrate; in this case it is the minor 12-dioxygenase activity of 15-LOX-1 that comes into play, as well recognized before in the 15-LOX-1-catalyzed transformations of 15S-hydroperoxyeicosatetraenoic acid (HPETE) to leukotriene epoxides (19) and the related transformations catalyzed by the leukocyte-type 12-LOX (20).
Reactions with mouse platelet-type 12-LOX
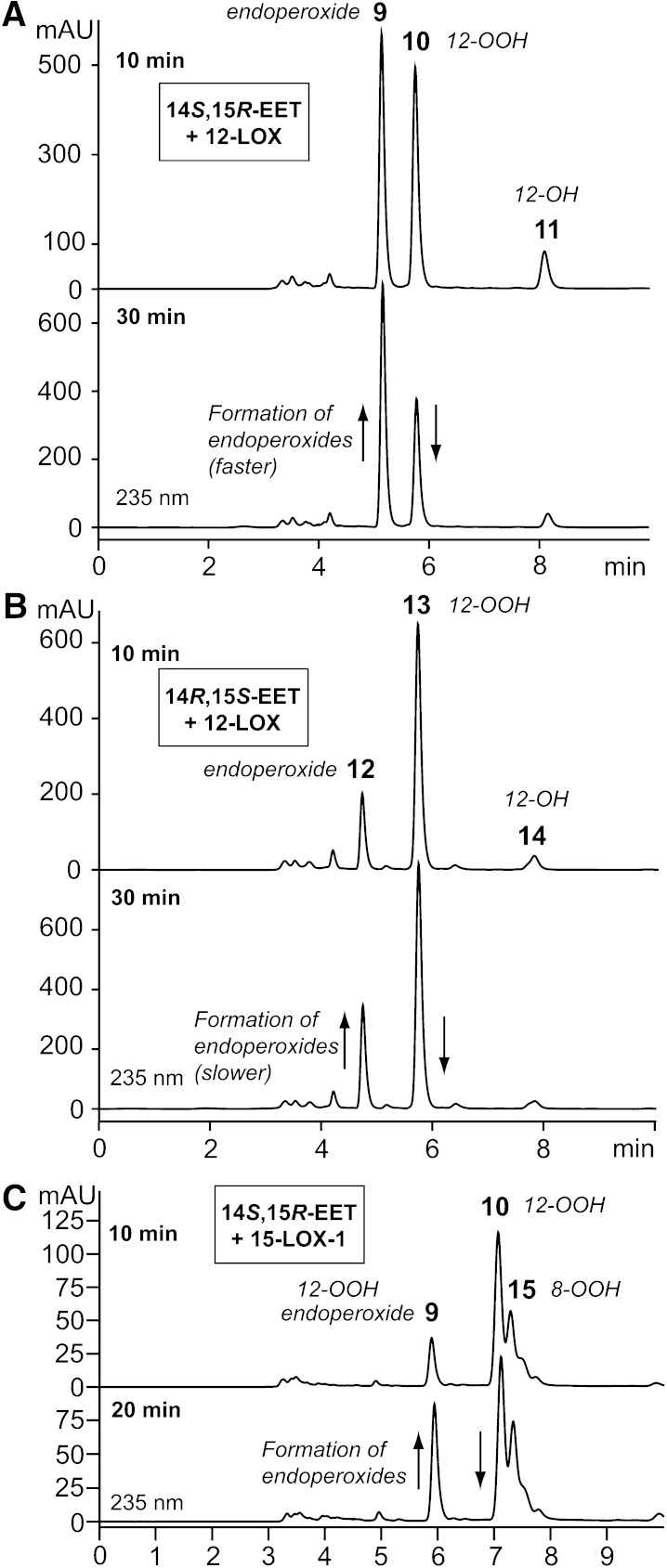
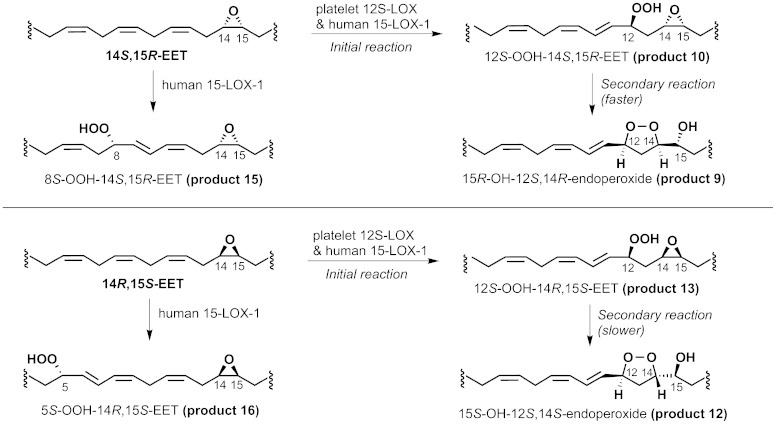
Reaction of recombinant platelet-type 12-LOX with the enantiomers of 14,15-EET was observed by repetitive UV scanning, which showed the appearance of a conjugated diene chromophore (compare Fig. 1A, B) and rates similar for the two enantiomers and comparable to the rate of reaction of arachidonic acid. The turnover values (kcat) for 14S,15R-EET and 14R,15S-EET were 1.3 and 0.95 s−1, respectively, and 1.8 s−1 for arachidonic acid. SP-HPLC analysis of the products revealed the formation of two major products from each 14,15-EET enantiomer, Fig. 4A, B. As found before with the α-linolenate epoxide products, there is a time-dependent transformation of the more polar products 10 and 13 to the earlier-eluting products 9 and 12. NMR analysis (supplementary data, pages 14–17) of all four main compounds confirmed what would be predicted, namely that the more polar products 10 and 13 are 12-hydroperoxy-14,15-epoxides, and the time-dependent secondary products 9 and 12 are 12,14-endoperoxy-15-hydroxy derivatives from 14S,15R-EET and 14R,15S-EET, respectively. Whereas the two 14,15-EET isomers react similarly with the 12-LOX, the secondary transformation to the endoperoxides is faster with the 14S,15R enantiomer (compare upper and lower chromatograms of Fig. 4A, B), compatible with its more favorable stereochemistry for the SNi attack of the hydroperoxide on the epoxide carbon at C14 (see the Discussion).
Fig. 4.
SP-HPLC analyses for products (analyzed as methyl esters) from incubation of platelet 12-LOX with 14S,15R-EETs (A) and 14R,15S-EETs (B) after 10 and 30 min, and the products from incubation of human 15-LOX-1 with 14S,15R-EET (C). (Incubation of 15-LOX-1 with 14R,15S-EET is given in the supplementary Fig. V.) Chromatographic conditions were as in Fig. 2. The structures of the products are shown in Scheme 2.
The supplementary data also illustrate that the UV spectra of the four conjugated dienes show distinct differences with a higher lambda-max and smoother profile for the earlier eluting endoperoxide derivatives (supplementary Fig. IIIA), and that in the presence of SnCl2, only a single product, the corresponding hydroxy-epoxide (products 11 and 14) is formed from each 14,15-EET enantiomer (supplementary Fig. IV).
Reactions with human 15-LOX-1
Using arachidonic acid as substrate, 15-LOX-1 displays a number of reactions and secondary transformations. The primary dioxygenase activities give 15S-HPETE and 12S-HPETE in ∼10:1 proportions (21) and secondary reactions give 5,15- and 8,15-diHPETEs as well as 14,15-leukotriene A4 and its hydrolysis products (19). Understanding these properties help in explaining a slightly more complex profile of products formed from the enantiomers of 14,15-EET. LOX catalysis of 14,15-EET to the 12-hydroperoxide requires an initial hydrogen abstraction from C10, which is a secondary property of 15-LOX-1, giving rise normally to the minor proportion of 12-HPETE from arachidonate. Therefore it was not unexpected that the two 14,15-EET enantiomers are transformed by recombinant human 15-LOX-1, albeit at a rate about an order of magnitude slower than using arachidonate as substrate. The products were separated by SP-HPLC and the structures determined by GC-MS of the methyl ester TMS derivative after reduction and hydrogenation of the double bonds (supplementary Fig. V).
The 14S,15R enantiomer is the slightly faster reacting (1.0 s−1 vs. 0.3 s−1 for 14R,15S-EET, and 10 s−1 for arachidonic acid), and it forms three main products (Fig. 4C). The tallest peak (product 10) is the 12-hydroperoxy-14,15-epoxide and it shows a time-dependent transformation to the earlier-eluting product 9, which was shown by UV analysis and HPLC mobility to be identical to the 12S,14R-endoperoxy-15R-hydroxy product of 12-LOX (product 9). The “extra” peak in Fig. 4C, product 15, was identified by GC-MS (after reduction of the hydroperoxide) as an 8-hydroxy-14,15-epoxide (Scheme 2, upper half).
Scheme 2.
Products formed from 14,15-EETs by platelet 12-LOX and human 15-LOX-1.
The second 14,15-EET enantiomer (14R,15S) was converted by 15-LOX-1 to product 13, identical to the 12S,14S-endoperoxy-15S-hydroxy product of 12-LOX, and which showed very little further transformation to an endoperoxide (product 12, a very small peak in supplementary Fig. VI, lower chromatogram). The major peak (product 16) was identified by GC-MS (after reduction) as a 5-hydroxy-14,15-epoxide (Scheme 2, lower half).
DISCUSSION
Routes to hydroxy-endoperoxides
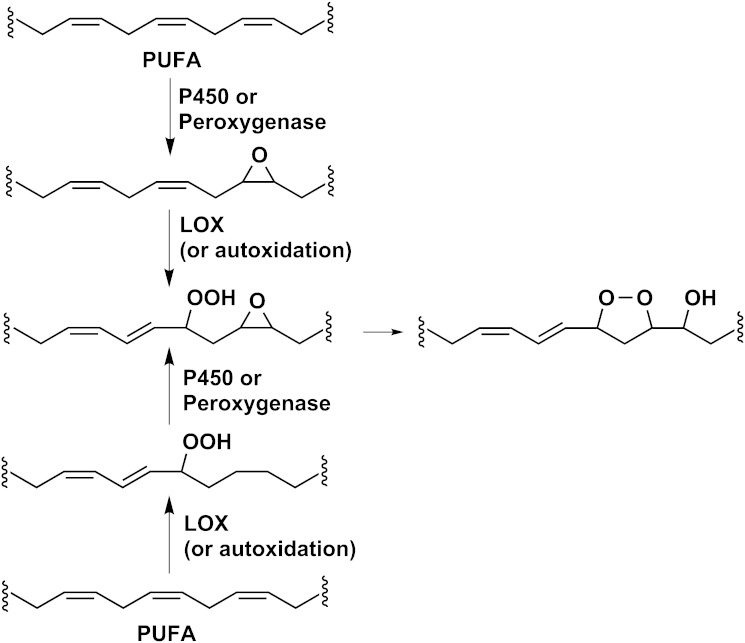
Fatty acid epoxides are typical products of P450 enzymes (22–24), and we describe a generally applicable reaction in which the fatty acid epoxide is further oxygenated by a LOX enzyme followed by the nonenzymatic formation of a monocyclic endoperoxide (Scheme 3). In principle, the order of the first two reactions could be reversed, with initial formation of the hydroperoxide and secondary epoxidation (Scheme 3, lower half). Peroxygenase enzymes are an alternative source of the fatty acid epoxide: typical plant peroxygenases use a fatty acid hydroperoxide to “charge” the enzyme and affect intermolecular oxygen transfer and epoxidation (5–7, 25). This co-occurrence of the LOX and epoxygenase activities together presents favorable circumstances for the further transformation to hydroxy-endoperoxides. It is interesting to consider that the cyclooxygenase-catalyzed oxygenation of 8,9-EET referred to in the Introduction sets up the potential for the synthesis of an 8-hydroxy-9,11-endoperoxide synthesis; however, the concomitant peroxidase activity of PGH synthase rapidly reduces the initial 11-hydroperoxy moiety, and the product recovered is the 8,9-epoxy-11-hydroxy derivative (3).
Scheme 3.
The potential interaction between epoxygenase and LOX pathways.
Mechanism of formation of the endoperoxides
The step of endoperoxide synthesis is understandable as an SNi type between the hydroperoxy nucleophile and the epoxy electrophile (Scheme 4). Differences in the rate of endoperoxide formation depend on the relative configuration of the hydroperoxy group to the epoxide moiety. The rate is faster when the hydroperoxide is more favorably positioned for SNi attack on the epoxide (Scheme 4, left side). For example, although the 15S,16R-epoxy isomer of α-linolenate (AD1) reacts more slowly with soybean LOX-1 (Fig. 1A, B), the oxygenation product (13S-hydroperoxy-15S,16R-epoxy) shows the faster nonenzymic transformation to the endoperoxide (Scheme 4, left side). The same observations applied to the step of endoperoxide synthesis involving the 12-hydroperoxy-14,15-epoxy products of 12-LOX and 15-LOX-1.
Scheme 4.
The reaction mechanism for the formation of endoperoxides from epoxy fatty acids.
There is a precedent for this fatty acid endoperoxide synthesis in the chemical literature. Porter et al. (26) used mCPBA and a catalytic amount of trichloroacetic acid in methylene chloride to convert 9-hydroperoxy-octadeca-6,10,12-trienoic acid to multiple products including a 6-hydroxy-7,9-endoperoxide. The mechanism clearly involved the intermediacy of the direct mCPBA-catalyzed formation of the 6,7-epoxide of the 9-hydroperoxide which was converted by the trace of acid to the corresponding hydroxy-endoperoxide. Earlier, Corey, Fleet, and Kato (27) had suggested a related theoretical pathway of prostaglandin H2 synthesis in which an arachidonate 14,15-epoxide could be oxygenated and cyclized to an endoperoxide with final opening of the epoxide ring to give the 9,11-endoperoxy-15-hydroxy product. Another related transformation involves formation of 5-hydroxy-prostaglandin I1 from 5,6-EET via COX-1 catalysis: synthesis of the corresponding endoperoxide and its reduction to a PGF2α analog permits nucleophilic attack of the C9 hydroxyl on the 6-carbon of the 5,6-epoxide, giving the 5-hydroxy-PGI1 isomers as final products (28).
The reactions we characterize take place in an aqueous environment and being enzyme-catalyzed, the products have a specific stereochemistry. Even though the endoperoxide formation is nonenzymatic, the reaction is stereoselective, a chiral epoxide and hydroperoxide giving a single endoperoxide diastereomer via intramolecular nucleophilic substitution (SNi). The reaction occurs most readily when the epoxide oxygen is on the opposite face of the carbon chain to the attacking hydroperoxide, allowing ready SN substitution and producing an endoperoxide with side chains in the cis configuration ( , left side). When the epoxide oxygen is on the same face as the hydroperoxide, the reaction is slower and the product is a trans endoperoxide ( , right side). Therefore under our aqueous conditions the rate of reaction is primarily determined by the stereochemistries of the epoxy and hydroperoxy moieties. Although Scheme 4 illustrates a hydroperoxy anion attacking the epoxide, the hydroperoxide (pKa 10 -12) is a sufficiently good nucleophile that its ionization may not be required.
Comparison and contrast with autoxidative formation of endoperoxides
It is well-recognized that nonenzymatic peroxidation can give rise to hydroperoxy-monocyclic endoperoxides (29, 30). Reduction of such hydroperoxy-endoperoxides will give a racemic version of the hydroxy-endoperoxide products we describe. In addition, several authors have used the initial LOX-catalyzed formation of 13-hydroperoxy-linolenic acid to study its further nonenzymatic transformation to hydroperoxy-endoperoxides (31, 32). The direct formation of hydroxy-endoperoxides is also theoretically feasible via lipid peroxidation because autoxidative reactions can include the production of fatty acid epoxides (33, 34), which are then open to a nonenzymatic peroxidation to the hydroxy-endoperoxides as we have characterized.
Trihydroxy fatty acids and other predicted derivatives
The fatty acid monocyclic endoperoxides are relatively stable under mild conditions (e.g., HPLC at room temperature). Also, unlike hydroperoxides, the monocyclic endoperoxide moiety is resistant to reduction using mild reducing agents such as triphenylphosphine and even sodium borohydride (31). Nonetheless, in biological systems opening of the epoxide ring is likely to occur on exposure to peroxidases, in which case the products will be fatty acid triols. The same triols could arise also from hydrolysis of the precursor hydroperoxy-epoxides, which are likely substrates for epoxide hydrolase (35).
Epoxy fatty acids as LOX substrates
A side issue of some interest in relation to our results is the suitability of specific fatty acid epoxides as substrates for LOX catalysis. One of the central concepts of substrate binding in LOX enzymes is the head-to-tail orientation of the fatty acid, which in the case of the soybean LOX-1, human 15-LOX-1 and the platelet-type 12-LOX involves a “tail-first” entry into the LOX active site (36). Our results indicate that the presence of an epoxide on the tail carbons slows the rate of reaction, but does not interfere with the specificity of the oxygenation. As the reaction rate of the platelet-type 12-LOX with 14,15-EET is comparable to the rate with arachidonic acid, this raises the possibility of their co-occurrence and interaction in the cardiovascular system.
CONCLUSION
The dioxygenation of epoxy fatty acids by LOXs give rise to hydroperoxy- and hydroxy-epoxy derivatives. Subsequent transformation of hydroperoxy-epoxides to hydroxy-endoperoxides as a result of intramolecular nucleophilic substitution between hydroperoxy and epoxy moieties is an alternative way of synthesizing monocyclic peroxides in vitro. In vivo, the crosstalk between epoxygenase and LOX pathways might result in hydroperoxy-epoxy and corresponding endoperoxy derivatives that might undergo reduction, giving a variety of hydroxy fatty acids. The presence and biological significance of the described fatty acid derivatives might be a matter of future research in lipidomics.
Supplementary Material
Acknowledgments
The authors thank Professor Nigulas Samel for encouragement and support for these studies and Professor Ned Porter for providing helpful mechanistic insights. The authors also thank Dr. Wade Calcutt and Brian Hachey for assistance with the mass spectrometric analyses.
Footnotes
Abbreviations:
- AD1/AD2
- 15,16-epoxy-linolenate enantiomers eluted from Chiralpak AD chiral column
- EET
- epoxyeicosatrienoic acid
- HAc
- glacial acetic acid
- HPETE
- hydroperoxyeicosatetraenoic acid
- LOX
- lipoxygenase
- mCPBA
- meta-chloroperoxybenzoic acid
- P450
- cytochrome P450
- PG
- prostaglandin
- RP-HPLC
- reversed phase HPLC
- SNi
- intramolecular nucleophilic substitution
- SP-HPLC
- straight phase HPLC
- TPP
- triphenylphosphine
This work was supported by National Institutes of Health Grants GM15431 and GM74888, by a voucher for use of the mass spectrometry resource through the Vanderbilt-Ingram Cancer Center Support Grant (P30 CA068485), and by the Institutional Research Grant IUT19-9 from the Estonian Ministry of Education and Research.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form six figures and NMR data.
REFERENCES
- 1.Hecker M., Ullrich V. 1989. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J. Biol. Chem. 264: 141–150. [PubMed] [Google Scholar]
- 2.Brash A. R. 2009. Mechanistic aspects of CYP74 allene oxide synthases and related cytochrome P450 enzymes. Phytochemistry. 70: 1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J. Y., Prakash C., Yamashita K., Blair I. A. 1992. Regiospecific and enantioselective metabolism of 8,9-epoxyeicosatrienoic acid by cyclooxygenase. Biochem. Biophys. Res. Commun. 183: 138–143. [DOI] [PubMed] [Google Scholar]
- 4.Feussner I., Wasternack C. 2002. The lipoxygenase pathway. Annu. Rev. Plant Biol. 53: 275–297. [DOI] [PubMed] [Google Scholar]
- 5.Blée E., Wilcox A. L., Marnett L. J., Schuber F. 1993. Mechanism of reaction of fatty acid hydroperoxides with soybean peroxygenase. J. Biol. Chem. 268: 1708–1715. [PubMed] [Google Scholar]
- 6.Hamberg M., Hamberg G. 1996. Peroxygenase-catalyzed fatty acid epoxidation in cereal seeds (sequential oxidation of linoleic acid into 9(S),12(S),13(S)-trihydroxy-10(E)-octadecenoic acid). Plant Physiol. 110: 807–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blée E., Flenet M., Boachon B., Fauconnier M. L. 2012. A non-canonical caleosin from Arabidopsis efficiently epoxidizes physiological unsaturated fatty acids with complete stereoselectivity. FEBS J. 279: 3981–3995. [DOI] [PubMed] [Google Scholar]
- 8.Boeglin W. E., Brash A. R. 2012. Cytochrome P450-type hydroxylation and epoxidation in a tyrosine-liganded hemoprotein, catalase-related allene oxide synthase. J. Biol. Chem. 287: 24139–24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin J., Zheng Y., Boeglin W. E., Brash A. R. 2013. Biosynthesis, isolation, and NMR analysis of leukotriene A epoxides: substrate chirality as a determinant of the cis or trans epoxide configuration. J. Lipid Res. 54: 754–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X-S., Brash A. R., Funk C. D. 1993. Purification and characterization of recombinant histidine-tagged human platelet 12-lipoxygenase expressed in a baculovirus/insect cell system. Eur. J. Biochem. 214: 845–852. [DOI] [PubMed] [Google Scholar]
- 11.Cui P. H., Duke R. K., Duke C. C. 2008. Monoepoxy octadecadienoates and monoepoxy octadecatrienoates 1: NMR spectral characterization. Chem. Phys. Lipids. 152: 122–130. [DOI] [PubMed] [Google Scholar]
- 12.Schneider C., Boeglin W. E., Brash A. R. 2000. Enantiomeric separation of hydroxy eicosanoids by chiral column chromatography: effect of the alcohol modifier. Anal. Biochem. 287: 186–189. [DOI] [PubMed] [Google Scholar]
- 13.Gao B., Boeglin W. E., Brash A. R. 2010. Omega-3 fatty acids are oxygenated at the n-7 carbon by the lipoxygenase domain of a fusion protein in the cyanobacterium Acaryochloris marina. Biochim. Biophys. Acta. 1801: 58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laethem R. M., Balazy M., Koop D. R. 1996. Epoxidation of C18 unsaturated fatty acids by cytochromes P4502C2 and P4502CAA. Drug Metab. Dispos. 24: 664–668. [PubMed] [Google Scholar]
- 15.Wei S., Brittin J. J., Falck J. R., Anjaiah S., Nithipatikom K., Cui L., Campbell W. B., Capdevila J. H. 2006. Chiral resolution of the epoxyeicosatrienoic acids, arachidonic acid epoxygenase metabolites. Anal. Biochem. 352: 129–134. [DOI] [PubMed] [Google Scholar]
- 16.Vliegenthart J. F. G., Veldink G. A. 1982. Lipoxygenases. In Free Radicals in Biology. W. A. Pryor, editor. Academic Press Inc., New York. 29–64. [Google Scholar]
- 17.Mihelich E. D. 1980. Structure and stereochemistry of novel endoperoxides isolated from the sensitized photooxidation of methyl linoleate. Implications for prostaglandin biosynthesis. J. Am. Chem. Soc. 102: 7141–7143. [Google Scholar]
- 18.Carless H. A. J., Batten R. J. 1987. Photosensitised oxidation of model unsaturated lipid systems: (4Z,7Z)-undeca-4,7-diene and (4Z)-undec-4-en-7-yne. J. Chem. Soc., Perkin Trans. 1. 1987: 1999–2007. [Google Scholar]
- 19.Bryant R. W., Schewe T., Rapoport S. M., Bailey J. M. 1985. Leukotriene formation by a purified reticulocyte lipoxygenase enzyme. Conversion of arachidonic acid and 15-hydroperoxyeicosatetraenoic acid to 14,15-leukotriene A4. J. Biol. Chem. 260: 3548–3555. [PubMed] [Google Scholar]
- 20.Yokoyama C., Shinjo F., Yoshimoto T., Yamamoto S., Oates J. A., Brash A. R. 1986. Arachidonate 12-lipoxygenase purified from porcine leukocytes by immunoaffinity chromatography and its reactivity with hydroperoxyeicosatetraenoic acids. J. Biol. Chem. 261: 16714–16721. [PubMed] [Google Scholar]
- 21.Bryant R. W., Bailey J. M., Schewe T., Rapoport S. M. 1982. Positional specificity of a reticulocyte lipoxygenase. Conversion of arachidonic acid to 15S-hydroperoxy-eicosatetraenoic acid. J. Biol. Chem. 257: 6050–6055. [PubMed] [Google Scholar]
- 22.Oliw E. H. 1994. Oxygenation of polyunsaturated fatty acids by cytochrome P450 monooxygenases. Prog. Lipid Res. 33: 329–354. [DOI] [PubMed] [Google Scholar]
- 23.Capdevila J. H., Falck J. R., Harris R. C. 2000. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 41: 163–181. [PubMed] [Google Scholar]
- 24.Konkel A., Schunck W. H. 2011. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim. Biophys. Acta. 1814: 210–222. [DOI] [PubMed] [Google Scholar]
- 25.Hamberg M., Hamberg G. 1990. Hydroperoxide-dependent epoxidation of unsaturated fatty acids in the broad bean (Vicia faba L.). Arch. Biochem. Biophys. 283: 409–416. [DOI] [PubMed] [Google Scholar]
- 26.Porter N. A., Funk M. O., Gilmore D., Isaac R., Nixon J. 1976. The formation of cyclic peroxides from unsaturated hydroperoxides: Models for prostaglandin biosynthesis. J. Am. Chem. Soc. 98: 6000–6005. [DOI] [PubMed] [Google Scholar]
- 27.Corey E. J., Fleet G. W. J., Kato M. 1973. Biogenetic approach to synthesis of a prostanoid precursor. Tetrahedron Lett. 14: 3963–3966. [Google Scholar]
- 28.Oliw E. H. 1984. Biosynthesis of 5,6-dihydroxyprostaglandin E1 and F1α from 5,6-dihydroxyeicosatrienoic acid by ram seminal vesicles. Biochim. Biophys. Acta. 795: 384–391. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor D. E., Mihelich E. D., Coleman M. C. 1984. Stereochemical course of the autoxidative cyclization of lipid hydroperoxides to prostaglandin-like bicyclo endoperoxides. J. Am. Chem. Soc. 106: 3577–3584. [Google Scholar]
- 30.Gardner H. W. 1989. Oxygen radical chemistry of polyunsaturated fatty acids. Free Radic. Biol. Med. 7: 65–86. [DOI] [PubMed] [Google Scholar]
- 31.Roza M., Francke A. 1978. Cyclic peroxides from a soya lipoxygenase-catalysed oxygenation of methyl linolenate. Biochim. Biophys. Acta. 528: 119–126. [DOI] [PubMed] [Google Scholar]
- 32.Chan H. W. S., Matthew J. A., Coxon D. T. 1980. A hydroperoxy-epidioxide from the autoxidation of a hydroperoxide of methyl linolenate. J. Chem. Soc. Chem. Comm. 1980: 235–236. [Google Scholar]
- 33.Wu G. S., Stein R. A., Mead J. F. 1977. Autoxidation of fatty acid monolayers adsorbed on silica gel: II. Rates and products. Lipids. 12: 971–978. [DOI] [PubMed] [Google Scholar]
- 34.Sevanian A., Mead J. F., Stein R. A. 1979. Epoxides as products of lipid autoxidation in rat lungs. Lipids. 14: 634–643. [DOI] [PubMed] [Google Scholar]
- 35.Decker M., Arand M., Cronin A. 2009. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch. Toxicol. 83: 297–318. [DOI] [PubMed] [Google Scholar]
- 36.Coffa G., Schneider C., Brash A. R. 2005. A comprehensive model of positional and stereo control in lipoxygenases. Biochem. Biophys. Res. Commun. 338: 87–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.