Abstract
Nonalcoholic steatohepatitis (NASH) is associated with increased synthesis of triglycerides and cholesterol coupled with increased VLDL synthesis in the liver. In addition, increased cholesterol content in the liver associates with NASH. Here we study the association of lipoprotein subclass metabolism with NASH. To this aim, liver biopsies from 116 morbidly obese individuals [age 47.3 ± 8.7 (mean ± SD) years, BMI 45.1 ± 6.1 kg/m2, 39 men and 77 women] were used for histological assessment. Proton NMR spectroscopy was used to measure lipid concentrations of 14 lipoprotein subclasses in native serum samples at baseline and after obesity surgery. We observed that total lipid concentration of VLDL and LDL subclasses, but not HDL subclasses, associated with NASH [false discovery rate (FDR) < 0.1]. More specifically, total lipid and cholesterol concentration of VLDL and LDL subclasses associated with inflammation, fibrosis, and cell injury (FDR < 0.1), independent of steatosis. Cholesterol concentration of all VLDL subclasses also correlated with total and free cholesterol content in the liver. All NASH-related changes in lipoprotein subclasses were reversed by obesity surgery. High total lipid and cholesterol concentration of serum VLDL and LDL subclasses are linked to cholesterol accumulation in the liver and to liver cell injury in NASH.
Keywords: high density lipoprotein, low density lipoprotein, liver, lipids, lipoproteins/metabolism, nuclear magnetic resonance, obesity, obesity surgery, very low density lipoprotein
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the Western world (1). It is estimated to affect 10–50% of the population (2). The disease presents primarily as steatosis contributing to metabolic alterations such as insulin resistance, hyperglycemia, and hyperlipidemia (3–5). However, NAFLD can also lead to nonalcoholic steatohepatitis (NASH) with a potential of leading to liver cirrhosis and liver failure (6).
Increased triglyceride synthesis and accumulation are characteristic in a steatotic liver (7). In addition, cholesterol synthesis is also increased in NAFLD (8), and cholesterol accumulation has been suggested to contribute to liver cell injury in NASH (9). The related overproduction of VLDL particles in the metabolic syndrome (10) and NAFLD (4) has been thought to be a consequence of this increased lipid production in the liver. It has also been suggested that high LDL cholesterol relates to NASH and liver fibrosis (11, 12), suggesting that the association of NASH with serum lipids is not only related to increased VLDL synthesis.
We investigated the association between serum lipoprotein subclasses and NASH. Our hypotheses were that dyslipidemia in NASH includes altered lipoprotein subclass lipid profile, and specifically that changes in cholesterol metabolism associate with liver inflammation and injury. To answer these questions, we investigated serum lipoprotein subclasses using NMR spectroscopy (13) in 116 obese individuals with detailed liver histology. The main finding was that the levels of total lipids and cholesterol in VLDL and LDL subclasses were linked to inflammation and cell injury in NASH, but not to simple steatosis. These changes were corrected with obesity surgery.
MATERIALS AND METHODS
Subjects
All patients undergoing obesity surgery in Kuopio University Hospital are recruited into our ongoing study investigating metabolic consequences of obesity surgery (Kuopio Obesity Surgery Study) (14, 15). The study group included 116 consecutive subjects [age 47.3 ± 8.7 (mean ± SD) years, BMI 45.1 ± 6.1 kg/m2, 39 men and 77 women], who were accepted for the Roux-en-Y gastric bypass (RYGB) operation over the years 2005–2010. Fifty-nine patients had serum NMR data available also 1 year after the surgery. The study protocol was approved by the Ethics Committee of the Northern Savo Hospital District (54/2005,104/2008, and 27/2010), and it was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from the subjects.
Laboratory determinations
Diabetes was defined by WHO’s criteria of diabetes (16). Plasma glucose, insulin, serum total cholesterol, HDL cholesterol, and triglyceride levels were analyzed as described before (14, 15).
Serum lipoprotein subclass analysis by NMR spectroscopy
Fasting concentrations of lipoprotein subclass particles and their main lipid components were analyzed by proton NMR spectroscopy in native serum samples (13, 17, 18). The details of this methodology have been described previously (13, 19), and this platform has recently been applied in various large-scale epidemiological and genetic studies (20–22). The lipoprotein subclass data available are as follows: chylomicrons and largest VLDL particles (average particle diameter at least 75 nm); five different VLDL subclasses: very large VLDL (average particle diameter of 64.0 nm), large VLDL (53.6 nm), medium VLDL (44.5 nm), small VLDL (36.8 nm), and very small VLDL (31.3 nm); IDL (28.6 nm); three LDL subclasses: large LDL (25.5 nm), medium LDL (23.0 nm), and small LDL (18.7 nm); and four HDL subclasses: very large HDL (14.3 nm), large HDL (12.1 nm), medium HDL (10.9 nm), and small HDL (8.7 nm). The following components of the lipoprotein particles were quantified: phospholipids, triglycerides, and cholesterol. Due to resolution and concentration issues, all of these components are not available for every subclass (17, 18, 20). The VLDL cholesterol concentration was calculated by subtracting the LDL, IDL, and HDL cholesterol from the total cholesterol. The total cholesterol content of chylomicrons and extremely large VLDL, very large VLDL and very small VLDL was calculated by subtracting the triglyceride and phospholipid concentrations from the total lipids of each subclass. Lipid composition as a percentage of each available lipid of the total lipid content in each lipoprotein subclass was also calculated.
Liver histology
Liver biopsies were obtained using a Tru-Cut needle (Radiplast AB, Uppsala, Sweden) during elective gastric bypass operations. Overall histological assessment of liver biopsy samples was performed by one pathologist according to the standard criteria (23, 24), and histological diagnosis was divided into three categories: 1) normal liver without any steatosis, inflammation, ballooning, or fibrosis; 2) simple steatosis (steatosis >5%) without evidence of hepatocellular ballooning, inflammation, or fibrosis; and 3) NASH (supplementary Tables I, II). When analyzing the effects of the gastric bypass operation, a different histological characterization was used to separate normal from probable and definite NASH (supplementary Table III): steatosis was graded into four categories (<5, 5–33, 33–66, and >66%); lobular inflammation was graded into four categories (no foci, <2, 2–4, and >4 per 200× field); fibrosis was staged from 1 to 4 and ballooning from 0 to 2. When all four variables were present, the diagnosis was “definite steatohepatitis,” and when variables one to three were positive, the diagnosis was “probable steatohepatitis.” In the absence of lobular inflammation and steatosis, the diagnosis was “not steatohepatitis”. All patients with alcohol consumption >2 doses per day were excluded from the study. Chronic hepatitis B and C (HBV and HCV) were excluded using serology if alanine aminotransferase values were elevated prior to surgery (HCV and HBV infections are rare in Finland). Hemochromatosis was excluded by histological analysis of liver biopsies, and by normal serum ferritin levels in subjects that had an elevated serum alanine aminotransferase level.
Liver cholesterol content with gas-liquid chromatography
Liver total cholesterol content (per 100 mg liver tissue) was quantified with gas-liquid chromatography (GLC) on a 50 m long capillary column (Ultra 2, Agilent Technologies, Wilmington, DE) using 5α-cholestane as the internal standard (25).
Liver free cholesterol content with NMR spectroscopy
Liver samples (approximately 50 mg) were homogenized in 1.5 ml Eppendorf tubes in NaCl solution (150 μl of 150 mM NaCl in D2O) by pestle. After homogenization, 300 μl of CD3OD and 600 μl of CDCl3 were added and samples were mixed vigorously using a vortex mixer and sonicated for 15 min (indirect sonication) in an ice bath. After mixing, the samples were centrifuged (5,000 g, 10 min, 4°C) to separate the organic and water phase. The lower organic phase was recovered and the aqueous layer was extracted again, first with 600 μl and then with 300 μl CDCl3 to standardize the yield. The separated organic layers were combined and evaporated to dryness under a gentle flow of dried air. Prior to NMR analysis, the extracted lipids were redissolved into 600 μl of CDCl3 containing 0.03% of tetramethylsilane as a reference substance.
1H NMR spectra of extracted lipids were recorded on a Bruker Avance III HD 600 NMR spectrometer operating at 600.28 MHz and equipped with a Prodigy TCI 5 mm cryogenically cooled probe head. Standard 1D 1H NMR spectra were recorded with 96,000 data points using 32 transients and applying a standard Bruker zg pulse sequence. The acquisition time was 5 s and the relaxation delay was 15 s. The spectra were measured at 295,000.
Data processing.
The free induction decays (FIDs) with 96,000 data points were zero-filled to 256,000 data points and multiplied by an exponential window function with a 0.3 Hz line-broadening. The areas of the known lipid resonances (26) in the spectra were determined using a constrained total-lineshape fitting approach to enable quantitative analysis of severely overlapping peaks and to increase the quantification accuracy (27). This methodology allowed us to get information on the amounts of several lipid components in the extracted samples, e.g., free cholesterol and total triglycerides. The PERCH NMR software was used for all the lineshape fitting analyses.
Liver gene expression and genotyping
Quantitative real-time PCR was carried out in the Applied Biosystems 7500 real-time PCR system using KAPA SYBR FAST qPCR Universal Master Mix (Kapa Biosystems, Woburn, MA). Primers are available in supplementary Table III. We genotyped rs738409 SNP of patatin-like phospholipase domain containing 3 (PNPLA3) (28) (using the TaqMan SNP genotyping assay (Applied Biosystems) according to the manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± SD. Differences between the study groups were examined with the χ2 test (in categorical variables) and by nonparametric Kruskall-Wallis test (continuous variables). Spearman rank correlation was used for correlation analysis. General linear model analysis was used for studying the independent contributions of variables on liver histology. Analyses were conducted with the SPSS version 19 (IBM SPSS). Multiple testing of several related lipid phenotypes was corrected using false discovery rate (FDR) calculated by the Benjamini-Hochberg method as implicated in the “p adjust method” in R (version 2.0.1) (29). FDR values <0.1 were considered to indicate a statistically significant difference.
RESULTS
Subject characteristics
Seventy-six out of the 116 patients had a clearly defined liver phenotype: 32 had normal liver, 19 had simple steatosis, and 25 had NASH (Table 1). Levels of fasting insulin and total and LDL cholesterol (P = 0.006, P = 0.004, and P = 0.010, Kruskall-Wallis test) differed between study groups. Total and LDL cholesterol were higher in individuals with NASH compared to those with simple steatosis (P = 0.002 and P = 0.007). The results were essentially the same if individuals using cholesterol-lowering medication (n = 21) were excluded (supplementary Table IV).
TABLE 1.
Clinical characteristics based on liver phenotype
| Normal Liver (n = 32) | Simple Steatosis (n = 19) | NASH (n = 25) | Pa | |
| Sex (male/female) | 11/21 | 4/15 | 10/15 | 0.404 |
| Age (y) | 47.9 ± 9.7 | 45.8 ± 9.8 | 46.7 ± 8.0 | 0.725 |
| Weight (kg) | 127.2 ± 19.4 | 126.2 ± 14.8 | 132.1 ± 24.5 | 0.676 |
| BMI (kg/m2) | 44.1 ± 6.8 | 44.8 ± 4.3 | 44.3 ± 6.9 | 0.716 |
| Fasting glucose (mmol/l) | 6.1 ± 0.9 | 6.3 ± 1.2 | 6.5 ± 1.6 | 0.929 |
| Fasting insulin (mU/l) | 14.5 ± 9.0 | 19.7 ± 10.1 | 25.4 ± 17.1b | 0.006 |
| Total cholesterol (mmol/l) | 4.23 ± 0.8 | 3.80 ± 0.9 | 4.74 ± 1.0b,c | 0.004 |
| LDL cholesterol (mmol/l) | 2.48 ± 0.7 | 2.11 ± 0.8 | 2.89 ± 1.0c | 0.010 |
| HDL cholesterol (mmol/l) | 1.07 ± 0.3 | 1.02 ± 0.2 | 1.06 ± 0.4 | 0.539 |
| Total triglycerides (mmol/l) | 1.49 ± 0.7 | 1.46 ± 0.6 | 1.74 ± 0.6 | 0.103 |
| Non-HDL cholesterol (mmol/l) | 2.23 ± 0.6 | 2.02 ± 0.6 | 2.67 ± 0.8b,c | 0.012 |
| Steatosis grade (n) | 8.0 × 10−17 | |||
| <5% | 32 | 0 | 0 | |
| 5–33% | 0 | 15 | 10 | |
| 33–66% | 0 | 2 | 10 | |
| >66% | 0 | 2 | 5 | |
| Lobular inflammation (n) | 1.2 × 10−15 | |||
| None | 32 | 19 | 0 | |
| <2 Foci per 200× field | 0 | 0 | 17 | |
| 2–4 Foci per 200× field | 0 | 0 | 8 | |
| >4 Foci per 200× field | 0 | 0 | 0 | |
| Fibrosis (n) | 6.6 × 10−11 | |||
| 0 | 32 | 19 | 4 | |
| 1 | 0 | 0 | 19 | |
| 2 | 0 | 0 | 1 | |
| 3 | 0 | 0 | 0 | |
| 4 | 0 | 0 | 1 |
P value is over all groups. Kruskal-Wallis test for continuous variables and chi-square test for categorical variables.
Normal liver versus NASH, P < 0.05.
Steatosis versus NASH, P < 0.05.
Serum lipids in relation to steatosis, inflammation, and fibrosis
Next, we investigated to determine whether the association between NASH and serum lipids is related to steatosis, inflammation, or fibrosis in the liver. To this aim, obese patients were divided into four groups based on severity of steatosis (steatosis grades: <5%, 5–33%, 33–66%, and >66%; supplementary Table II, upper part); into three groups based on lobular inflammation (no inflammatory cells, <2 cells per 200× field, and 2–4 cells per 200× field; supplementary Table II, middle part); and into three groups based on fibrosis stage (by combining stages 2–4; supplementary Table II, lower part). Steatosis associated with higher fasting insulin levels (P = 0.002), but not with serum lipids (supplementary Fig. I). In contrast, lobular inflammation and stage 1 fibrosis associated with total and LDL cholesterol (P = 0.0001–0.022; supplementary Fig. IB, C). In addition, individuals with stage 1 fibrosis had higher total triglycerides (P = 0.008) than individuals without any sign of fibrosis (supplementary Fig. IC). There was no difference when comparing individuals without fibrosis to those with grades 2–4 fibrosis, suggesting a decline in serum lipids when moving from stage 1 to a more advanced stage of fibrosis.
VLDL and LDL lipid concentration associates with inflammation, fibrosis, and liver cell injury
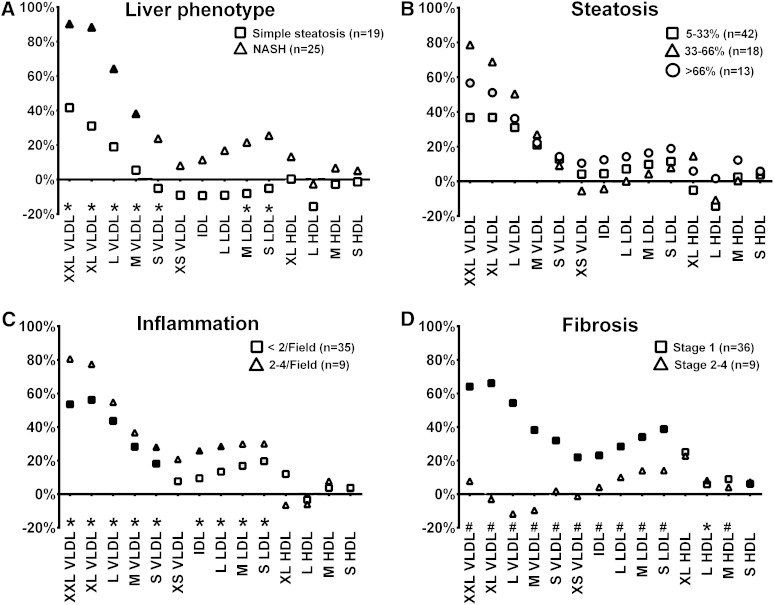
The serum lipid and lipoprotein analysis was extended to a more detailed lipoprotein subclass analysis using NMR spectroscopy (13, 19) (Fig. 1A). Total lipid concentration of VLDL (excluding very small VLDL) and medium and small LDL associated with NASH (FDR < 0.1, Table 2). More specifically, total lipid concentration of VLDL and LDL subclasses was increased in individuals with NASH, but not significantly in those with simple steatosis (Fig. 1A).
Fig. 1.
Lipoprotein subclass lipid concentration in individuals divided into groups by liver phenotype divided to those with normal liver histology (n = 32), simple steatosis without inflammation and cell injury (n = 19) and to those with NASH (n = 25) (A), steatosis grade (B), lobular inflammation (C), and fibrosis stage (D). Percentage changes comparing to the group without the pathology (set to 0%) have been calculated. Statistical significance over all groups (normal and all degrees of pathology in each panel) are visualized with FDR < 0.05 (*) and FDR < 0.01 (#) compared with individuals without the pathology below the horizontal axis in each panel. The color of the symbol indicates subgroup analysis comparing a given group to the group without pathology (gray indicates P < 0.05, black indicates P < 0.01).
TABLE 2.
Serum lipoprotein subclass data according to liver phenotype
| Normal Liver (n = 32) | Simple Steatosis (n = 19) | NASH (n = 25) | Pa | FDR | |
| Total lipids in (mmol/l) | |||||
| Chylomicrons and extremely large VLDL | 0.025 ± 0.021 | 0.036 ± 0.031 | 0.048 ± 0.026 | 0.0007 | 0.0065 |
| Very large VLDL | 0.069 ± 0.050 | 0.090 ± 0.069 | 0.129 ± 0.061 | 0.0004 | 0.0065 |
| Large VLDL | 0.277 ± 0.153 | 0.330 ± 0.211 | 0.456 ± 0.185 | 0.0017 | 0.0074 |
| Medium VLDL | 0.566 ± 0.198 | 0.597 ± 0.266 | 0.783 ± 0.246 | 0.0022 | 0.0077 |
| Small VLDL | 0.629 ± 0.145 | 0.596 ± 0.159 | 0.778 ± 0.205 | 0.0013 | 0.0065 |
| Very small VLDL | 0.508 ± 0.124 | 0.462 ± 0.096 | 0.549 ± 0.172 | 0.1255 | 0.1515 |
| Total IDL | 1.033 ± 0.255 | 0.936 ± 0.237 | 1.151 ± 0.344 | 0.0651 | 0.0872 |
| Large LDL | 1.206 ± 0.301 | 1.095 ± 0.310 | 1.409 ± 0.416 | 0.0154 | 0.0250 |
| Medium LDL | 0.694 ± 0.181 | 0.638 ± 0.203 | 0.844 ± 0.258 | 0.0095 | 0.0179 |
| Small LDL | 0.457 ± 0.120 | 0.433 ± 0.156 | 0.574 ± 0.184 | 0.0097 | 0.0179 |
| Very large HDL | 0.266 ± 0.135 | 0.267 ± 0.160 | 0.301 ± 0.193 | 0.6399 | 0.6399 |
| Large HDL | 0.463 ± 0.264 | 0.390 ± 0.249 | 0.451 ± 0.300 | 0.3278 | 0.3585 |
| Medium HDL | 0.844 ± 0.175 | 0.821 ± 0.107 | 0.901 ± 0.188 | 0.2808 | 0.3170 |
| Small HDL | 1.110 ± 0.090 | 1.095 ± 0.168 | 1.167 ± 0.128 | 0.0781 | 0.0976 |
| Total cholesterol in (mmol/l) | |||||
| Large VLDL | 0.062 ± 0.035 | 0.071 ± 0.046 | 0.100 ± 0.040 | 0.0011 | 0.0065 |
| Medium VLDL | 0.160 ± 0.053 | 0.159 ± 0.063 | 0.214 ± 0.066 | 0.0028 | 0.0082 |
| Small VLDL | 0.232 ± 0.060 | 0.207 ± 0.054 | 0.277 ± 0.082 | 0.0028 | 0.0082 |
| Total IDL | 0.633 ± 0.156 | 0.567 ± 0.148 | 0.703 ± 0.205 | 0.0440 | 0.0616 |
| Large LDL | 0.797 ± 0.213 | 0.712 ± 0.222 | 0.937 ± 0.293 | 0.0157 | 0.0250 |
| Medium LDL | 0.453 ± 0.131 | 0.406 ± 0.107 | 0.558 ± 0.188 | 0.0096 | 0.0179 |
| Small LDL | 0.283 ± 0.083 | 0.260 ± 0.103 | 0.357 ± 0.127 | 0.0141 | 0.0247 |
| Very large HDL | 0.151 ± 0.080 | 0.161 ± 0.095 | 0.175 ± 0.093 | 0.5106 | 0.5256 |
| Large HDL | 0.217 ± 0.118 | 0.183 ± 0.130 | 0.208 ± 0.162 | 0.2098 | 0.2448 |
| Medium HDL | 0.415 ± 0.094 | 0.401 ± 0.063 | 0.439 ± 0.098 | 0.3646 | 0.3867 |
| Triglycerides in (mmol/l) | |||||
| Chylomicrons and extremely large VLDL | 0.019 ± 0.015 | 0.027 ± 0.022 | 0.035 ± 0.019 | 0.0009 | 0.0065 |
| Very large VLDL | 0.045 ± 0.032 | 0.060 ± 0.044 | 0.084 ± 0.040 | 0.0004 | 0.0065 |
| Large VLDL | 0.163 ± 0.089 | 0.198 ± 0.125 | 0.269 ± 0.111 | 0.0019 | 0.0074 |
| Medium VLDL | 0.292 ± 0.111 | 0.321 ± 0.156 | 0.415 ± 0.139 | 0.0035 | 0.0094 |
| Small VLDL | 0.253 ± 0.067 | 0.255 ± 0.087 | 0.329 ± 0.088 | 0.0012 | 0.0065 |
| Very small VLDL | 0.119 ± 0.028 | 0.112 ± 0.023 | 0.142 ± 0.040 | 0.0095 | 0.0179 |
| Total IDL | 0.120 ± 0.032 | 0.111 ± 0.020 | 0.138 ± 0.041 | 0.0313 | 0.0457 |
| Very large HDL | 0.010 ± 0.004 | 0.009 ± 0.004 | 0.013 ± 0.005 | 0.0079 | 0.0179 |
| Small HDL | 0.042 ± 0.010 | 0.042 ± 0.010 | 0.048 ± 0.011 | 0.0673 | 0.0872 |
| Apolipoproteins (g/l) | |||||
| Apolipoprotein A-I | 1.367 ± 0.200 | 1.311 ± 0.186 | 1.476 ± 0.231 | 0.0179 | 0.0272 |
| Apolipoprotein B | 0.860 ± 0.179 | 0.821 ± 0.189 | 1.023 ± 0.246 | 0.0060 | 0.0150 |
Kruskal-Wallis test. FDR with Benjamini-Hochberg method.
Next, we investigated the association of total lipoprotein lipid concentration with steatosis, inflammation, or fibrosis (detailed results in supplementary Tables V–VII). No significant associations were observed between subclass lipid concentration and steatosis (FDR > 0.1, Fig. 1B), while total lipid concentration in all VLDL, IDL, and LDL subclasses (excluding very small VLDL) was increased by 20–80% in relation to inflammation (Fig. 1C) and grade 1 fibrosis (Fig. 1D). Stage 2–4 fibrosis was characterized with lower lipoprotein lipid concentrations than grade 1 (Fig. 1D). Furthermore, the total lipid concentration of all VLDL and LDL particles associated with the NAFLD activity score [that measures histological injury in NASH combining information about steatosis, inflammation, and liver cell injury (24)] and with ballooning [a histological marker of liver cell injury in NASH (FDR < 0.1, data not shown)]. The total lipid concentration of HDL subclasses was not altered in relation to steatosis or inflammation, but stage 1 fibrosis related to higher HDL lipid concentration (Fig. 1D).
We also evaluated whether the lipid composition (as a percentage of individual lipids from total lipids) would alter in NASH. There were no differences in the lipid composition of any lipoprotein subclass in relation to steatosis and inflammation, and only small changes in relation to fibrosis (supplementary Table VIII).
VLDL and LDL cholesterol associates with liver inflammation independent of steatosis and serum triglycerides
The liver cholesterol accumulation has been associated with NASH (9, 12, 30), and our traditional lipid analysis supported an association between cholesterol metabolism and NASH (supplementary Fig. I). The cholesterol concentration of VLDL (except in small VLDL), IDL, and LDL associated with inflammation and fibrosis (FDR < 0.05; supplementary Fig. II; supplementary Tables V–VII), but not with steatosis. Importantly, in multivariate analysis the association of VLDL and LDL cholesterol concentration with liver inflammation was independent of steatosis and serum total triglycerides (P = 0.024–0.049 for small, medium, and large VLDL and for large and medium LDL subclasses). We also demonstrated that the association of lipoprotein cholesterol concentrations with liver inflammation remained significant when adjusted for fasting insulin (P = 0.012–0.045).
VLDL cholesterol correlates with liver total and free cholesterol content
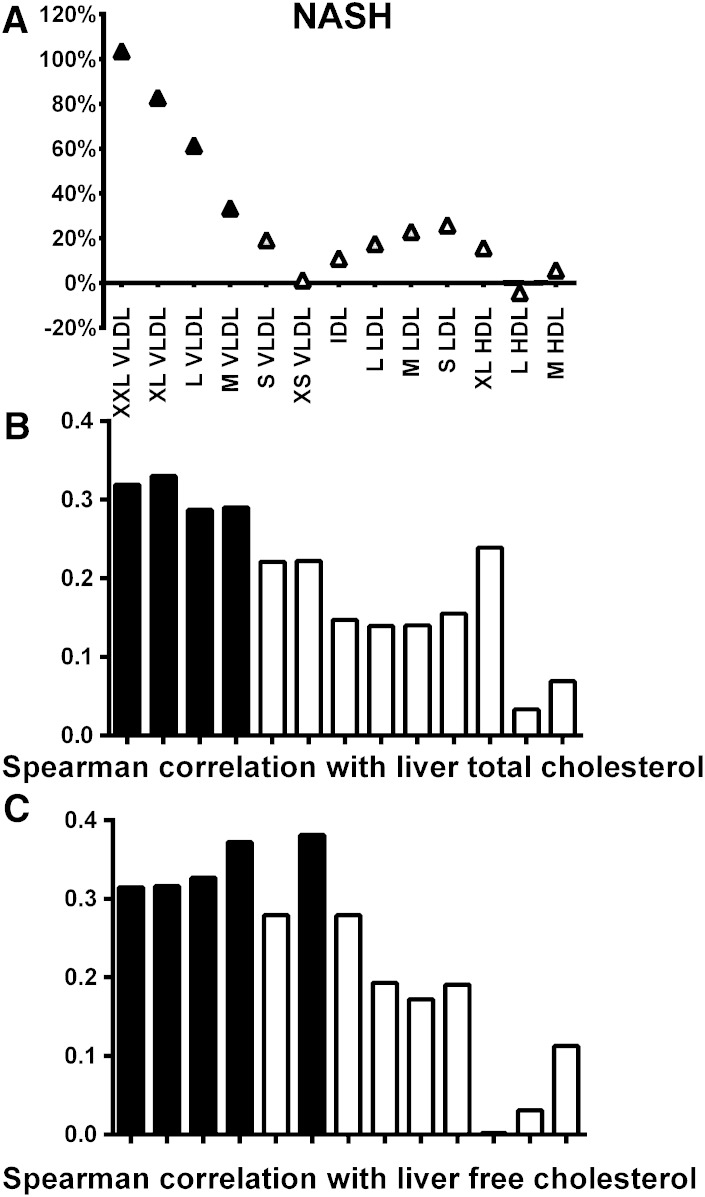
To explain the link between lipoprotein cholesterol metabolism and liver disease, we analyzed the association between lipoprotein cholesterol concentrations and liver cholesterol content (n = 52). Liver cholesterol content measured by GLC correlated with lobular inflammation (rs = 0.393, P = 0.004), as expected, but not significantly with steatosis (rs = 0.258, P = 0.065) and fibrosis (rs = −0.186, P = 0.221). Interestingly, liver cholesterol content also correlated with VLDL cholesterol, but not with LDL and HDL cholesterol (Fig. 2B). To further study the interaction between liver cholesterol metabolism and NASH, we measured liver free cholesterol content with NMR in the available liver samples (n = 45). First, our observation that liver triglyceride content measured with NMR correlated with steatosis determined by histology validates our NMR methodology (rs = 0.772, P = 5.4 × 10−10). Second, free cholesterol content measured with NMR correlated with liver total cholesterol content measured with GLC (rs = 0.419, P = 0.024), but not with steatosis, lobular inflammation, or fibrosis (supplementary Table IX). Similarly to total liver cholesterol, liver free cholesterol correlated with all VLDL subclasses (rs = 0.315–0.381, P < 0.005), except with small VLDL (rs = 0.279, P = 0.063), and not with LDL and HDL cholesterol (Fig. 2C).
Fig. 2.
A: Lipoprotein subclass cholesterol concentration in individuals with NASH compared with those with normal liver histology. Percentage changes comparing to the group without the pathology (set to 0%) have been calculated and statistical significance is shown as in Fig. 1. Spearman correlation between lipoprotein subclass cholesterol concentration and liver cholesterol content (B) and liver free cholesterol content (C) (black bars, P < 0.05).
Because high VLDL cholesterol is linked with increased cholesterol synthesis in the liver, we also explored mRNA expression of genes regulating cholesterol synthesis (CYP51A1, DHCR7, DHCR24, HMGCR, LSS, SC4MOL, SC5D, SREBP1a, SREBP1c, SREBP2, and TM7SF2) and uptake (LDLR) in the liver. Although mRNA expression of some genes regulating synthesis tended to correlate with VLDL and LDL cholesterol subclasses (supplementary Table X), we could not show any association between the expression of cholesterol synthesis genes and steatosis, inflammation, or fibrosis (all P > 0.3, data not shown). Thus, the transcriptional upregulation of the cholesterol synthesis genes does not explain the association between VLDL cholesterol and NASH. Interestingly, we found that X-box binding protein 1 (XBP1) splicing in the liver correlated with lobular inflammation (rs = 0.272, P = 0.014) and ballooning (rs = 0.243, P = 0.029), but not with steatosis (rs = 0.080, P = 0.479). Because XBP1 splicing also associated with cholesterol concentration of small VLDL, IDL, LDL subclasses and medium HDL (rs = 0.220–0.313, P < 0.05), endoplasmic reticulum stress could potentially be a link between cholesterol metabolism and NASH.
Although the PNPLA3 genotype has been associated with liver fat (15, 31), and also tended to associate with steatosis in our study (P = 0.080, χ2 test), there was no association of the PNPLA3 genotype with serum lipids (all P > 0.2, data not shown).
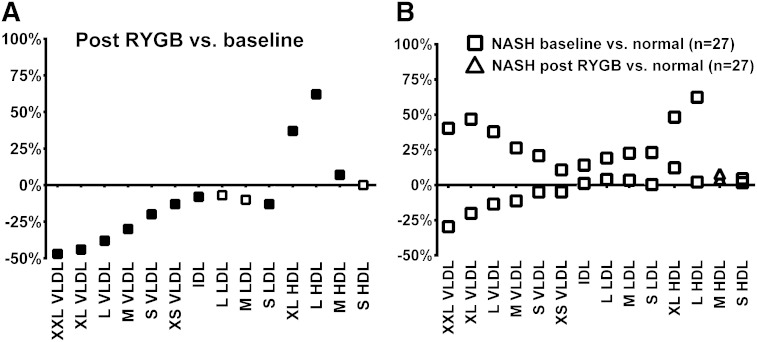
Obesity surgery normalizes NASH-related lipoprotein abnormalities
To determine whether obesity surgery normalizes NASH-related lipoprotein abnormalities, we analyzed serum NMR results before and after RYGB. The effect of RYGB on total lipid concentration of different lipoprotein subclasses is shown in Fig. 3A (more detailed in supplementary Table XI). The VLDL and LDL lipid accumulations that were associated with NASH (Fig. 3B) decreased significantly after surgery. In contrast, HDL lipid concentration that was not associated with simple steatosis or NASH clearly increased with weight loss. To investigate whether obesity surgery corrects NASH-related dyslipidemia, we divided patients not using cholesterol lowering medication to those without NASH (n = 27) at baseline and to those with possible/definite NASH (n = 20, for definition see Materials and Methods) at baseline. The increases in VLDL, IDL, and LDL cholesterol and serum triglycerides in individuals with NASH normalized after surgery to levels comparable with the levels in individuals without NASH (all changes P < 0.05) (Table 3).
Fig. 3.
Changes in lipoprotein subclass lipid concentrations (A) in response to RYGB in the whole study group compared with baseline. B: Shows the effect of RYBG specifically in individuals with NASH by showing the difference between the NASH group and the normal group at baseline (squares) and the NASH group after RYGB compared with the normal group at baseline (triangles). The color of the symbol indicates subgroup analysis comparing a given group to the group without pathology (A) or to baseline (B) (gray indicates P < 0.05, black indicates P < 0.01).
TABLE 3.
Effect of obesity surgery on NASH-related alterations in cholesterol concentration of different lipoproteins and serum total triglycerides in patients not using cholesterol lowering medication
| Not NASH (n = 27) | Possible/Definite NASH (n = 20) | Pb | |
| Total cholesterol | |||
| Before | 4.27 ± 0.9 | 4.98 ± 0.9 | 0.009 |
| After | 4.48 ± 1.0 | 4.43 ± 0.8 | 0.914 |
| Change | 4.9% | −11.0% | 0.008 |
| P value for changea | 0.189 | 0.016 | |
| VLDL cholesterol | |||
| Before | 0.76 ± 0.2 | 0.82 ± 0.2 | 0.237 |
| After | 0.69 ± 0.3 | 0.63 ± 0.2 | 0.813 |
| Change | −9.2% | −23.2% | 0.169 |
| P value for changea | 0.073 | 3.0 × 10−5 | |
| IDL cholesterol | |||
| Before | 0.66 ± 0.2 | 0.78 ± 0.2 | 0.023 |
| After | 0.67 ± 0.2 | 0.64 ± 0.2 | 0.621 |
| Change | 1.5% | −17.9% | 0.013 |
| P value for changea | 0.709 | 0.003 | |
| LDL cholesterol | |||
| Before | 1.67 ± 0.5 | 2.04 ± 0.6 | 0.019 |
| After | 1.65 ± 0.6 | 1.57 ± 0.5 | 0.949 |
| Change | −1.2% | −23.0% | 0.022 |
| P value for changea | 0.752 | 0.001 | |
| HDL cholesterol | |||
| Before | 1.18 ± 0.2 | 1.34 ± 0.3 | 0.037 |
| After | 1.48 ± 0.3 | 1.60 ± 0.3 | 0.085 |
| Change | 25.4% | 19.4% | 0.111 |
| P value for changea | 3.0 × 10−6 | 0.0002 | |
| Triglycerides | |||
| Before | 1.60 ± 0.5 | 1.75 ± 0.5 | 0.143 |
| After | 1.25 ± 0.6 | 1.16 ± 0.4 | 0.966 |
| Change | −21.9% | −33.7% | 0.288 |
| P value for changea | 0.002 | 2.1 × 10−5 |
Paired samples t-test for within subjects comparison.
Kruskall-Wallis independent samples test for between groups comparison.
DISCUSSION
Our study was set to assess whether lipoprotein subclass metabolism is linked with NAFLD or NASH. High serum VLDL and LDL lipid concentration related to liver inflammation and fibrosis more than to steatosis (Fig. 1). More specifically, we explored the association of cholesterol metabolism with liver injury in NASH (9, 12, 32). Both VLDL and LDL cholesterol concentrations associated with liver inflammation and fibrosis (Fig. 2), and VLDL subclass cholesterol concentrations associated also with liver cholesterol content. This association was independent of steatosis and serum triglycerides (Fig. 2 and multivariate analysis) suggesting an independent link between VLDL and LDL cholesterol and NASH.
A key finding in this study was that high serum VLDL and LDL lipid concentration related to lobular inflammation in the liver independent of steatosis (Table 2, Fig. 1C, and multivariate analysis). We also found a link between VLDL and LDL lipid concentration and cell injury in NASH, quantified by the NAFLD score and ballooning (24, 33). These data give a more detailed view on lipoprotein subclass metabolism in NASH than earlier findings demonstrating increased VLDL synthesis in steatosis (4, 8), leading to the suggestion that high serum VLDL and LDL lipid concentrations are also related to the progression from simple steatosis to NASH. This is in line with previous findings suggesting that nonHDL cholesterol (VLDL + LDL) is a biomarker for NASH (34).
One potential link between the VLDL and LDL lipid contents and NASH is altered cholesterol metabolism in the liver. Accumulation of liver cholesterol has been suggested to be important in the pathophysiology of NASH (9). Cellular free cholesterol, but not free fatty acids or triglycerides, sensitizes to TNF- and Fas-induced steatohepatitis (35). The possible explanation is that free cholesterol is cytotoxic and can activate both Fas-independent and Fas-triggering mitochondrial dysfunction in macrophages (36). Thus, it has been proposed that hepatic accumulation of free cholesterol results in cytotoxicity which mediates transition from steatosis to NASH (37). It has recently been suggested that the cause for cholesterol accumulation in NASH is increased cholesterol synthesis (30). Our gene expression findings in the liver support the suggestion that endoplasmic reticulum stress may be a link between cholesterol metabolism and NASH. We observed that altered splicing of XBP1 related to both NASH and to changes in cholesterol metabolism linked with NASH. Earlier, the IREα/XBP1 pathway has been suggested to regulate hepatic lipid homeostasis and its dysregulation has been associated with NASH (38).
Although both VLDL and LDL cholesterol associated with inflammation and fibrosis, only VLDL cholesterol correlated significantly with the liver cholesterol content in our study (Fig. 2B, C). This suggests that increased synthesis of VLDL particles, rather than decreased release of VLDL (39), is the key finding in NASH. Although we could not link liver cholesterol accumulation, or increased VLDL cholesterol concentrations, to increased expression of genes regulating cholesterol metabolism, as recently suggested (30, 40), our results support the view that changes in lipoprotein subclass metabolism in NASH relate to inflammation and cell injury in NASH (8, 9, 30, 40–42).
Importantly, all NASH-related lipoprotein abnormalities were abolished by obesity surgery (Fig. 3). The decrease in serum LDL cholesterol and total triglycerides, and the increase HDL cholesterol, in response to RYGB has been well-characterized (43). In this study we demonstrate that the lipoprotein abnormalities related to NASH can be corrected with RYGB, also at a subclass level. We assume that this is related to the amelioration of NASH. Unfortunately, we had only 11 liver biopsies taken at 1 year follow-up. As expected (44–46), they consistently showed improved histology (data not shown).
Although the study population was limited to 116 patients, the detailed histological characterization is more extensive than in most previous studies (9, 11, 12, 30). In addition, the analysis of serum concentrations of lipoprotein subclasses, as opposed to using only standard enzymatic methods, was a strength in our study. For example, we could not identify the expected association between serum total triglycerides and NASH (Table 1), but could clearly demonstrate that triglyceride levels of all VLDL subclasses had association with NASH diagnosis (Table 2). Because of ethical difficulties to obtain liver biopsies in healthy lean individuals, we only had biopsies from obese individuals. On the other hand, by studying obese individuals, we increased the possibility to find subjects with NASH.
We conclude that VLDL and LDL subclass lipid concentrations are associated with lobular inflammation and liver cell injury in NASH. More specifically, our results support an independent link between VLDL cholesterol, liver cholesterol, and NASH. Finally, we demonstrated that lipoprotein subclass abnormalities in NASH can be reversed with obesity surgery.
Supplementary Material
Acknowledgments
The authors thank Päivi Turunen, Tiina Sistonen, and Matti Laitinen for their careful work in patient recruitment and laboratory analyses.
Footnotes
Abbreviations:
- CYP51A1
- cytochrome P450 family 51 subfamily A polypeptide 1
- DHCR7
- 7-dehydrocholesterol reductase
- DHCR24
- 24-dehydrocholesterol reductase
- FDR
- false discovery rate
- GLC
- gas-liquid chromatography
- HMGCR
- HMG-CoA reductase
- LDLR
- LDL receptor
- LSS
- lanosterol synthase
- NAFLD
- nonalcoholic fatty liver disease
- NASH
- nonalcoholic steatohepatitis
- PNPLA3
- patatin-like phospholipase domain containing 3
- RYGB
- Roux-en-Y gastric bypass
- SC4MOL
- methylsterol monooxygenase
- SC5D
- sterol-C5-desaturase
- SREBP1a
- sterol regulatory element binding transcription factor 1a
- SREBP1c
- sterol regulatory element binding transcription factor 1c
- SREBP2
- sterol regulatory element binding transcription factor 2
- TM7SF2
- transmembrane 7 superfamily member 2
- XBP1
- X-box binding protein 1
This study was supported by the Finnish Diabetes Research Foundation (J.P.), Academy of Finland Clinical Researcher Fellowship Grants (120979 2008-2010 and 138006 2011-2013) (J.P.), the Academy of Finland (P.S.), TEKES-the Finnish Funding Agency for Technology and Innovation (M.A-K.), the Sigrid Juselius Foundation (M.A-K.), and Strategic Research Funding from the University of Oulu (M.A-K.). A.J.K., P.S., and M.A-K. are shareholders of Brainshake Ltd., a startup company offering NMR-based metabolite profiling.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two figures and eleven tables.
REFERENCES
- 1.Musso G., Gambino R., Cassader M. 2010. Non-alcoholic fatty liver disease from pathogenesis to management: an update. Obes. Rev. 11: 430–445. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G., Baranova A., Younossi Z. M. 2011. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 34: 274–285. [DOI] [PubMed] [Google Scholar]
- 3.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adiels M., Taskinen M. R., Packard C., Caslake M. J., Soro-Paavonen A., Westerbacka J., Vehkavaara S., Hakkinen A., Olofsson S. O., Yki-Jarvinen H., et al. 2006. Overproduction of large VLDL particles is driven by increased liver fat content in man. Diabetologia. 49: 755–765. [DOI] [PubMed] [Google Scholar]
- 5.Kotronen A., Yki-Järvinen H. 2008. Fatty liver: a novel component of the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 28: 27–38. [DOI] [PubMed] [Google Scholar]
- 6.Ekstedt M., Franzen L. E., Mathiesen U. L., Thorelius L., Holmqvist M., Bodemar G., Kechagias S. 2006. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 44: 865–873. [DOI] [PubMed] [Google Scholar]
- 7.Fabbrini E., Mohammed B. S., Magkos F., Korenblat K. M., Patterson B. W., Klein S. 2008. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 134: 424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simonen P., Kotronen A., Hallikainen M., Sevastianova K., Makkonen J., Hakkarainen A., Lundbom N., Miettinen T. A., Gylling H., Yki-Järvinen H. 2011. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J. Hepatol. 54: 153–159. [DOI] [PubMed] [Google Scholar]
- 9.Puri P., Baillie R. A., Wiest M. M., Mirshahi F., Choudhury J., Cheung O., Sargeant C., Contos M. J., Sanyal A. J. 2007. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 46: 1081–1090. [DOI] [PubMed] [Google Scholar]
- 10.Kotronen A., Westerbacka J., Bergholm R., Pietiläinen K. H., Yki-Järvinen H. 2007. Liver fat in the metabolic syndrome. J. Clin. Endocrinol. Metab. 92: 3490–3497. [DOI] [PubMed] [Google Scholar]
- 11.Alkhouri N., Tamimi T. A., Yerian L., Lopez R., Zein N. N., Feldstein A. E. 2010. The inflamed liver and atherosclerosis: a link between histologic severity of nonalcoholic fatty liver disease and increased cardiovascular risk. Dig. Dis. Sci. 55: 2644–2650. [DOI] [PubMed] [Google Scholar]
- 12.Koruk M., Savas M. C., Yilmaz O., Taysi S., Karakok M., Gundogdu C., Yilmaz A. 2003. Serum lipids, lipoproteins and apolipoproteins levels in patients with nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 37: 177–182. [DOI] [PubMed] [Google Scholar]
- 13.Soininen P., Kangas A. J., Wurtz P., Tukiainen T., Tynkkynen T., Laatikainen R., Järvelin M. R., Kähönen M., Lehtimäki T., Viikari J., et al. 2009. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst. 134: 1781–1785. [DOI] [PubMed] [Google Scholar]
- 14.Pihlajamäki J., Grönlund S., Simonen M., Käkelä P., Moilanen L., Pääkkönen M., Pirinen E., Kolehmainen M., Kärjä V., Kainulainen S., et al. 2010. Cholesterol absorption decreases after Roux-en-Y gastric bypass but not after gastric banding. Metabolism. 59: 866–872. [DOI] [PubMed] [Google Scholar]
- 15.Pihlajamäki J., Kuulasmaa T., Kaminska D., Simonen M., Kärjä V., Grönlund S., Käkelä P., Pääkkönen M., Kainulainen S., Punnonen K., et al. 2012. Serum interleukin 1 receptor antagonist as an independent marker of nonalcoholic steatohepatitis in humans. Int. J. Hepatol. 56: 663–670. [DOI] [PubMed] [Google Scholar]
- 16.Alberti K. G., Zimmet P. Z. 1998. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 15: 539–553. [DOI] [PubMed] [Google Scholar]
- 17.Ala-Korpela M. 2008. Critical evaluation of 1H NMR metabonomics of serum as a methodology for disease risk assessment and diagnostics. Clin. Chem. Lab. Med. 46: 27–42. [DOI] [PubMed] [Google Scholar]
- 18.Ala-Korpela M., Soininen P., Savolainen M. J. 2009. Letter by Ala-Korpela et al. regarding article, “Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women”. Circulation. 120: e149. [DOI] [PubMed] [Google Scholar]
- 19.Inouye M., Kettunen J., Soininen P., Silander K., Ripatti S., Kumpula L. S., Hämäläinen E., Jousilahti P., Kangas A. J., Männistö S., et al. 2010. Metabonomic, transcriptomic, and genomic variation of a population cohort. Mol. Syst. Biol. 6: 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tukiainen T., Kettunen J., Kangas A. J., Lyytikäinen L. P., Soininen P., Sarin A. P., Tikkanen E., O’Reilly P. F., Savolainen M. J., Kaski K., et al. 2012. Detailed metabolic and genetic characterization reveals new associations for 30 known lipid loci. Hum. Mol. Genet. 21: 1444–1455. [DOI] [PubMed] [Google Scholar]
- 21.Chambers J. C., Zhang W., Sehmi J., Li X., Wass M. N., Van der Harst P., Holm H., Sanna S., Kavousi M., Baumeister S. E., et al. 2011. Genome-wide association study identifies loci influencing concentrations of liver enzymes in plasma. Nat. Genet. 43: 1131–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Würtz P., Raiko J. R., Magnussen C. G., Soininen P., Kangas A. J., Tynkkynen T., Thomson R., Laatikainen R., Savolainen M. J., Laurikka J., et al. 2012. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur. Heart J. 33: 2307–2316. [DOI] [PubMed] [Google Scholar]
- 23.Brunt E. M., Janney C. G., Di Bisceglie A. M., Neuschwander-Tetri B. A., Bacon B. R. 1999. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 94: 2467–2474. [DOI] [PubMed] [Google Scholar]
- 24.Kleiner D. E., Brunt E. M., Van Natta M., Behling C., Contos M. J., Cummings O. W., Ferrell L. D., Liu Y. C., Torbenson M. S., Unalp-Arida A., et al. ; Nonalcoholic Steatohepatitis Clinical Research Network. 2005. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 25.Miettinen T. A. 1988. Cholesterol metabolism during ketoconazole treatment in man. J. Lipid Res. 29: 43–51. [PubMed] [Google Scholar]
- 26.Tukiainen T., Tynkkynen T., Makinen V. P., Jylanki P., Kangas A., Hokkanen J., Vehtari A., Grohn O., Hallikainen M., Soininen H., et al. 2008. A multi-metabolite analysis of serum by 1H NMR spectroscopy: early systemic signs of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 375: 356–361. [DOI] [PubMed] [Google Scholar]
- 27.Soininen P., Haarala J., Vepsäläinen J., Niemitz M., Laatikainen R. 2005. Strategies for organic impurity quantification by 1H NMR spectroscopy: constrained total-line-shape fitting. Anal. Chim. Acta. 542: 178–185. [Google Scholar]
- 28.Romeo S., Kozlitina J., Xing C., Pertsemlidis A., Cox D., Pennacchio L. A., Boerwinkle E., Cohen J. C., Hobbs H. H. 2008. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 40: 1461–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benjamini Y., Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 57: 289–300. [Google Scholar]
- 30.Min H. K., Kapoor A., Fuchs M., Mirshahi F., Zhou H., Maher J., Kellum J., Warnick R., Contos M. J., Sanyal A. J. 2012. Increased hepatic synthesis and dysregulation of cholesterol metabolism is associated with the severity of nonalcoholic fatty liver disease. Cell Metab. 15: 665–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speliotes E. K., Butler J. L., Palmer C. D., Voight B. F.; GIANT Consortium; MIGen Consortium; NASH CRN; and J. N. Hirschhorn. 2010. PNPLA3 variants specifically confer increased risk for histologic nonalcoholic fatty liver disease but not metabolic disease. Hepatology. 52: 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fon Tacer K., Rozman D. 2011. Nonalcoholic fatty liver disease: focus on lipoprotein and lipid deregulation. J. Lipids. 2011: 783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCullough A. J. 2011. Epidemiology of the metabolic syndrome in the USA. J. Dig. Dis. 12: 333–340. [DOI] [PubMed] [Google Scholar]
- 34.Corey K. E., Lai M., Gelrud L. G., Misdraji J., Barlow L. L., Zheng H., Andersson K. L., Thiim M., Pratt D. S., Chung R. T. 2012. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 10: 651–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marí M., Caballero F., Colell A., Morales A., Caballeria J., Fernandez A., Enrich C., Fernandez-Checa J. C., Garcia-Ruiz C. 2006. Mitochondrial free cholesterol loading sensitizes to TNF- and Fas-mediated steatohepatitis. Cell Metab. 4: 185–198. [DOI] [PubMed] [Google Scholar]
- 36.Yao P. M., Tabas I. 2001. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J. Biol. Chem. 276: 42468–42476. [DOI] [PubMed] [Google Scholar]
- 37.Van Rooyen D. M., Larter C. Z., Haigh W. G., Yeh M. M., Ioannou G., Kuver R., Lee S. P., Teoh N. C., Farrell G. C. 2011. Hepatic free cholesterol accumulates in obese, diabetic mice and causes nonalcoholic steatohepatitis. Gastroenterology. 141: 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henkel A., Green R. M. 2013. The unfolded protein response in fatty liver disease. Semin. Liver Dis. 33: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujita K., Nozaki Y., Wada K., Yoneda M., Fujimoto Y., Fujitake M., Endo H., Takahashi H., Inamori M., Kobayashi N., et al. 2009. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 50: 772–780. [DOI] [PubMed] [Google Scholar]
- 40.Caballero F., Fernandez A., De Lacy A. M., Fernandez-Checa J. C., Caballeria J., Garcia-Ruiz C. 2009. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J. Hepatol. 50: 789–796. [DOI] [PubMed] [Google Scholar]
- 41.Ordovas-Montanes J. M., Ordovas J. M. 2012. Cholesterol, inflammasomes, and atherogenesis. Curr. Cardiovasc. Risk Rep. 6: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Im S. S., Yousef L., Blaschitz C., Liu J. Z., Edwards R. A., Young S. G., Raffatellu M., Osborne T. F. 2011. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 13: 540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tice J. A., Karliner L., Walsh J., Petersen A. J., Feldman M. D. 2008. Gastric banding or bypass? A systematic review comparing the two most popular bariatric procedures. Am. J. Med. 121: 885–893. [DOI] [PubMed] [Google Scholar]
- 44.de Almeida S. R., Rocha P. R., Sanches M. D., Leite V. H., da Silva R. A., Diniz M. T., Diniz Mde F., Rocha A. L. 2006. Roux-en-Y gastric bypass improves the nonalcoholic steatohepatitis (NASH) of morbid obesity. Obes. Surg. 16: 270–278. [DOI] [PubMed] [Google Scholar]
- 45.Clark J. M., Alkhuraishi A. R., Solga S. F., Alli P., Diehl A. M., Magnuson T. H. 2005. Roux-en-Y gastric bypass improves liver histology in patients with non-alcoholic fatty liver disease. Obes. Res. 13: 1180–1186. [DOI] [PubMed] [Google Scholar]
- 46.Barker K. B., Palekar N. A., Bowers S. P., Goldberg J. E., Pulcini J. P., Harrison S. A. 2006. Non-alcoholic steatohepatitis: effect of Roux-en-Y gastric bypass surgery. Am. J. Gastroenterol. 101: 368–373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.