Abstract
N-acylethanolamines (NAEs) are endogenous lipid-signaling molecules involved in satiety and energetics; however, how diet impacts circulating NAE concentrations and their downstream metabolic actions in humans remains unknown. Objectives were to examine effects of diets enriched with high-oleic canola oil (HOCO) or HOCO blended with flaxseed oil (FXCO), compared with a Western diet (WD), on plasma NAE levels and the association with energy expenditure and substrate oxidation. Using a randomized controlled crossover design, 36 hypercholesterolemic participants consumed three isoenergetic diets for 28 days, each containing 36% energy from fat, of which 70% was HOCO, FXCO, or WD. Ultra-performance liquid chromatography-MS/MS was used to measure plasma NAE levels and indirect calorimetry to assess energy expenditure and substrate oxidation. After 28 days, compared with WD, plasma oleoylethanolamide (OEA) and alpha-linolenoyl ethanolamide (ALEA) levels were significantly increased in response to HOCO and FXCO (P = 0.002, P < 0.001), respectively. Correlation analysis demonstrated an inverse association between plasma OEA levels and percent body fat (r = −0.21, P = 0.04), and a positive association was observed between the plasma arachidonoyl ethanolamide (AEA)/OEA ratio and android:gynoid fat (r = 0.23, P = 0.02), respectively. Results suggest that plasma NAE levels are upregulated via their dietary lipid substrates and may modulate regional and total fat mass through lipid-signaling mechanisms.
Keywords: endocannabinoids, clinical trials, fatty acid metabolism, fatty acid oxidation, peroxisome proliferator-activated receptor, oleoylethanolamide, canola oil, oleic acid, arachidonoylethanolamide, body composition
The global impact of obesity and its comorbidities is widespread, signaling a need to identify key metabolic targets and molecular mechanisms involved in energy balance in response to altered nutrition. One such target is the group of N-acylethanolamines (NAEs), which are endogenous chemical signaling lipids identified as regulating lipid metabolism and feeding behavior and, thus, possibly important in the etiology of obesity (1, 2). NAEs are a type of endogenous fatty acid amide that contain several types of acyl groups linked to the nitrogen atom of ethanolamine (3). NAEs are synthesized from fatty acid substrates via intestinal phospholipase enzymes N-acylphosphatidylethanolamine and N-acyl phosphatidylethanolamine-specific phospholipase D (3). Concentrations of circulating NAEs are present at very low levels in tissues and biofluids due to the action of fatty acid amide hydrolase enzyme, which degrades NAEs into their fatty acid substrate and ethanolamine (4). Specific NAEs, namely arachidonoyl ethanolamide (AEA), or anandamide, oleoylethanolamide (OEA), and palmitoylethanolamide (PEA), have been demonstrated to regulate lipolysis, lipogenesis, and satiety through their activation of lipometabolic genes (5). These pathways are primarily mediated by the central nervous system or periphery by cannabinoid (CB) receptors, CB1 and CB2, when bound to anandamide; whereas OEA and PEA as PPAR-α agonists act to activate these genes via PPAR-α activation (6, 7). Accordingly, understanding the action of circulating NAE concentrations on energy balance, and whether specific fatty acids may alter these concentrations in humans, is of considerable interest.
The lipometabolic and feeding behavioral roles of AEA and OEA are increasingly well documented as several animal models have demonstrated the orexigenic and anorexigenic properties of AEA and OEA, respectively. In mice fed a high-fat diet, hepatic AEA levels were shown to increase, stimulating an increase in CB1-mediated fatty acid synthesis via upregulated lipogenic gene expression resulting in diet-induced obesity (DIO) and fatty liver, whereas CB1-deficient mice were resistant to DIO (8). In contrast, OEA administration has been documented to reduce food intake and lower body-weight gain in a dose-dependent manner through selective activation of PPAR-α in high-fat-fed wild-type but not PPAR-α-deficient mice (7). This effect of OEA on feeding behavior and body-weight gain is also replicated with PEA, but with less potency, potentially through increased leptin sensitivity (7, 9). Similarly, overweight/obese T2D patients have increased circulating levels of AEA and 2-arachidonoylglycerol (2-AG), an AEA-related endocannabinoid, as well as increased subcutaneous white adipose tissue AEA levels confirming the link between AEA levels and adiposity and also suggesting a link between AEA levels and insulin resistance (10, 11). The reactive roles of endocannabinoids and their response to energy balance are well established; however, the important impact of diet fat selection as proactive precursors to circulating endocannabinoids and their role in metabolic control are still widely unknown (12, 13). Previous research has confirmed that a linoleic-rich diet elevates AEA levels, whereas a diet supplemented with n-3 PUFAs DHA and EPA reduced AEA levels along with improving metabolic profiles in obese rats (14, 15). For instance, one study indicated that consumption of krill oil (2 g/day) over 4 weeks significantly reduced plasma 2-AG levels and was directly correlated to the plasma n-6/n-3 ratio in obese subjects (15). However, the impact of different dietary fatty acids, from saturated fats to polyunsaturated fats, on circulating NAE levels in humans and their impact on energy metabolism has yet to be elucidated. The primary objective of this study was to examine the effects of specific dietary fatty acid consumption on plasma NAE concentrations and whether associations exist between modulated circulating NAE levels in response to diet and shifts in body composition, substrate oxidation, and energy expenditure in hypercholesterolemic, but healthy, humans.
MATERIALS AND METHODS
Participant characteristics
The design and selection criteria of this study have been described elsewhere (16). Thirty-nine participants (males = 14, females = 25), aged 18 to 65 years, were screened and found eligible based on inclusion and exclusion criteria as previously described (16). Participant baseline characteristics are summarized in Table 1. Written informed consent was obtained from all participants prior to study commencement. The study was conducted according to the principles outlined in the Declaration of Helsinki. The University of Manitoba Biomedical Research Ethics Board approved all study procedures as the institutional review board authority, reference number B2007:071. This clinical study is registered with the US National Library of Medicine public trials registry record #NCT00927199.
TABLE 1.
Baseline characteristics of study volunteers
| Anthropometric Measurement | All Subjects |
| Age (y) | 48.24 ± 11.88 |
| Body weight (kg) | 77.09 ± 16.02 |
| BMI (kg/m2) | 28.12 ± 4.49 |
| Body fat (%) | 38.01 ± 7.36 |
| FFM (kg) | 47.56 ± 10.47 |
| Android (% of fat) | 45.81 ± 6.07 |
| Gynoid (% of fat) | 42.75 ± 8.98 |
| RMR (kcal/min) | 0.812 ± 0.176 |
Means ± SD (n = 36); baseline characteristics were previously published (19). FFM, fat-free mass; RMR, resting metabolic rate.
Study design
This study, a randomized, single-blind, crossover, controlled-diet clinical investigation using a 3 × 3 Latin square sequence design, consisted of three 29-day phases, each separated by a 4- to 8-week washout period. Participants were randomized to one of three treatment arms per phase: a) control, a blend of oils typical of a Western diet (WD); b) high-oleic canola oil (HOCO); or c) a 1:1 blend of HOCO and flaxseed oil (FXCO). Each participant received all treatments during the study. During each treatment phase, participants consumed one of these three diets fed at levels commensurate with calculated caloric requirements, consisting of three isoenergetic meals using a 3-day rotation menu, which were prepared by the metabolic kitchen at the Richardson Centre for Functional Foods and Nutraceuticals of the University of Manitoba. Food ingredients were weighed within 0.5 g accuracy. Participants’ individual energy requirements for weight maintenance were determined using the Mifflin equation (17) and multiplied by an activity factor of 1.7. If a participant’s body weight fluctuated within the first week of the study phase, then his or her energy intake was corrected by adjusting the activity factor to maintain body weight. Participants consumed their breakfast meal under supervision on a daily basis to ensure compliance. The remaining meals were packed for takeaway, and empty containers were returned to ensure compliance. Further details of the trial design have been previously described (16).
Experimental diets
Experimental diets were designed to contain energy levels consistent with subjects’ needs and have an identical macronutrient profile, providing 50% energy as carbohydrate, 15% as protein, and 35% as fat as described previously (16). Seventy percent of total fat intake was provided by the experimental treatment oils: 1) control (WD), a blend of nonsalted butter (12%), extravirgin olive oil (35%), vegetable lard (35%), and sunflower oil (>60% linoleic acid [LA]) (18%); 2) HOCO, contributing ∼70% oleic acid (Canola Harvest HiLo® Richardson Oilseed Limited, Lethbridge, Alberta, Canada); or 3) a 1:1 blend of the HOCO and FXCO, ∼55% α-linoleic acid (ALA) and no lignans (Bioriginal Food and Science Corporation, Saskatoon, SK, Canada). Experimental oils were incorporated into milkshakes at breakfast and puddings at lunch and dinner. Table 2 outlines the fatty acid profile of each experimental diet.
TABLE 2.
Fatty acid profile of treatment diets
| WD | FXCO | HOCO | ||||
| Fatty Acid | % Energy | g/day | % Energy | g/day | g/day | % Energy |
| Energy (kcal) | 2,500 | |||||
| SFA | 11.2 | 31.2 | 6.14 | 5.64 | 15.7 | 17.0 |
| 12:0 | 0.23 | 0.64 | 0.13 | 0.13 | 0.35 | 0.37 |
| 14:0 | 0.93 | 2.58 | 0.52 | 0.50 | 1.38 | 1.45 |
| 16:0 | 6.65 | 18.5 | 3.39 | 3.07 | 8.53 | 9.42 |
| 18:0 | 2.69 | 7.48 | 1.51 | 1.24 | 3.45 | 4.19 |
| MUFA | 16.1 | 44.8 | 15.9 | 22.9 | 63.5 | 44.2 |
| 16:1 | 0.53 | 1.48 | 0.23 | 0.27 | 0.75 | 0.63 |
| 18:1 | 15.3 | 42.6 | 15.3 | 22.1 | 61.4 | 42.6 |
| PUFA | 6.49 | 18.0 | 12.3 | 5.74 | 15.9 | 34.1 |
| 18:2n-6 | 5.97 | 16.5 | 4.85 | 4.84 | 13.3 | 13.5 |
| 18:3n-3 | 0.45 | 1.26 | 7.35 | 0.78 | 2.40 | 20.6 |
| 20:4n-6 | 0.02 | 0.06 | 0.02 | 0.02 | 0.06 | 0.06 |
| 20:5n-3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 22:5n-3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 |
| 22:6n-3 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.01 |
SFA, saturated fatty acid. The macronutrient profile of the three experimental diets was estimated using FOOD PROCESSOR software (version 7.81; Food Processor, Salem, OR).
Plasma fatty acid composition
Fasting blood samples were drawn on days 1 and 29 of each phase into BD Vacutainer blood collection tubes (EDTA and heparin), then separated into plasma and red blood cells (RBCs) by centrifugation at 3,000 rpm for 20 min at 4°C. Procedures used for plasma lipid extraction and plasma fatty acid composition analysis have been published previously (16, 18).
Plasma N-acylethanolamine extraction
NAE extraction was executed via solid-phase extraction using a vacuum manifold (Agilent, Willington, CT) based on the method previously described (12). Plasma samples were blended with a mix of 6-deuterium NAE standards. Methanol and deionized water were added to precondition the Oasis HLB 1 cc, 30 mg cartridge. Samples were then introduced into a preconditioned cartridge followed by washing with 40% methanol and then eluting with acetonitrile. Eluted samples were dried under nitrogen and dissolved in acetonitrile.
UPLC-MS/MS measurement of NAE concentrations
Sample analyses were carried out using a Waters Acquity UPLC system coupled to a Quattro micro API mass spectrometer (Waters Corp., Milford, MA). The chromatographic separation of all NAEs in plasma was achieved using a Kinetex XB-C18 column (100 × 2.1 mm, 1.7 µm; Phenomenex, CA) at a flow rate of 0.2 ml/min. The ultra-performance liquid chromatography-MS/MS conditions were as previously described (12). Multiple reaction monitoring (MRM) mode was used to monitor the precursor ion to product ion transition under optimized MS/MS conditions. The MRM transitions selected were as previously described (12). Data were acquired and analyzed using Masslynx version 4.1 (Waters Corp.). Validation of UPLC-MS/MS measurements, including limits of detection, precision, and accuracy of analyzed NAE based on nonextracted standards, was as previously described in detail (12).
Body composition measurement
Body composition of study participants was measured using dual-energy X-ray absorptiometry (General Electric Lunar Digital Prodigy Advance, Madison, WI) on days 1 and 29 of each phase to assess percentage of total fat mass, lean body mass, fat-free mass (lean body mass plus bone mineral content), android fat mass, and gynoid fat mass, which in turn was used to determine android:gynoid fat ratio, as described (19). Resulting data were analyzed with corresponding volume integration software (Encore 2005, version 9.30.044; General Electric Lunar Prodigy Advance).
Indirect calorimetry measurements
Energy expenditure and substrate oxidation were analyzed by indirect calorimetry using an open-circuit ventilated canopy (Vmax Encore software; Summit Technologies Inc., Burlington, ON, Canada), recording the rate (l/min) of oxygen consumption (VO2) and carbon dioxide production (VCO2) on 1 day during the first week of phase 1 (baseline) and 1 day of the fourth week of each phase (phase end points). The detailed indirect calorimetry measurement method has been previously described (19). Energy expenditure (fasting and postprandial) as well as carbohydrate and fat oxidation rates were calculated using recorded VO2 and VCO2 values (l/min) (19).
Statistical analysis
Statistical analysis was performed using SPSS 18.0 (SPSS Inc., Chicago, IL). Results are expressed as means ± SEM unless otherwise noted. Effects of dietary treatment were examined using a mixed-model ANOVA procedure with diet, sequence, phase, and gender as fixed factors and participant as the random factor. Significant treatment effects were examined using Bonferroni post hoc tests for multiple comparisons. Pearson correlation analyses were conducted to test associations between individual NAE and participant characteristics, body composition, substrate oxidation, and energy expenditure in each participant. The strength of association of coefficient (r) was proposed as weak (r = 0.1 to 0.3 or r = −0.1 to −0.3), moderate (r = 0.3 to 0.5 or r = −0.3 to −0.5), or strong (r = 0.5 to 1.0 or r = −0.5 to −1.0). Statistical significance was set at P < 0.05 for all analyses.
RESULTS
Participant characteristics
Participant baseline characteristics are displayed in Table 1. Thirty-six participants (male = 13; women = 23, postmenopausal = 5) completed the entire intervention study. Three participants withdrew due to residence relocation and work-related issues. Two participants did not complete the indirect calorimetry procedure due to discomfort with the ventilated canopy. Participants reported no changes in physical activity across the study engagement period, as well as no side effects and good tolerance to the experimental diets during the study protocol.
Plasma fatty acid composition in response to treatment
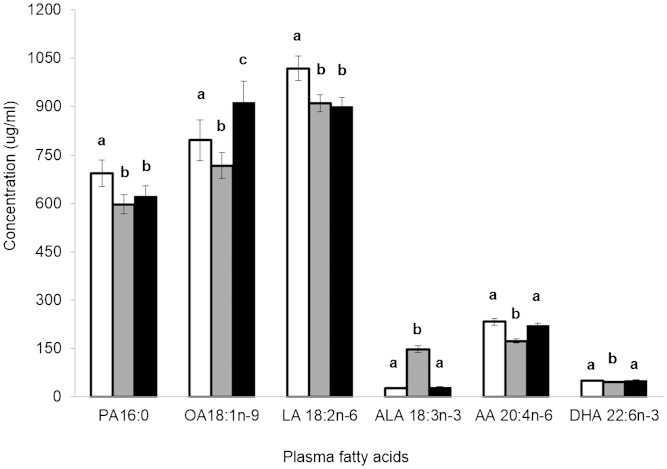
The pattern of plasma fatty acid composition was consistent with the dietary fat compositional data. Fig. 1 presents selected plasma fatty acid concentrations of interest after consumption of the three treatment diets. WD resulted in the highest (P < 0.001) concentrations of plasma PA and LA compared with FXCO and HOCO treatments. Plasma AA concentrations were lowest (P < 0.001) after consumption of the FXCO treatment compared with HOCO and WD. Consumption of HOCO resulted in higher concentrations of plasma OA compared with FXCO (P < 0.001) and WD (P < 0.022). Plasma OA concentrations also differed (P < 0.031) between consumption of FXCO and WD treatments. Finally, consumption of FXCO resulted in the highest (P < 0.001) concentrations of plasma ALA and the lowest (P < 0.01) concentrations of plasma DHA compared with HOCO and WD.
Fig. 1.
Selected plasma fatty acid concentrations after consumption of each of the three treatment diets: WD (white bars), FXCO (gray bars), and HOCO (black bars). Values are presented as means ± SEM (n = 36); mean values with different lowercase letters are significantly different between treatment groups (P < 0.05) as analyzed by mixed-model ANOVA (with the Bonferroni adjustment for multiple comparisons). AA, arachidonic acid; OA, oleic acid; PA, palmitic acid.
Plasma N-acylethanolamine in response to treatment
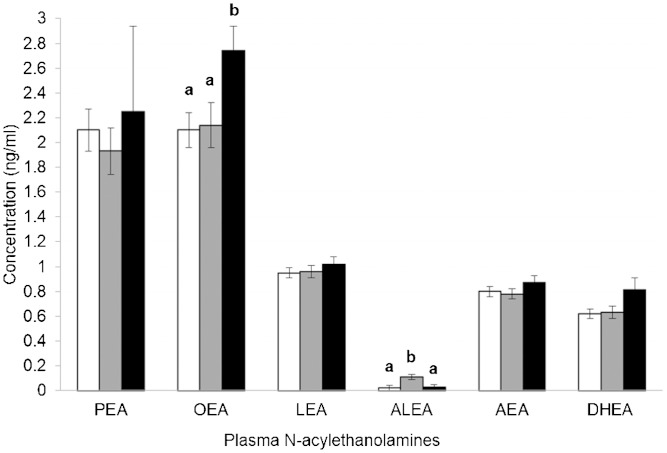
Figure 2 outlines plasma NAE concentrations after consumption of the three treatment diets. Plasma OEA concentrations were higher (P = 0.002) after consumption of HOCO compared with the other two treatment diets. Consumption of FXCO resulted in higher (P < 0.001) concentrations of plasma ALEA compared with the other two treatment diets. Considering plasma NAE ratios of interest, after consumption of FXCO, plasma ALEA/AEA and ALEA/OEA ratios were higher (P < 0.001) compared with the other two treatment diets. No differences in plasma PEA, LEA, AEA, and DHEA concentrations, nor in LEA/ALEA, DHEA/OEA, and AEA/OEA ratios, were observed between the three treatments.
Fig. 2.
Plasma N-acylethanolamine concentrations after consumption of each of the three treatment diets: WD (white bars), FXCO (gray bars), and HOCO (black bars). Values are presented as means ± SEM (n = 36 for all NAEs, except n = 34 for ALEA); mean values with different lowercase letters are significantly different between treatment groups (P < 0.05) as analyzed by mixed-model ANOVA (with the Bonferroni adjustment for multiple comparisons). ALEA, α-linolenoylethanolamide; DHEA, docosahexaenoylethanolamide; LEA, linoleoylethanolamide.
Correlational analysis for all subjects across every treatment (composite group) showed that plasma ALEA concentrations were positively associated with those of plasma ALA (r = 0.54, r2 = 0.29, P < 0.001) and negatively associated with plasma PA (r = −0.20, r2 = 0.04, P = 0.047), OA (r = −0.21, r2 = 0.04, P = 0.038), AA (r = −0.39, r2 = 0.15, P < 0.001), and DHA (r = −0.22, r2 = 0.05, P = 0.023). Plasma LEA levels were negatively associated with those of plasma PA (r = −0.21, r2 = 0.04, P = 0.026). No other correlations were observed between plasma NAEs and fatty acid concentrations. The overall correlational analysis is summarized in Table 3.
TABLE 3.
Pearson correlation coefficients (r) and coefficient of determination (r2) between composite NAE and NAE ratios and multiple variables
| NAE | NAE Ratio | ||||||||||
| Parameter | PEA | OEA | LEA | ALEA | AEA | DHEA | AEA/OEA | ALEA/OEA | DHEA/OEA | ALEA/AEA | LEA/ALEA |
| n | 108 | 108 | 108 | 103 | 108 | 107 | — | — | — | — | — |
| PA | |||||||||||
| r | −0.02 | −0.11 | −0.21a | −0.20a | −0.08 | 0.01 | — | — | — | — | — |
| r2 | 0.00 | 0.01 | 0.04 | 0.04 | 0.01 | 0.00 | |||||
| OA | |||||||||||
| r | 0.01 | 0.01 | −0.15 | −0.21a | 0.00 | 0.05 | — | — | — | — | — |
| r2 | 0.00 | 0.00 | 0.02 | 0.04 | 0.00 | 0.00 | |||||
| LA | |||||||||||
| r | −0.05 | −0.12 | −0.15 | −0.13 | −0.04 | −0.05 | — | — | — | — | — |
| r2 | 0.00 | 0.01 | 0.02 | 0.02 | 0.00 | 0.00 | |||||
| ALA | |||||||||||
| r | −0.13 | −0.16 | −0.13 | 0.54a | −0.12 | −0.10 | — | — | — | — | — |
| r2 | 0.02 | 0.03 | 0.02 | 0.29 | 0.01 | 0.01 | |||||
| AA | |||||||||||
| r | −0.06 | −0.10 | −0.16 | −0.39a | −0.02 | 0.06 | — | — | — | — | — |
| r2 | 0.00 | 0.01 | 0.03 | 0.15 | 0.00 | 0.00 | |||||
| DHA | |||||||||||
| r | −0.12 | −0.14 | −0.19 | −0.22a | −0.10 | 0.17 | — | — | — | — | — |
| r2 | 0.01 | 0.02 | 0.04 | 0.05 | 0.01 | 0.03 | |||||
| n | 102 | 102 | 102 | 97 | 102 | 101 | 102 | 98 | 101 | 98 | 97 |
| %Fat | |||||||||||
| r | −0.13 | −0.21a | −0.17 | −0.03 | −0.31a | 0.08 | −0.07 | 0.07 | 0.30a | 0.02 | 0.04 |
| r2 | 0.02 | 0.04 | 0.03 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.09 | 0.00 | 0.00 |
| %LM | |||||||||||
| r | 0.09 | 0.12 | 0.13 | −0.00 | 0.33a | −0.01 | 0.17 | −0.00 | −0.08 | −0.05 | −0.18 |
| r2 | 0.00 | 0.01 | 0.02 | 0.00 | 0.10 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.03 |
| %FM | |||||||||||
| r | 0.09 | −0.10 | −0.06 | −0.01 | −0.11 | 0.08 | −0.00 | 0.09 | 0.21a | 0.02 | −0.05 |
| r2 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | 0.05 | 0.00 | 0.00 |
| %FFM | |||||||||||
| r | −0.03 | 0.12 | 0.13 | −0.00 | 0.33a | −0.01 | 0.17 | −0.00 | −0.08 | −0.05 | −0.17 |
| r2 | 0.00 | 0.01 | 0.02 | 0.00 | 0.11 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.03 |
| %AF | |||||||||||
| r | −0.08 | −0.16 | −0.11 | −0.00 | −0.21a | 0.09 | −0.05 | 0.09 | 0.28a | 0.02 | 0.05 |
| r2 | 0.00 | 0.03 | 0.01 | 0.00 | 0.04 | 0.01 | 0.00 | 0.01 | 0.08 | 0.00 | 0.0 |
| %GF | |||||||||||
| r | −0.04 | −0.14 | −0.19 | −0.03 | −0.41a | 0.07 | −0.19 | 0.04 | 0.18 | 0.05 | 0.09 |
| r2 | 0.00 | 0.02 | 0.03 | 0.00 | 0.17 | 0.00 | 0.04 | 0.00 | 0.03 | 0.00 | 0.01 |
| A:G | |||||||||||
| r | −0.06 | 0.00 | 0.10 | 0.02 | 0.33a | −0.03 | 0.23a | 0.03 | 0.06 | −0.05 | −0.08 |
| r2 | 0.00 | 0.00 | 0.01 | 0.00 | 0.10 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.01 |
| n | 102 | 102 | 102 | 97 | 102 | 101 | 102 | 98 | 101 | 98 | 97 |
| RMREE | |||||||||||
| r | 0.07 | 0.06 | 0.04 | −0.04 | 0.08 | 0.14 | 0.11 | −0.03 | 0.15 | −0.02 | −0.16 |
| r2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 | 0.02 | 0.00 | 0.03 |
| PPEE | |||||||||||
| r | 0.04 | 0.02 | −0.00 | −0.05 | 0.11 | 0.08 | 0.15 | −0.03 | 0.11 | −0.03 | −0.14 |
| r2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.02 |
| TEEE | |||||||||||
| r | −0.07 | −0.10 | −0.11 | −0.01 | 0.08 | −0.16 | 0.09 | −0.01 | −0.09 | −0.02 | 0.05 |
| r2 | 0.00 | 0.01 | 0.01 | 0.00 | 0.01 | 0.03 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
| n | 102 | 102 | 102 | 97 | 102 | 101 | 102 | 98 | 101 | 98 | 97 |
| RMRFatOx | |||||||||||
| r | −0.09 | −0.12 | −0.13 | −0.07 | −0.17 | 0.01 | 0.01 | 0.00 | 0.07 | 0.02 | −0.06 |
| r2 | 0.01 | 0.01 | 0.02 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PPFatOx | |||||||||||
| r | −0.08 | −0.12 | −0.21a | −0.08 | −0.21a | −0.04 | −0.02 | −0.01 | −0.00 | 0.03 | −0.02 |
| r2 | 0.01 | 0.01 | 0.04 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| TEFatOx | |||||||||||
| r | 0.04 | 0.03 | −0.10 | 0.01 | −0.02 | −0.08 | −0.05 | −0.03 | −0.14 | 0.02 | 0.08 |
| r2 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.01 |
| RMRChoOx | |||||||||||
| r | 0.18 | 0.22a | 0.21a | 0.07 | 0.31a | 0.09 | 0.07 | −0.03 | 0.01 | −0.04 | −0.04 |
| r2 | 0.03 | 0.05 | 0.04 | 0.00 | 0.09 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| PPChoOx | |||||||||||
| r | 0.14 | 0.18 | 0.27a | 0.07 | 0.37a | 0.11 | 0.15 | −0.01 | 0.09 | −0.07 | −0.09 |
| r2 | 0.02 | 0.03 | 0.07 | 0.00 | 0.14 | 0.01 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 |
| TEChoOx | |||||||||||
| r | −0.07 | −0.08 | 0.06 | −0.01 | 0.05 | 0.02 | 0.10 | 0.03 | 0.12 | −0.03 | −0.07 |
| r2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 |
AF, android fat; A:G, android:gynoid fat mass; FM, fat mass; GF, gynoid fat; LM, lean mass; PPChoOx, postprandial carbohydrate oxidation; PPEE, postprandial energy expenditure; PPFatOx, postprandial fat oxidation; RMRChoOx, resting metabolic rate carbohydrate oxidation; RMREE, resting metabolic rate energy expenditure; RMRFatOx, resting metabolic rate fat oxidation; TEChoOx, total energy carbohydrate oxidation; TEEE, total energy energy expenditure; TEFatOx, total energy fat oxidation. Values are expressed as Pearson correlation coefficients (r) and coefficients of determination (r2).
P < 0.05.
Correlations between N-acylethanolamine levels and body composition
Table 3 outlines correlations observed between NAE concentrations and body composition measurements for the composite group. Correlational analysis for the composite group showed an inverse relationship between percent body fat on the final week of treatment and plasma OEA concentrations measured on day 29 (r = −0.21, r2 = 0.04, P = 0.036). In addition, plasma AEA concentrations at day 29 were moderately negatively associated with percent body fat (r = −0.31, r2 = 0.10, P = 0.001) and positively associated with lean mass (r = 0.33, r2 = 0.10, P = 0.001) and fat-free mass (r = 0.33, r2 = 0.11, P = 0.001) assessed on the final days of treatment. Considering regional fat mass, plasma AEA concentrations at day 29 exhibited a weak negative association with android fat mass (r = −0.21, r2 = 0.04, P = 0.033) and a moderate negative association with gynoid fat mass (r = −0.41, r2 = 0.17, P < 0.001) measured at the end of treatment, while moderately positively associated with the android:gynoid fat ratio (r = 0.33, r2 = 0.10, P = 0.001). Furthermore, plasma AEA/OEA ratio had a weak positive association with android:gynoid fat ratio (r = 0.23, r2 = 0.05, P = 0.023), and plasma DHEA/OEA ratio was moderately positively associated with percent body fat (r = 0.30, r2 = 0.09, P = 0.003), fat mass (r = 0.21, r2 = 0.05, P = 0.031), and android fat mass (r = 0.28, r2 = 0.08, P = 0.005) across all diets. However, all correlations were weak to moderate with low correlation of determinations. In comparison, when treatment-specific NAE levels were tested against body composition parameters, plasma AEA levels exhibited an inverse association between plasma percent body fat and gynoid fat (r = −0.35, r2 = −0.12, P = 0.042; r = −0.44, r2 = −0.20, P = 0.009), as well as a positive association between AEA and lean mass, fat-free mass, and android:gynoid fat ratio (r = 0.33, r2 = 0.11, P = 0.054; r = 0.33, r2 = 0.11, P = 0.054; r = 0.37, r2 = 0.14, P = 0.029) with HOCO consumption. However, the inverse association between plasma OEA levels and percent body fat observed in the composite group was not replicated when tested by treatment. Comparatively, similar correlations were observed between plasma AEA levels in response to WD consumption with an inverse association between plasma percent body fat and android and gynoid fat (r = −0.44, r2 = 0.19, P = 0.01; r = −0.35, r2 = 0.12, P = 0.04; r = −0.54, r2 = 0.29, P = 0.001), and a positive association between plasma AEA levels and percent lean mass, fat-free mass, and android:gynoid fat ratio (r = 0.49, r2 = 0.24, P = 0.003; r = 0.49, r2 = 0.24, P = 0.003; r = 0.36, r2 = 0.13, P = 0.04). No additional correlations were observed between plasma PAE, LEA, ALEA, or DHEA levels, nor LEA/ALEA, ALEA/AEA, or ALEA/OEA ratios, and body composition measures across the composite group as well as the individual treatments.
Correlations between N-acylethanolamine levels and energy expenditure and substrate oxidation
Correlational analyses for all subjects across all dietary treatments (composite group) failed to reveal any statistically significant relationship between plasma NAE concentrations measured at day 29 and energy expenditure measurements assessed by indirect calorimetry on the final days of treatment for both the composite and treatment-specific groups. For the composite group, plasma LEA and AEA concentrations were negatively correlated with PPFatOx (r = −0.21, r2 = 0.04, P = 0.03; r = −0.21, r2 = 0.05, P = 0.03). Furthermore, it was observed that in response to HOCO consumption, a similar moderate inverse association was detected between plasma LEA and PPFatOx (r = −0.34, r2 = 0.12, P = 0.05) but not in other treatments (Table 4). Similarly, the inverse association between plasma AEA levels and PPFatOx observed in the composite group was only replicated in the FXCO group (r = −0.41, r2 = 0.17, P = 0.02) when analyzed separately. Comparatively, a moderate inverse association was observed between plasma ALEA levels and RMRFatOx and PPFatOx (r = −0.37, r2 = 0.14, P = 0.04; r = −0.40, r2 = 0.16, P = 0.03) in response to HOCO consumption but was not observed in the composite group.
TABLE 4.
Pearson correlation coefficients (r) and coefficient of determination (r2) between plasma fatty acids and NAE and multiple variables by treatment
| WD | FXCO | HOCO | ||||||||||||||||
| Parameter | PEA | OEA | LEA | ALEA | AEA | DHEA | PEA | OEA | LEA | ALEA | AEA | DHEA | PEA | OEA | LEA | ALEA | AEA | DHEA |
| n | 36 | 36 | 36 | 34 | 36 | 36 | 36 | 36 | 36 | 35 | 36 | 35 | 36 | 36 | 36 | 34 | 36 | 36 |
| PA | ||||||||||||||||||
| r | −0.11 | −0.17 | −0.25 | −0.22 | −0.11 | −0.05 | −0.04 | −0.15 | −0.31 | −0.24 | −0.12 | 0.00 | 0.10 | 0.01 | −0.13 | −0.12 | −0.04 | 0.09 |
| r2 | 0.01 | 0.03 | 0.06 | 0.05 | 0.01 | 0.00 | 0.00 | 0.02 | 0.01 | 0.06 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 | 0.01 | 0.00 | 0.01 |
| OA | ||||||||||||||||||
| r | −0.07 | −0.10 | −0.18 | −0.26 | −0.04 | −0.10 | −0.03 | −0.09 | −0.25 | −0.19 | 0.00 | −0.02 | 0.05 | 0.00 | −0.14 | −0.07 | −0.03 | 0.05 |
| r2 | 0.00 | 0.01 | 0.03 | 0.07 | 0.00 | 0.01 | 0.00 | 0.00 | 0.06 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 |
| LA | ||||||||||||||||||
| r | −0.17 | −0.20 | −0.19 | −0.27 | −0.05 | −0.15 | 0.00 | −0.05 | −0.10 | −0.05 | 0.00 | 0.06 | 0.07 | 0.00 | −0.13 | −0.05 | −0.05 | 0.04 |
| r2 | 0.03 | 0.04 | 0.04 | 0.07 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.00 | 0.0 | 0.00 |
| ALA | ||||||||||||||||||
| r | −0.01 | −0.09 | −0.18 | −0.13 | −0.08 | −0.03 | −0.15 | −0.19 | −0.27 | −0.09 | −0.13 | −0.11 | 0.01 | 0.044 | −0.09 | 0.05 | 0.02 | 0.08 |
| r2 | 0.00 | 0.00 | 0.03 | 0.02 | 0.00 | 0.00 | 0.02 | 0.04 | 0.07 | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| AA | ||||||||||||||||||
| r | −0.24 | −0.31 | −0.31 | −0.17 | −0.23 | −0.23 | −0.09 | −0.18 | −0.28 | −0.24 | −0.16 | 0.04 | −0.01 | −0.02 | −0.05 | −0.15- | 0.13 | 0.16 |
| r2 | 0.06 | 0.10 | 0.10 | 0.03 | 0.05 | 0.05 | 0.00 | 0.03 | 0.08 | 0.06 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.03 |
| DHA | ||||||||||||||||||
| r | −0.37 | −0.42 | −0.30 | −0.31 | −0.17 | −0.13 | −0.11 | −0.17 | −0.23 | −0.24 | −0.19 | 0.24 | 0.07 | 0.01 | −0.10 | −0.14 | −0.02 | 0.27 |
| r2 | 0.14 | 0.18 | 0.09 | 0.10 | 0.03 | 0.02 | 0.01 | 0.03 | 0.05 | 0.06 | 0.04 | 0.06 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.07 |
| n | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 |
| %Fat | ||||||||||||||||||
| r | −0.10 | −0.20 | −0.25 | 0.06 | −0.44a | 0.04 | −0.18 | −0.20 | −0.03 | −0.09 | −0.15 | 0.05 | −0.09 | −0.25 | −0.23 | −0.18 | −0.35a | 0.13 |
| r2 | 0.01 | 0.04 | 0.06 | 0.00 | 0.19 | 0.00 | 0.03 | 0.04 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.06 | 0.05 | 0.03 | 0.12 | 0.02 |
| %LM | ||||||||||||||||||
| r | 0.07 | 0.08 | 0.13 | 0.07 | 0.49a | 0.07 | 0.02 | 0.04 | −0.03 | −0.10 | 0.19 | 0.00 | 0.19 | 0.25 | 0.28 | 0.09 | 0.33a | −0.05 |
| r2 | 0.00 | 0.00 | 0.02 | 0.00 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.04 | 0.06 | 0.08 | 0.00 | 0.11 | 0.00 |
| %FFM | ||||||||||||||||||
| r | 0.07 | 0.08 | 0.14 | 0.07 | 0.49a | 0.07 | −0.02 | 0.04 | −0.02 | −0.10 | 0.19 | 0.01 | 0.20 | 0.25 | 0.27 | 0.10 | 0.33a | −0.05 |
| r2 | 0.00 | 0.00 | 0.02 | 0.00 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.04 | 0.00 | 0.04 | 0.06 | 0.07 | 0.01 | 0.11 | 0.00 |
| %FM | ||||||||||||||||||
| r | −0.02 | −0.10 | −0.12 | 0.17 | −0.13 | 0.10 | −0.13 | −0.13 | −0.01 | −0.11 | −0.04 | 0.11 | 0.05 | −0.08 | −0.06 | −0.09 | −0.15 | 0.09 |
| r2 | 0.00 | 0.01 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.02 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.02 | 0.01 |
| %AF | ||||||||||||||||||
| r | −0.10 | −0.21 | −0.25 | 0.08 | −0.35a | 0.04 | −0.12 | −0.14 | 0.08 | −0.05 | −0.02 | 0.09 | −0.01 | −0.16 | −0.17 | −0.09 | −0.25 | 0.13 |
| r2 | 0.01 | 0.04 | 0.06 | 0.00 | 0.12 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.03 | 0.008 | 0.06 | 0.02 |
| %GF | ||||||||||||||||||
| r | 0.07 | 0.00 | −0.20 | 0.11 | −0.54a | 0.11 | −0.10 | −0.16 | −0.06 | −0.05 | −0.28 | −0.00 | −0.10 | −0.26 | −0.30 | −0.17 | −0.44a | 0.10 |
| r2 | 0.00 | 0.00 | 0.04 | 0.01 | 0.29 | 0.01 | 0.01 | 0.03 | 0.00 | 0.00 | 0.08 | 0.00 | 0.01 | 0.07 | 0.09 | 0.03 | 0.20 | 0.01 |
| A:G | ||||||||||||||||||
| r | −0.24 | −0.26 | −0.02 | −0.18 | 0.36a | −0.16 | −0.02 | 0.04 | 0.05 | −0.00 | 0.28 | 0.02 | 0.11 | 0.20 | 0.27 | 0.09 | 0.37a | 0.02 |
| r2 | 0.06 | 0.07 | 0.00 | 0.03 | 0.13 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | 0.00 | 0.01 | 0.04 | 0.07 | 0.01 | 0.14 | 0.00 |
| RMREE | ||||||||||||||||||
| r | 0.06 | 0.06 | −0.04 | 0.14 | 0.15 | 0.28 | 0.05 | 0.03 | −0.01 | −0.10 | 0.00 | 0.15 | 0.08 | 0.06 | 0.12 | −0.14 | 0.01 | 0.09 |
| r2 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.08 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 |
| PPEE | ||||||||||||||||||
| r | −0.02 | −0.05 | −0.08 | 0.07 | 0.17 | 0.16 | 0.06 | 0.05 | −0.04 | −0.10 | 0.03 | 0.06 | 0.06 | 0.03 | 0.08 | −0.14 | 0.12 | 0.06 |
| r2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.03 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.02 | 0.01 | 0.00 |
| TEEE | ||||||||||||||||||
| r | −0.17 | −0.25 | −0.11 | −0.13 | 0.09 | −0.24 | 0.01 | 0.04 | −0.08 | −0.00 | 0.07 | −0.22 | −0.05 | −0.10 | −0.14 | 0.01 | 0.08 | −0.12 |
| r2 | 0.03 | 0.06 | 0.01 | 0.02 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.01 | 0.02 | 0.00 | 0.00 | 0.01 |
| RMRFatOx | ||||||||||||||||||
| r | −0.24 | −0.10 | 0.08 | −0.01 | 0.11 | 0.04 | 0.11 | 0.00 | −0.15 | −0.07 | −0.27 | 0.05 | −0.18 | −0.28 | −0.27 | −0.37a | −0.31 | −0.02 |
| r2 | 0.06 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 | 0.01 | 0.00 | 0.02 | 0.00 | 0.07 | 0.00 | 0.03 | 0.08 | 0.07 | 0.14 | 0.10 | 0.00 |
| PPFatOx | ||||||||||||||||||
| r | −0.16 | −0.07 | −0.04 | −0.07 | −0.01 | 0.07 | 0.11 | −0.00 | −0.21 | −0.04 | −0.41a | −0.04 | −0.24 | −0.31 | −0.34a | −0.40a | −0.22 | −0.10 |
| r2 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.04 | 0.00 | 0.17 | 0.00 | 0.06 | 0.01 | 0.12 | 0.16 | 0.05 | 0.01 |
| TEFatOx | ||||||||||||||||||
| r | 0.21 | 0.08 | −0.22 | 0.09 | −0.23 | 0.03 | −0.04 | −0.01 | −0.05 | 0.06 | −0.13 | −0.15 | −0.07 | 0.00 | −0.07 | 0.05 | 0.24 | −0.13 |
| r2 | 0.04 | 0.00 | 0.05 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.02 |
| RMRChoOx | ||||||||||||||||||
| r | 0.33 | 0.16 | −0.12 | 0.20 | −0.04 | 0.13 | −0.13 | 0.02 | 0.22 | 0.03 | 0.42a | 0.05 | 0.34a | 0.48a | 0.52a | 0.45a | 0.56a | 0.12 |
| r2 | 0.11 | 0.03 | 0.01 | 0.04 | 0.00 | 0.02 | 0.02 | 0.00 | 0.05 | 0.00 | 0.18 | 0.00 | 0.12 | 0.23 | 0.27 | 0.20 | 0.31 | 0.01 |
| PPChoOx | ||||||||||||||||||
| r | 0.18 | 0.04 | −0.01 | 0.14 | 0.15 | 0.05 | −0.09 | 0.04 | 0.22 | −0.02 | 0.52a | 0.10 | 0.41a | 0.48a | 0.57a | 0.44a | 0.44a | 0.20 |
| r2 | 0.03 | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.27 | 0.01 | 0.17 | 0.23 | 0.32 | 0.19 | 0.19 | 0.04 |
| TEChoOx | ||||||||||||||||||
| r | −0.30 | −0.21 | 0.19 | −0.16 | 0.29 | −0.15 | 0.05 | 0.03 | 0.01 | −0.07 | 0.16 | 0.07 | 0.06 | −0.05 | 0.02 | −0.06 | −0.25 | 0.10 |
| r2 | 0.09 | 0.04 | 0.04 | 0.03 | 0.08 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.01 |
Values are expressed as Pearson correlation coefficients (r) and coefficients of determination (r2).
P < 0.05
For carbohydrate oxidation, RMRChoOx was positively correlated with plasma OEA, LEA, and AEA concentrations (r = 0.22, r2 = 0.05, P = 0.029; r = 0.21, r2 = 0.04, P = 0.035; r = 0.31, r2 = 0.09, P = 0.002) in the composite group. Interestingly, a significant moderate to strong positive association was demonstrated between RMRChoOx and plasma PEA, OEA, LEA, ALEA, and AEA levels in response to HOCO consumption (r = 0.34, r2 = 0.12, P = 0.05; r = 0.48, r2 = 0.23, P = 0.004; r = 0.52, r2 = 0.27, P = 0.002; r = 0.45, r2 = 0.20, P = 0.01; r = 0.56, r2 = 0.31, P = 0.001). The positive association between RMRChoOx was also observed with plasma AEA levels but only in the FXCO group (r = 0.42, r2 = 0.18, P = 0.01). Further, a significant moderate positive association was also demonstrated between PPChoOx and LEA and AEA levels in the composite group (r = 0.27, r2 = 0.07, P = 0.007; r = 0.37, r2 = 0.14, P < 0.001). Again, the positive association with PPChoOx was further demonstrated with plasma PEA, OEA, LEA, ALEA, and AEA levels in response to HOCO consumption (r = 0.41, r2 = 0.17, P = 0.02; r = 0.48, r2 = 0.23, P = 0.004; r = 0.57, r2 = 0.32, P < 0.001; r = 0.44, r2 = 0.19, P = 0.01; r = 0.44, r2 = 0.19, P = 0.01) but was only observed in plasma AEA levels in response to FXCO consumption (r = 0.52, r2 = 0.27, P = 0.002). No correlations were observed between plasma NAE levels or NAE ratios and thermic effect of food fat oxidation or carbohydrate oxidation across all groups.
DISCUSSION
The major finding from this controlled feeding study is that circulating NAE concentrations are modulated by the type of dietary fat consumed in humans. Specifically, consumption of diets enriched in monounsaturated fat (OA) from HOCO, or enriched in omega-3 fat (ALA) from FXCO, resulted in elevated concentrations of plasma OEA and ALEA, respectively. Although this is the first human intervention study measuring plasma NAE levels in response to different dietary fat interventions, previous animal studies have been conducted examining similar questions. For instance, it has been observed that plasma and tissue levels of NAEs in hamsters are modified in response to dietary fatty acid composition and are observed to specifically activate lipid metabolism pathways through NAE-regulated mechanisms (5). Although it has been demonstrated that overall high-fat feeding modulates circulating and peripheral NAE levels irrespective of their fatty acid composition (20, 21), the present human study shows that different dietary fatty acid compositions are capable of altering plasma NAE content in a composition-dependent manner, irrespective of the isoenergetic high-fat content (34% energy), which is consistent with previous animal studies examining this question (5, 13). In fact, the plasma NAE levels were observed to shift solely in response to the type of fatty acid, rather than the quantity of overall dietary fat, suggesting that NAE levels can be significantly modulated through dietary fat choice. Furthermore, the pattern of both plasma fatty acid composition and plasma NAE modifications are consistent with the fatty acid profiles presented in the individual diets, where consumption of the OA-rich HOCO diet stimulated a significant increase in circulating OEA levels, while the ALA-rich FXCO diet increased circulating ALEA levels. This association is consistent with recent work by Alvheim and colleagues (22), in which mice fed a low-fat diet supplemented with dietary LA exhibited elevated levels of RBC-phospholipid AA, a precursor for AEA and 2-AG synthesis, and thus also demonstrated increased levels of liver AEA and 2-AG and subsequent weight gain. However, the direct association between increased dietary LA within our WD and increasing levels of AA and subsequent AEA levels shown by Alvheim et al. (22) was not observed in our cohort. In fact, the PUFA-rich FXCO diet exhibited significantly lower plasma AA levels than other treatments, but this did not translate into significantly modified plasma AEA levels. To our knowledge, shifts in circulating AEA and 2-AG levels have only been observed in response to changes in body weight or in a comparison between obese and lean humans (23, 24). These shifts have not been reported in weight-maintained humans as in this study and may not necessarily be altered considerably without significant changes in body weight or direct feeding of AA to induce synthesis of its product, AEA. Based on the response of OEA and ALEA levels observed following feeding of OA- and ALA-rich diets, these results indicate that NAE synthesis is highly dependent on the availability of its respective fatty acid substrate.
The second novel finding presently is the proposed correlation between plasma NAE levels, body composition, and substrate oxidation in humans. A moderate inverse relationship was observed between percent body fat and plasma OEA concentrations, which is consistent with multiple previously published reports (7, 25). It has been described that OEA exerts an inhibitory effect on body-weight gain due to the role of OEA as an endogenous PPAR-α agonist, which regulates the expression of genes involved in fatty acid absorption, transport, utilization, storage, and energy balance (26, 27). Previous animal studies have reported that increased levels of OEA demonstrate enhanced lipolysis and fatty acid oxidation via simultaneous stimulation of PPAR-α and the upregulation of the respective lipid catabolic genes, ultimately resulting in a decrease in body weight (5, 7, 25–27). Additionally, activation of PPAR-α via OEA has been shown to increase satiety, resulting in decreased food intake and adipose tissue mass, all contributing to the effect OEA has on body weight and energy balance (28, 29). However, the present study only exhibited a slight correlation between percent body fat and plasma OEA concentrations with no significant change in body weight and was only observed in the composite group and showed no correlation when analyzed by treatment. Further, this slight correlation between OEA and percent body fat occurred with no correlations to fat oxidation but demonstrated a direct correlation to carbohydrate oxidation along with other NAEs. Most interestingly, plasma AEA concentrations were also moderately inversely associated with percent body, android, and gynoid fat, but directly associated with lean and fat-free mass, which is an atypical correlation between AEA and fat deposition as AEA is generally associated with increased adiposity (1). However, when regional fat distribution was analyzed, android:gynoid fat mass ratio showed a stronger positive association with AEA levels, as well as the AEA/OEA ratio, indicating that although both AEA and OEA levels were inversely associated with percent body fat, AEA levels appeared to correlate stronger with android fat distribution more so than gynoid fat. Furthermore, a similar trend was exhibited with DHEA/OEA and percent android fat, indicating that OEA levels have less affinity for android fat distribution when compared with both AEA and DHEA. It has been identified that the effect of AEA on weight gain can be reduced with the inclusion of dietary EPA and DHA and can reduce visceral fat mass in rats (15, 30, 31), thus the observation that DHEA, the NAE-analog of DHA, was correlated with android fat deposition more so than OEA suggests that circulating OEA levels may contribute to a more desirable regional body fat deposition profile than do other NAEs. Moreover, it was also observed that plasma NAE levels exhibited a moderate direct correlation with carbohydrate oxidation but were inversely correlated with fat oxidation, both resting and postprandial, of which plasma AEA levels showed the strongest correlation. This observation is contradictory to the effects on regional adiposity as inverse associations between fat mass and NAE levels demonstrated in our cohort would suggest that a direct association with fat oxidation exists. However, the opposite is evident with a positive association with carbohydrate oxidation and was most pronounced in response to the HOCO treatment. This anomaly may be explained with the observation that this moderate direct association was demonstrated across multiple NAEs, particularly OEA and AEA, which typically act as opposite metabolic regulators, with OEA linked to lipolysis and AEA to lipogenesis (1, 26), suggesting that a factor other than circulating NAEs within the HOCO is driving the carbohydrate oxidation association in the HOCO treatment. As it has been shown in this study that feeding MUFA-rich OA through HOCO increases circulating OEA levels and PUFA-rich ALA through FXCO increases circulating ALEA levels, it is of interest to define whether an LA-rich diet or AA supplementation exacerbates the observed correlations between AEA levels and fat deposition and substrate oxidation establishing a connection between dietary fat type on AEA levels with regional adiposity and energetics.
Overall, the present results suggest that plasma NAE levels are modifiable by dietary fatty acid type, and, as such, the shift in plasma NAE levels acts to induce modest shifts in processes that regulate body composition and substrate oxidation. However, the mechanisms regulating these effects, dietary and cellular, require further investigation.
Acknowledgments
The authors thank the study participants for their cooperation and the invaluable technical and coordination assistance from the laboratory and clinical staff at the Richardson Centre for Functional Foods and Nutraceuticals (RCFFN), including Khatima Khalloufi, Vanu Ramprasath, Jen Gustafson, Kimberley Robinson, and the staff of the RCFFN Metabolic Kitchen. The authors acknowledge Bioriginal Food & Science Corporation and Dow AgroSciences LLC for the kind donation of the FXCO and HOCO, respectively.
Footnotes
Abbreviations:
- 2-AG
- 2-arachidonoylglycerol
- AA
- arachidonic acid
- AEA
- arachidonoyl ethanolamide
- ALA
- α-linoleic acid
- ALEA
- α-linolenoyl ethanolamide
- CB
- cannabinoid
- DHEA
- docosahexaenoyl hanolamide
- FXCO
- flaxseed high-oleic canola oil
- HOCO
- high-oleic canola oil
- LA
- linoleic acid
- LEA
- linoleoyl ethanolamide
- NAE
- N-acylethanolamine
- OA
- oleic acid
- OEA
- oleoylethanolamide
- PA
- palmitic acid
- PEA
- palmitoylethanolamide
- PPChoOx
- postprandial carbohydrate oxidation
- PPFatOx
- postprandial fat oxidation
- RMRChoOx
- resting metabolic rate carbohydrate oxidation
- RMRFatOx
- resting metabolic rate fat oxidation
- UPLC
- ultra-performance liquid chromatography
- WD
- Western diet.
This work was supported in part by grants from Flax Canada 2015, Canola Council of Canada, and Agri-Food Research & Development Initiative.
REFERENCES
- 1.Hansen H. S., Diep T. A. 2009. N-acylethanolamines, anandamide and food intake. Biochem. Pharmacol. 78: 553–560. [DOI] [PubMed] [Google Scholar]
- 2.Coulon D., Laure L., Salmon M., Wattelet V., Bessoule J-J. 2012. N-Acylethanolamines and related compounds: aspects of metabolism and functions. Plant Sci. 184: 129–140. [DOI] [PubMed] [Google Scholar]
- 3.Thabuis C., Destaillats F., Tissot-Favre D., Martin J-C. 2007. Oleoyl-ethanolamide (OEA): a bioactive lipid derived from oleic acid and phosphatidylethanol-amine. Lipid Technology. 19: 225–227. [Google Scholar]
- 4.Fu J., Kim J., Oveisi F., Astarita G., Piomelli D. 2008. Targeted enhancement of oleoylethanolamide production in proximal small intestine induces across-meal satiety in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295: R45–R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin L., Rideout T., Yurkova N., Yang H., Eck P., Jones P. J. H. 2013. Fatty acid ethanolamides modulate CD36-mRNA through dietary fatty acid manipulation in Syrian Golden hamsters. Appl. Physiol. Nutr. Metab. 38: 870–878. [DOI] [PubMed] [Google Scholar]
- 6.Pertwee R. G., Ross R. A. 2002. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fatty Acids. 66: 101–121. [DOI] [PubMed] [Google Scholar]
- 7.Lo Verme J., Fu J., Astarita G., La Rana G., Russo R., Calignano A., Piomelli D. 2005. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol. Pharmacol. 67: 15–19. [DOI] [PubMed] [Google Scholar]
- 8.Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., et al. 2005. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 115: 1298–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mattace Raso G., Santoro A., Russo R., Simeoli R., Paciello O., Di Carlo C., Diano S., Calignano A., Meli R. 2014. Palmitoylethanolamide prevents metabolic alterations and restores leptin sensitivity in ovariectomized rats. Endocrinology. 155: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Marzo V., Côté M., Matias I., Lemieux I., Arsenault B. J., Cartier A., Piscitelli F., Petrosino S., Alméras N., Després J. P. 2009. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia. 52: 213–217. [DOI] [PubMed] [Google Scholar]
- 11.Abdulnour J., Yasari S., Rabasa-Lhoret R., Faraj M., Petrosino S., Piscitelli F., Prud’ Homme D., Di Marzo V. 2014. Circulating endocannabinoids in insulin sensitive vs. insulin resistant obese postmenopausal women. A MONET group study. Obesity (Silver Spring). 22: 211–216. [DOI] [PubMed] [Google Scholar]
- 12.Lin L., Yang H., Jones P. J. H. 2012. Quantitative analysis of multiple fatty acid ethanolamides using ultra-performance liquid chromatography-tandem mass spectrometry. Prostaglandins Leukot. Essent. Fatty Acids. 87: 189–195. [DOI] [PubMed] [Google Scholar]
- 13.Artmann A., Petersen G., Hellgren I., Boberg J., Skonberg C., Nellemann C., Hansen S. H., Hansen H. S. 2008. Influence of dietary fatty acids on endocannabinoid and N-acylethanolamine levels in rat brain, liver and small intestine. Biochim. Biophys. Acta. 1781: 200–212. [DOI] [PubMed] [Google Scholar]
- 14.Alvheim A. R., Malde M. K., Osei-Hyiaman D., Lin Y. H., Pawlosky R. J., Madsen L., Kristiansen K., Frøyland L., Hibbeln J. R. 2012. Dietary linoleic acid elevates endogenous 2-AG and anandamide and induces obesity. Obesity (Silver Spring). 20: 1984–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banni S., Carta G., Murru E., Cordeddu L., Giordano E., Sirigu A. R., Berge K., Vik H., Maki K. C., Di Marzo V., et al. 2011. Krill oil significantly decreases 2-arachidonoylglycerol plasma levels in obese subjects. Nutr. Metab. (Lond.). 8: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gillingham L. G., Gustafson J. A., Han S-Y., Jassal D. S., Jones P. J. H. 2011. High-oleic rapeseed (canola) and flaxseed oils modulate serum lipids and inflammatory biomarkers in hypercholesterolaemic subjects. Br. J. Nutr. 105: 417–427. [DOI] [PubMed] [Google Scholar]
- 17.Mifflin M. D., St. Jeor S. T., Hill L. A., Scott B. J., Daugherty S. A., Koh Y. O. 1990. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 51: 241–247. [DOI] [PubMed] [Google Scholar]
- 18.Folch J., Lees M., Sloane G. H. 1957. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226: 497–509. [PubMed] [Google Scholar]
- 19.Gillingham L. G., Robinson K. S., Jones P. J. H. 2012. Effect of high-oleic canola and flaxseed oils on energy expenditure and body composition in hypercholesterolemic subjects. Metabolism. 61: 1598–1605. [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo V., Capasso R., Matias I., Aviello G., Petrosino S., Borrelli F., Romano B., Orlando P., Capasso F., Izzo A. A. 2008. The role of endocannabinoids in the regulation of gastric emptying: alterations in mice fed a high-fat diet. Br. J. Pharmacol. 153: 1272–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diep T. A., Madsen A. N., Holst B., Kristiansen M. M., Wellner N., Hansen S. H., Hansen H. S. 2011. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 25: 765–774. [DOI] [PubMed] [Google Scholar]
- 22.Alvheim A. R., Torstensen B. E., Lin Y. H., Lillefosse H. H., Lock E. J., Madsen L., Frøyland L., Hibbeln J. R., Malde M. K. 2014. Dietary linoleic acid elevates the endocannabinoids 2-AG and anandamide and promotes weight gain in mice fed a low fat diet. Lipids. 49: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blüher M., Engeli S., Kloting N., Berndt J., Fasshauer M., Bátkai S., Pacher P., Schön M. R., Jordan J., Stumvoll M. 2006. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 55: 3053–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J. P., Di Marzo V. 2007. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. (Lond.). 31: 692–699. [DOI] [PubMed] [Google Scholar]
- 25.Fu J., Gaetani S., Oveisi F., Lo Verme J., Serrano A., Rodríguez De Fonseca F., Rosengarth A., Luecke H., Di Giacomo B., Tarzia G., et al. 2003. Oleoylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 425: 90–93. [DOI] [PubMed] [Google Scholar]
- 26.Guzmán M., Lo Verme J., Fu J., Oveisi F., Blázquez C., Piomelli D. 2004. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J. Biol. Chem. 279: 27849–27854. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz G. J., Fu J., Astarita G., Li X., Gaetani S., Campolongo P., Cuomo V., Piomelli D. 2008. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 8: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen G., Sørensen C., Schmid P. C., Artmann A., Tang-Christensen M., Hansen S. H., Larsen P. J., Schmid H. H., Hansen H. S. 2006. Intestinal levels of anandamide and oleoylethanolamide in food-deprived rats are regulated through their precursors. Biochim. Biophys. Acta. 1761: 143–150. [DOI] [PubMed] [Google Scholar]
- 29.Thabuis C., Destaillats F., Landrier J. F., Tissot-Favre D., Martin J-C. 2010. Analysis of gene expression pattern reveals potential targets of dietary oleoylethanolamide in reducing body fat gain in C3H mice. J. Nutr. Biochem. 21: 922–928. [DOI] [PubMed] [Google Scholar]
- 30.Di Marzo V., Griinari M., Carta G., Murru E., Ligresti A., Cordeddu L., Giordano E., Bisogno T., Collu M., Batetta B., et al. 2010. Dietary krill oil increases docosahexaenoic acid and reduces 2-arachidonoyl-glycerol but not N-acylethanolamine levels in the brain of obese Zucker rats. Int. Dairy J. 20: 231–235. [Google Scholar]
- 31.Alvheim A. R., Torstensen B. E., Lin Y. H., Lillefosse H. H., Lock E. J., Madsen L., Hibbeln J. R., Malde M. K. 2013. Dietary linoleic acid elevates endogenous 2-arachidonoylglycerol and anandamide in Atlantic salmon (Salmo salar L.) and mice, and induces weight gain and inflammation in mice. Br. J. Nutr. 109: 1508–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]