Abstract
Within recent years, ganglioside patterns have been increasingly analyzed by MS. However, internal standards for calibration are only available for gangliosides GM1, GM2, and GM3. For this reason, we prepared homologous internal standards bearing nonnatural fatty acids of the major mammalian brain gangliosides GM1, GD1a, GD1b, GT1b, and GQ1b, and of the tumor-associated gangliosides GM2 and GD2. The fatty acid moieties were incorporated after selective chemical or enzymatic deacylation of bovine brain gangliosides. For modification of the sphingoid bases, we developed a new synthetic method based on olefin cross metathesis. This method was used for the preparation of a lyso-GM1 and a lyso-GM2 standard. The total yield of this method was 8.7% for the synthesis of d17:1-lyso-GM1 from d20:1/18:0-GM1 in four steps. The title compounds are currently used as calibration substances for MS quantification and are also suitable for functional studies.
Keywords: brain lipids, chemical synthesis, glycolipids, lipidomics, mass spectrometry, sphingolipids, olefin metathesis
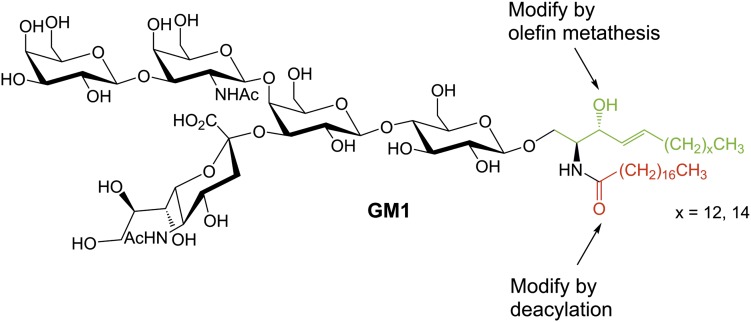
Gangliosides are sialic acid-containing glycosphingolipids (GSLs). They can be found in vertebrates and, with a few exceptions, not in invertebrates (1). Gangliosides are especially abundant in neuronal tissues, where their content is one to two orders of magnitude higher than in extraneural tissues (2). In the brain, gangliosides, together with other GSLs, are the main glycan carriers (80% of the total glycan mass in adult rat brain) (3). The main gangliosides in adult mammalian brain are GM1 (Fig. 1), GD1a, GD1b, GT1b, 9-O-Ac-GT1b, and GQ1b (4). They contain mostly C18- and C20-sphingosine acylated with stearic acid, which constitutes more than 80% of the total ganglioside fatty acid content in the nervous system (4). In mammals, C20-sphingsosine-containing gangliosides can only be found in significant amounts in the nervous system (5). In contrast to adult brain, different GSL and ganglioside series are expressed in the developing nervous system (6).
Fig. 1.
Possible concepts for the synthesis of internal ganglioside standards.
Subcellularly, the majority of gangliosides are localized in the outer leaflet of the plasma membrane. They are anchored to the membrane by their hydrophobic ceramide part, while their hydrophilic carbohydrate part extrudes into the extracellular space. They are part of the cellular glycocalyx and interact with molecules outside the cell (“trans”-interaction) and within the same membrane (“cis”-interaction) (1). Examples are the trans-interaction of ganglioside GM1 with cholera toxin (7) and of NeuAcα2-3Galβ1-3GalNAc termini on axonal gangliosides such as GD1a, GT1b, and GM1b with the myelin-associated glycoprotein (8), and the cis-interaction of GM3 with the receptors for insulin (9), epidermal growth factor (10), or vascular endothelial growth factor (11).
In the past, ganglioside patterns have been largely determined by TLC followed by densitometric quantification (12, 13). Within recent years, ganglioside determinations by mass spectrometric methods have been increasingly applied (14). In addition to ganglioside classes, which are defined by the carbohydrate head group, MS also allows profiling of ganglioside lipoforms (15) with different structure of acyl chain and sphingoid base. Ganglioside quantification is applied to ganglioside pattern investigation (16) in food analyses (17, 18), but is also used in the analysis of lysosomal storage diseases (19) and in quantitative imaging MS (20). Lysogangliosides play a role in the pathogenesis of gangliosidoses. Elevated levels of lyso-GM2 and lyso-GA2 are present in the brains of patients with GM2 gangliosidoses (21), and elevated levels of lyso-GM1 and lyso-GA1 are present in patients with GM1 gangliosidosis (22). Both substances are potential biomarkers for these diseases (23), and the lysoganglioside standards prepared in this work can be used for their quantitative analysis.
Quantification of gangliosides by MS, including quantitative imaging MS, requires calibration substances. Hereby, calibration is defined as the establishment of a correlation between analyte concentration and mass spectrometer response. Three principle methods can be distinguished: external calibration, standard addition calibration, and internal standard calibration. The internal standard calibration shows the highest accuracy and precision because standard and analyte are measured in the same sample at the same time, and the internal standard acts as a self-correcting system for analyte losses during purification steps and for fluctuations in mass spectrometer response (24). Internal standards are chemically and physically similar, but nonisobaric derivatives of the analyte. Stable isotope-labeled derivatives (type 2 internal standards) or homologous derivatives (type 3) of the analyte are suitable. Type 1 internal standards, which are any nonisobaric compounds with similar physical and chemical properties, are less accurate. Usually, the standard is added in a defined amount to the sample at an early stage of sample handling. After purification of the sample, the ratio of the MS response of analyte and standard is measured. The concentration of the analyte can be calculated by the following equation (24): can = (Ian/IIS) × cis = CFIS × Ian where I is the intensity, an is the analyte, IS is the internal standard, c is the concentration, and CFIS is the calibration factor.
We report on the preparation of a set of new ganglioside internal standards for the major mammalian brain gangliosides and the tumor-associated gangliosides, GM2 and GD2. The title substances were prepared from a mixture of gangliosides from bovine brain, which was separated first into ganglioside classes and then into pure lipoforms. Nonnatural chain lengths in the fatty acid part were introduced by selective chemical and enzymatic deacylation and reacylation. Furthermore, we developed a new method for the modification of the sphingosine chain length by olefin cross metathesis (Fig. 1). The method was applied for the synthesis of a lyso-GM1 and a lyso-GM2 standard.
MATERIALS AND METHODS
Materials
All chemicals were of analytical grade or the highest purity available. Water (H2O) used for buffers and solutions was purified by an ultrapure H2O system (EASYpure UV/UF D8612, Werner Reinstwassersysteme, Leverkusen, Germany). Solvents used in reactions of oxygen- and moisture-sensitive compounds were in anhydrous form. 1-Propanol, methanol (MeOH), pyridine, and acetic anhydride were degassed before use. The native mixture of bovine brain gangliosides, Cronassial®, which consists of 21% GM1, 40% GD1a, 16% GD1b, 19% GT1b, and 4% other gangliosides (25), was available in our lab. Sphingolipid ceramide N-deacylase (SCDase) and β-galactosidase from bovine testes were from Sigma-Aldrich (Schnelldorf, Germany). Triton™ X-100, sodium taurodeoxycholate, Grubbs catalyst 2nd generation, Hoveyda-Grubbs catalyst 2nd generation, and Stewart-Grubbs catalyst were also from Sigma-Aldrich. For normal phase (NP) column chromatography, silica gel 60 (0.040–0.063 mm or 0.015–0.040 mm) from Merck (Darmstadt, Germany) was used. For desalting and reversed phase (RP) column chromatography, LiChroprep® RP-18 (0.040–0.063 mm) from Merck was used. For TLC analysis, high-performance TLC (HPTLC) silica gel 60 F254 and HPTLC silica gel 60 RP-18 plates from Merck were used. For anion-exchange chromatography, DEAE Sephadex™ A-25 from Amersham Pharmacia Biotech AB (Uppsala, Sweden) was used.
Purification
Desalting was performed by the method of Wiliams and McCluer (26). The usage of polar eluents for column chromatography causes significant contamination of column material in the products. We reduced this contamination to a minimum by the following method. NP silica gel columns of a very small column volume (approximately 1 ml column volume per 5 mg of product) were prepared in filtration columns from Supelco (Bellefonte, PA) using a mobile phase in which the substance is retained on the column. The substance was applied to the column and washed with three column volumes of the same solvent. Then, the substance was eluted by an appropriate solvent of higher polarity.
Analytical procedures
For 1H- and 13C-NMR spectroscopy, Bruker Avance 300, 400, or 500 instruments (Billerica, MA) were used. For exchange of exchangeable protons by deuterium, gangliosides were dissolved in CD3OD and the solvent was removed in a stream of nitrogen. This process was repeated three times before measurement. For EI-MS, a MAT 95 XL sector field instrument (Thermo Finnigan MAT GmbH, Bremen, Germany) or a MAT 90 sector field instrument (Thermo Finnigan MAT GmbH) was used. For ESI-MS and ESI-MS/MS, a nano-ESI-QTOF mass spectrometer (Micromass, Manchester, UK) was used. In general, measurements were conducted in positive ion mode. For interpretation of the ESI-MS/MS spectra, the nomenclature for carbohydrate fragments by Domon and Costello (27) was applied. For GC-MS, a GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan) equipped with a ZB-5MSi column [l = 30 m, Ø = 0.25 mm, df (film thickness) = 0.25 μm] containing a 5% phenyl and 95% dimethylpolysiloxane phase was used. For carbon-hydrogen-nitrogen (CHN) microanalysis, a vario EL cube (Elementar Analysensysteme GmbH, Hanau, Germany) was used.
In general, ganglioside yields were determined by dissolving the ganglioside in a defined volume of MeOH or H2O and subsequent sialic acid determination. Ganglioside lipoforms, which were quantified by CHN analysis before, were used as external standards for the quantification of the synthesized ganglioside derivatives. Photometric sialic acid determination was performed by the resorcinol method of Svennerholm (28) modified by Mietinnen and Takkiluukkainen (29) using an Ultrospec III (Pharmacia Biotech, Uppsala, Sweden). Fluorimetric sialic acid determination was performed using the sialic acid assay kit EnzyChrom™ ESLA-100 of BioAssay Systems (Hayward, CA) and a Fluoroscan II (Labsystems Oy, Helsinki, Finland).
Purification of bovine brain gangliosides
To obtain pure natural ganglioside lipoforms as starting material, the ganglioside mixture Cronassial® was purified by anion-exchange chromatography, followed by desalting, NP column chromatography, and RP column chromatography. In the first step, a slightly modified method of Momoi, Ando, and Magai (30) was used. Briefly, 5.03 g of ganglioside mixture were applied to a DEAE Sephadex™ A-25 column (2.2 × 22 cm) and ganglioside classes were eluted by a step gradient of different ammonium acetate concentrations in MeOH. After desalting, the ganglioside classes were obtained as colorless solids. GQ1b was already obtained in pure form after these steps. From 5.03 g of starting material, 1.28 g of monosialogangliosides, 2.80 g of disialogangliosides, 0.674 g of trisialogangliosides, and 41.5 mg of GQ1b were obtained. Then, proportions of these ganglioside classes were separated by NP column chromatography. The monosialogangliosides were separated isocratically using the solvent system CHCl3/MeOH/0.2% CaCl2 (60:35:8, v/v/v) to obtain GM1. The disialogangliosides were separated by a step gradient of CHCl3/MeOH/2.5 M NH3 (60:35:6, v/v/v) and CHCl3/MeOH/2.5 M NH3 (60:35:8, v/v/v) to obtain GD1a and GD1b, respectively. Trisialogangliosides were separated isocratically using the solvent system CHCl3/MeOH/H2O (50:42:11, v/v/v) to obtain GT1b. Proportions of these five gangliosides were separated into their d18:1/18:0 and d20:1/18:0 lipoforms by RP column chromatography using the solvent system MeOH/H2O in appropriate compositions [90:25 (v/v) for GM1, 90:49 (v/v) for GD1a, 90:40 (v/v) for GD1b, 90:40 (v/v) for GT1b, and 90:50 (v/v) for GQ1b]. The obtained ganglioside lipoforms were analyzed by ESI-MS, ESI-MS/MS, 1H-NMR, and CHN analysis (Table 1).
TABLE 1.
Pure ganglioside lipoforms that were obtained by purification of a bovine brain gangliosides mixture
| Ganglioside | Isolated Amount (mg) | Purity (%) |
| d18:1/18:0-GM1·NH3 (1) | 64.8 | 88.5 |
| d20:1/18:0-GM1·NH3 (2) | 100 | 94.8 |
| d18:1/18:0-GD1a·2NH3 (3) | 89.5 | 90.4 |
| d20:1/18:0-GD1b·2NH3 (4) | 19.1 | 90.1 |
| d20:1/18:0-GT1b·3NH3 (5) | 40.4 | 86.9 |
| d20:1/18:0-GQ1b·4NH3 (6) | 18.8 | 85.7 |
The results of the CHN analysis are given in column 3. The gangliosides were not 100% pure due to residual H2O content. Compound 2 was dried in vacuum while the other compounds were dried by lyophilization.
Chemical preparation of lysogangliosides
The d18:1-lyso-GM1·NH32 (7) was prepared from d18:1/18:0-GM1·NH3 (1) by the method of Sonnino et al. (31). All steps were performed under an argon atmosphere. Twenty milligrams (12.8 μmol) of 1 were dissolved in 9.2 ml of dry propanol. Then, 2.3 ml of a 1 M potassium hydroxide solution in the same solvent were added and the reaction mixture was heated to 90°C for 20 h. After cooling to room temperature, the reaction mixture was brought to pH 9 by adding an aqueous ammonium chloride buffer. The solvent was removed in a stream of nitrogen, the crude product was dried in vacuum and subsequently purified by RP column chromatography [1.5 × 3.5 cm, MeOH/H2O (90:40, v/v)]. A second purification step was performed by NP column chromatography [2.0 × 22 cm, CHCl3/MeOH/2.5 M NH3 (60:40:9, v/v/v)]. After lyophilization, 7 was obtained as a colorless powder (8.69 mg, 52%), ESI-MS: m/z 1,280.62 [M + H]+, m/z 651.80 [M + Na + H]2+, m/z 640.81 [M + 2H]2+, Rf = 0.12 [CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. The method was also applied for the preparation of d18:1-lyso-GD1a·2NH3 (8) (1.23 mg, 4.8%) from d18:1/18:0-GD1a·2NH3 (3), ESI-MS: 1,571.75 [M + H]+, 797.36 [M + Na + H]2+, 794.88 [M + NH4 + H]2+, 786.37 [M + 2H]2+, Rf = 0.24 [CHCl3/MeOH/2.5 M NH3 (60:44:12, v/v/v)].
Enzymatic preparation of lysogangliosides
The enzymatic preparation of lysogangliosides was performed by reported methods (32, 33). Triton™ X-100 was used instead of sodium cholate because we observed higher conversions. d18:1/18:0-GD1a·2NH3 (3) [0.919 mg (491 nmol)] was dissolved in 500 μl of 50 mM sodium acetate buffer (pH 6.0), which contained 0.8% of Triton™ X-100. The mixture was exposed to an ultrasonic bath for 15 s. Then, 3.6 μl (18 mU) of a SCDase solution (in 50 mM sodium acetate buffer, pH 6.0) were added, the aqueous phase was covered with 5 ml of n-decane to remove the fatty acid from the equilibrium, and the mixture was incubated at 37°C. Every 2 h, the n-decane phase was replaced. To obtain a complete conversion of the educt, an additional 36 mU of enzyme were added over a period of 2 days. The reaction was stopped by the addition of a few drops of 2.5 M ammonia. The solvent was removed in a stream of nitrogen, the crude product was dried in vacuum and subsequently purified by NP column chromatography [2.0 × 23 cm, CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. After lyophilization, d18:1-lyso-GD1a·2NH3 (8) was obtained as a colorless powder (0.74 mg, 47% from two preparations), ESI-MS: m/z 1,571.67 [M + H]+, 915.46 [Y2α + 2H]+, 816.30 (1.6) [M + K + Na]2+, 797.32 [M + Na + H]2+, 794.84 [M + NH4 + H]2+, 786.33 [M + 2H]2+, Rf = 0.24 [CHCl3/MeOH/2.5 M NH3 (60:44:12, v/v/v)]. The method was also applied for the preparation of d20:1-lyso-GD1b·2NH3 (9) (1.72 mg, 53%) from d20:1/18:0-GD1b·2NH3 (4), ESI-MS: m/z 1,599.67 [M + H]+, 811.32 [M + Na + H]2+, 808.85 [M + NH4 + H]2+, 800.33 [M + 2H]2+, Rf = 0.18 [CHCl3/MeOH/2.5 M NH3 (60:44:12, v/v/v)], d20:1-lyso-GT1b·3NH3 (10) (1.16 mg, 61%) from d20:1/18:0-GT1b·3NH3 (5), ESI-MS: m/z 1,890.74 [M + H]+, 956.89 [M + Na + H]2+, 945.90 [M + 2H]2+, Rf = 0.26 [CHCl3/MeOH/2.5 M NH3 (50:50:16, v/v/v)], and d20:1-lyso-GQ1b·4NH3 (11) (0.97 mg, 44%) from d20:1/18:0-GQ1b·4NH3 (6), ESI-MS: m/z 1,113.92 [M + 2Na]2+, 1,102.93 [M + Na + H]2+, 1,099.95 [M + NH4 + H]2+, 1,091.93 [M + 2H]2+, Rf = 0.25 [CHCl3/MeOH/2.5 M NH3 (50:50:16, v/v/v)].
Synthesis of 2,5-dioxopyrrolidin-1-yl tetradecanoate (12)
Tetradecanoic acid [3.00 g (13.1 mmol)] and 3.24 g of N,N′-dicyclohexylcarbodiimide (15.7 mmol) were dissolved in 26.2 ml of dry tetrahydrofuran and stirred at room temperature for 10 min. Then, 1.81 (15.7 mmol) of N-hydroxysuccinimide in 14.1 ml of the same solvent were added and the reaction mixture was stirred at room temperature for 22.5 h. TLC analysis [CHCl3/MeOH (50:1, v/v)] revealed a complete conversion of the educt. After this, the reaction mixture was filtrated by suction. The solvent was removed under reduced pressure and the residue was recrystallized from ethanol containing a trace of H2O (34). The product was obtained as a colorless solid (3.08 g, 72%), 1H-NMR (300 MHz, CDCl3): δ [ppm] = 2.83 (s, 4H, H-3, 4), 2.60 (t, 2H, 3JH2′-H3′ = 7.5 Hz, H-2′), 1.74 (tt, 2H, 3JH3′-H4′ = 7.5 Hz, 3JH3′-H2′ = 7.5 Hz, H-3′), 1.46-1.18 (m, 20H), 0.88 (t, 3H, 3JH14′-H13′ = 6.7 Hz, H-14′), 13C-NMR (75 MHz, CDCl3): δ [ppm] = 169.15 (R C ON, C-2, 5), 168.68 (R C O2R′, C-1′), 31.90 (CH2, C-2′), 30.93 (CH2, C-12′), 29.63-28.77 (8 CH2), 25.57 (CH2, C-3, 4), 24.56 (CH2, C-3′), 22.67 (CH2, C-13′), 14.09 (CH3, C-14′), EI-MS: m/z 211.3 [M+·-C4H4NO3·], 129.1 [C8H17O+], 98.2 [C7H14+·], 84.1 [C6H12+·], CHN analysis: calc.: C, 66.43; H, 9.60; N, 4.30, found: C, 66.03; H, 9.63; N, 4.29. The method was also applied for the synthesis of 2,5-dioxopyrrolidin-1-yl heptadecanoate (13) (0.897 g, 66%), 1H-NMR (400 MHz, CDCl3): δ [ppm] = 2.83 (s, 4H, H-3, 4), 2.59 (t, 1H, 3JH2′-H3′ = 7.5 Hz, H-2′), 1.74 (tt, 2H, 3JH3′-H4′ = 7.5 Hz, 3JH3′-H2′ = 7,5 Hz, H-3′), 1.45 – 1.19 (m, 26H), 0.88 (t, 3H, 3JH17′-H16′ = 6.9 Hz, H-17′), 13C-NMR (75 MHz, CDCl3): δ [ppm] = 169.15 (R C ON, C-2, 5), 168.68 (R C O2R′, C-1′), 31.90 (CH2, C-2′), 30.93 (CH2, C-15′), 29.66-28.78 (11 CH2), 25.57 (CH2, C-3,4), 24.56 (C-3′), 22.67 (CH2, C-16′), 14.10 (CH3, C-17′), EI-MS: m/z 367.3 [M+·], 253.3 [M+·-C4H4NO3·], 98.1 [C7H14+·], 84.1 [C6H12+·], CHN analysis: calc.: C, 68.63; H, 10.15; N, 3.81, found: C, 68.63; H, 10.14; N, 3.75.
Reacylation of lysogangliosides
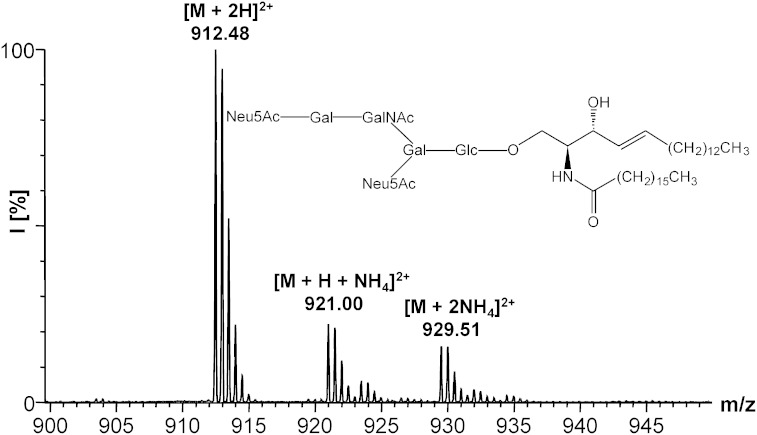
All steps were performed under an argon atmosphere. The d18:1-lyso-GD1a·2NH3 (8) [0.98 mg (0.610 μmol)] and 7.6 μl (44.7 μmol) of Hünig’s base were dissolved in 62 μl of dry dimethylformamide. Then, 38 μl of the same solvent containing 1.43 mg (3.89 μmol) of 2,5-dioxopyrrolidin-1-yl heptadecanoate (13) were added. The reaction mixture was stirred at 30°C for 28 h. Then, a few drops of 2.5 M ammonia were added, the solvent was removed in a stream of nitrogen, the crude product was dried in vacuum and subsequently purified by NP column chromatography [2.0 × 20 cm, CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. After lyophilization, d18:1/17:0-GD1a·2NH3 (16) was obtained as a colorless powder (0.98 mg, 86%), ESI-MS: m/z 929.51 [M + 2NH4]2+, 921.00 [M + NH4 + H]2+, 912.48 [M + 2H]2+, Rf = 0.18 [CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. The method was also applied for the synthesis of d18:1/14:0-GM1·NH3 (14) (6.32 mg, 28%) from 7, ESI-MS: m/z 1,490.72 [M + H]+, 764.83 [M + K + H]2+, 754.37 [M + NH4 + H]2+, 745.86 [M + 2H]2+, Rf = 0.22 [CHCl3/MeOH/2.5 M NH3 (60:40:9, v/v/v)], d18:1/14:0-GD1a·2NH3 (15) (0.88 mg, 42%) from 8, ESI-MS: m/z 918.86 [M + K + NH4]2+, 910.86 [M + Na + NH4]2+, 908.39 [M + 2NH4]2+, 899.88 [M + NH4 + H]2+, 891.37 [M + 2H]2+, Rf = 0.25 [CHCl3/MeOH/2.5 M NH3 (60:44:12, v/v/v)], d20:1/14:0-GD1b·2NH3 (17) (1.28 mg, 70%) from 9, ESI-MS: m/z 1809.92 [M + H]+, 916.44 [M + Na + H]2+, 905.45 [M + 2H]2+, Rf = 0.21 [CHCl3/MeOH/2.5 M NH3 (60:44:12, v/v/v)], d20:1/14:0-GT1b·3NH3 (18) (0.40 mg, 34%) from 10, ESI-MS: m/z 1,070.48 [M + K + H]2+, 1,070.99 [M + Na + NH4]2+, 1,062.49 [M + Na + H]2+, 1,051.50 [M + 2H]2+, Rf = 0.29 [CHCl3/MeOH/2.5 M NH3 (60:50:15, v/v/v)], and d20:1/14:0-GQ1b·4NH3 (19) (0.19 mg, 19%) from 11, ESI-MS: m/z 1,227.01 [M + K + Na]2+, 1,216.02 [M + K + H]2+, 1,208.03 [M + Na + H]2+, 1,205.55 [M + NH4 + H]2+, 1197.04 [M + 2H]2+, Rf = 0.27 [CHCl3/MeOH/2.5 M NH3 (50:50:16, v/v/v)].
Enzymatic degalactosylation of ganglioside standards
The d18:1/14:0-GM1·NH3 (14) [1.34 mg (0.889 μmol)] was dissolved in 410 μl of 100 mM McIlvaine buffer (pH 4.3), which contained 3.9 mM sodium taurodeoxycholate. Then, 200 μl of buffer containing 3.2 mM Triton™ X-100 and 41 μl of pure buffer were added. The mixture was exposed to an ultrasonic bath for 15 s. Then, 150 μl (134 mU) of β-galactosidase solution were added. After 1 day, TLC analysis [CHCl3/MeOH/2.5 M NH3 (50:40:9 v/v/v)] revealed a conversion of approximately 50%. An additional 150 μl of enzyme solution was added. After a further day, the conversion was complete and the reaction was stopped by addition of a few drops of 2.5 M ammonia. The solvent was removed in a stream of nitrogen and the residue was redissolved in 1 ml of MeOH. The enzyme was separated by centrifugation (12,100 g), which was repeated five times. The supernatant was separated and evaporated in a stream of nitrogen. The crude product was purified by preparative HPTLC [20 × 20 cm glass plate, CHCl3/MeOH/2.5 M NH3 (60:40:9, v/v/v)]. Silica gel was removed by the method described under Purification in the Materials and Methods. After lyophilization, d18:1/14:0-GM2·NH3 (20) was obtained as a colorless powder (1.84 mg, 77% from two preparations), ESI-MS: m/z 1,345.81 [M + NH4]+, 1,328.78 [M + H]+, 681.49 [M + 2NH4]2+, 664.89 [M + 2H]2+, Rf = 0.27 [CHCl3/MeOH/2.5 M NH3 (60:40:9, v/v/v)]. The method was also applied for the preparation of d20:1/14:0-GD2·2NH3 (21) from d20:1/14:0-GD1b·2NH3 (17). For this conversion, the concentrations of detergents and enzyme were doubled. Compound 21 was obtained as a colorless powder (0.25 mg, 61%), ESI-MS: m/z 846.43 [M + 2Na]2+, 835.44 [M + Na + H]2+, 841.47 [M + 2NH4]2+, 832.96 [M + NH4 + H]2+, 824.45 [M + 2H]2+, Rf = 0.30 [CHCl3/MeOH/2.5 M aq. NH3 (60:44:12, v/v/v)].
Synthesis of lysoganglioside standards
(E)-hexacos-13-ene (22).
All steps were performed under an argon atmosphere. 1-Tetradecene [200 mg (1.02 mmol)] and 36.7 mg (0.204 mmol, 20 mol%) of tetrafluoro-1,4-benzoquinone were dissolved in 2.8 ml of dry toluene. Hoveyda-Grubbs catalyst 2nd generation [1,3-bis-(2,4,6-trimethylphenyl)-2-imidazolidnylidene]dichloro(o-isopropoxyphenylmethylene)ruthenium(II) [6.33 mg (10.1 μmol, 0.99 mol%)] was added and the reaction mixture was stirred at room temperature for 3 h. Ethylene was removed by a stream of argon during the reaction. The solvent was removed in a stream of nitrogen and the catalyst was removed by NP column chromatography. The crude product was applied to the column (1.0 × 4.0 cm) in hexane/EtOAc (80:1, v/v). The column was eluted by four column volumes of the same solvent. Products containing fractions were pooled, the solvent was removed in a stream of nitrogen and subsequently dried in vacuum. After recrystallization from 4°C cold ethanol, 22 was obtained as a colorless solid (58.8 mg, 32%), 1H-NMR (400 MHz, CDCl3): δ [ppm] = 5.38 (tt, 2H, 3JH13-H12 = 3.6 Hz, 4JH13-H11 = 1.4 Hz, H-13, 14), 1.97 (m, 4H, H12, 15), 1.26 (m, 40H), 0.88 (t, 6H, 3JH1-H2 = 6.8 Hz, H-1, 26), EI-MS: m/z 364.45 [M+·], 111.15 [C8H15+], 97.10 [C7H13+], 83.10 [C6H11+], 69.00 [C5H9+], 57.10 [C4H9+], 55.05 [C4H7+], GC-MS: tR 14.14 min, 100%, M+· = 364.45, CHN analysis: calc.: C, 85.63; H, 14.37, found: C, 85.21; H, 14.11.
Pentadeca-O-acetyl-GM1 (d20:1/18:0) sialoyl-II2-lactone (23).
All steps were performed under an argon atmosphere. The d20:1/18:0-GM1·NH3 (2) [30.9 mg (19.4 μmol)] and 0.5 mg (4.09 μmol) of 4-dimethylaminopyridine were suspended in 832 μl of dry pyridine. Acetic anhydride (413 μl) was added and the reaction mixture was stirred at room temperature for 15 h. It took a few hours for the reactants to dissolve completely. The solvent was removed in a stream of nitrogen and the crude product was purified by NP column chromatography [2.0 × 21 cm, toluene/acetone (5:4, v/v)]. After drying in vacuum, 23 was obtained as a colorless solid (26.3 mg, 62%), 1H-NMR (400 MHz, CDCl3): see Table 2, ESI-MS: m/z 2,220.10 [M + MeOH + H]+, 2,188.07 [M + H]+, 1,127.56 [M + MeOH + 2NH4]2+, 1,119.05 [M + MeOH + NH4 + H]2+, 1,110.54 [M + MeOH + 2H]2+, 1,111.55 [M + 2NH4]2+, 1,103.04 [M + NH4 + H]2+, 1,094.53 [M + 2H]2+, Rf = 0.23 (toluene/acetone, 5:4, v/v).
TABLE 2.
Characteristic 1H-NMR chemical shifts of compounds 23, 24, and 25
| Protons | 23 | 24 | 25 |
| Olefinic | |||
| Cer, H-5 | 5.76 (3J = 15.1 Hz) | 6.65 (3J = 15.9 Hz) | 5.77 (3J = 15.2 Hz) |
| Cer, H-4 | Unresolved | 6.09 (3J = 15.9 Hz) | Unresolved |
| Anomeric | |||
| Glc | 4.76 | 4.76 | 4.76 |
| Gal (II) | Unresolved | Unresolved | Unresolved |
| Gal (IV) | 5.04 | 5.04 | 5.04 |
| GalNAc | 5.63 | 5.74 | 5.64 |
| Methyl | |||
| Alkyl chain | 0.87 | 0.87 | 0.87 |
| GalNAc | 1.87 | 1.89 | 1.89 |
| Neu5Ac | 1.96 | 1.96 | 1.96 |
| O-Ac | 2.01–2.18 | 2.02–2.18 | 2.02–2.18 |
The olefinic 3J coupling constants are given in parentheses. Ac, acetyl; Cer, ceramide; Gal, d-galactose; GalNAc, N-acetyl-d-galactosamine; Glc, d-glucose; Neu5Ac, N-acetylneuraminic acid.
O-(tetradeca-O-acetylmonosialogangliotetraosyl)-(1→1)-(2R,3S,4E)-3-O-acetyl-2-octadecanoylamino-5-phenylpent-4-en-1-ol sialoyl-II2-lactone (24).
All steps were performed under an argon atmosphere. A 1:1 mixture of pentadeca-O-acetyl-GM1 (d20:1/18:0) sialoyl-II2-lactone (23) [39.7 mg (18.3 μmol)] and pentadeca-O-acetyl-GM1 (d18:1/18:0) sialoyl-II2-lactone ( = 2173.41 gmol−1) was dissolved in 477 μl of dry dichloromethane in a 1 ml glass vial. (E)-stilbene [33.0 mg (183 μmol)] and 23.9 mg (28.2 μmol) of Grubbs catalyst 2nd generation [1,3-bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene]dichloro(phenylmethylene)(tricyclohexylphosphine)-ruthenium(II) were added and the reaction mixture was stirred at 43°C for 46 h. TLC monitoring [toluene/acetone (1:1, v/v)] revealed a conversion of more than 90%. The solvent was removed in a stream of nitrogen, the crude product was dried in vacuum and subsequently purified by NP column chromatography [2.0 × 22 cm, toluene/acetone (10:9, v/v)]. Traces of catalyst were removed by the method described under Purification in the Materials and Methods. After drying in vacuum, 24 was obtained as a colorless solid (25.7 mg, 68%), 1H-NMR (400 MHz, CDCl3): see Table 2, ESI-MS: m/z 2,085.94 [M + MeOH + H]+, 2,053.90 [M + H]+, 1,060.46 [M + MeOH + 2NH4]2+, 1,051.95 [M + MeOH + NH4 + H]2+, 1,043.44 [M + MeOH + 2H]2+, 1,044.45 [M + 2NH4]2+, 1,035.94 [M + NH4 + H]2+, 1,027.43 [M + 2H]2+, Rf = 0.15 (toluene/acetone, 5:4, v/v).
Pentadeca-O-acetyl-GM1 (d17:1/18:0) sialoyl-II2-lactone (25).
All steps were performed under an argon atmosphere. 15.5 mg (7.55 μmol) of 24, 55.0 mg (151 μmol) of (E)-hexacos-13-ene (22), and 3.43 mg (31.7 μmol, 20 mol%) of p-benzoquinone were dissolved in 271 μl of dry dichloromethane in a 1 ml glass vial. Then, 19.8 mg (31.6 μmol, 20 mol%) of Hoveyda-Grubbs catalyst 2nd generation were added and the reaction mixture was stirred at 40°C. After 1 day, TLC monitoring (toluene/acetone, 1:1, v/v) revealed a conversion of approximately 70%. Further, 19.8 mg of catalyst and 3.43 mg of p-benzoquinone were added and the reaction was stirred at 40°C for another day. After a total of 41 h, the solvent was removed in a stream of nitrogen and the crude product was dried in vacuum. Subsequently, it was purified by NP column chromatography [2.0 × 23 cm, toluene/acetone (10:9, v/v)]. Traces of catalyst were removed by the method described under Purification in the Materials and Methods. After drying in vacuum, 25 was obtained as a colorless solid (9.91 mg, 61%), 1H-NMR (400 MHz, CDCl3): see Table 2, ESI-MS: m/z 2,178.10 [M + MeOH + H]+, 2,146.05 [M + H]+, 1,106.56 [M + MeOH + 2NH4]2+, 1,098.04 [M + MeOH + NH4 + H]2+, 1,089.53 [M + MeOH + 2H]2+, 1,090.54 [M + 2NH4]2+, 1,082.02 [M + NH4 + H]2+, 1,073.52 [M + 2H]2+, Rf = 0.20 (toluene/acetone, 5:4, v/v).
Preparation of d17:1-lyso-GM1·NH3 (26)
In the next step, pentadeca-O-acetyl-GM1 (d17:1/18:0) sialoyl-II2-lactone (25) was deprotected and regioselectively deacylated by the method described under the heading Chemical preparation of lysogangliosides in the Materials and Methods. Briefly, 9.40 mg (4.38 μmol) of 25 were applied. The reaction time was 19 h. The crude product was purified by RP column chromatography [0.9 × 2.8 cm, MeOH/H2O (90:40, v/v)]. A second purification step was performed by NP column chromatography [2.0 × 19 cm, CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. MS analysis revealed that the product contained 4.8% of the d16:1-lyso-GM1 isomer (see Results section), so the product was purified by isocratic RP column chromatography [1.0 × 22 cm, MeOH/H2O (90:31, v/v)], again. After lyophilization, 26 was obtained as a colorless powder (1.93 mg, 34%), ESI-MS: m/z 1,266.60 [M + H]+, 1,104.55 [Y3α + 2H]+, 901.47 [Y2α + 2H]+, 652.78 [M + K + H]2+, 644.79 [M + Na + H]2+, 642.31 [M + NH4 + H]2+, 633.88 [M + 2H]2+, Rf = 0.14 [CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)].
Preparation of d17:1-lyso-GM2·NH3 (27)
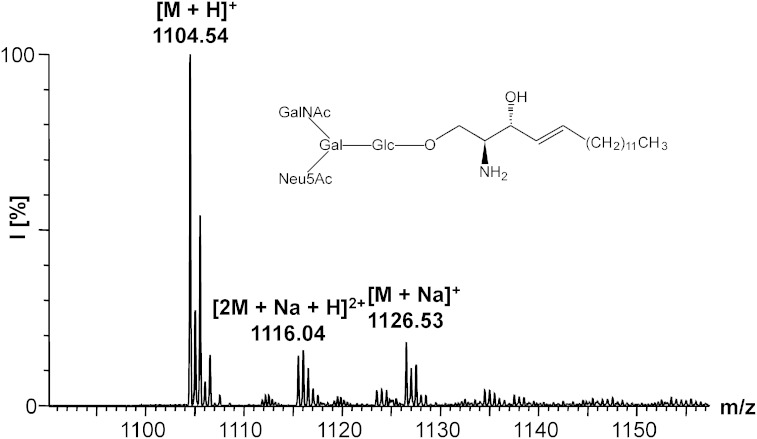
The d17:1-lyso-GM2·NH3 (27) was prepared by the method described under the heading Enzymatic degalactosylation of ganglioside standards in the Materials and Methods. Briefly, 1.09 mg (0.849 μmol) of d17:1-lyso-GM1·NH3 (26) and 314 mU of β-galactosidase were applied. The reaction time was 15.5 h. The crude product was purified by NP column chromatography [2.0 × 19 cm, CHCl3/MeOH/2.5 M NH3 (60:40:10, v/v/v)]. After lyophilization, 27 was obtained as a colorless powder (0.66 mg, 69%). ESI-MS: m/z 1,126.53 [M + Na]+, 1,104.54 [M + H]+, 563.76 [M + Na + H]2+, 561.28 [M + NH4 + H]2+, 552.77 [M + 2H]2+, Rf = 0.16 [CHCl3/MeOH/2.5 M NH3 (60:40:9 v/v/v)].
Validation of the lysoganglioside lipoforms as calibrators
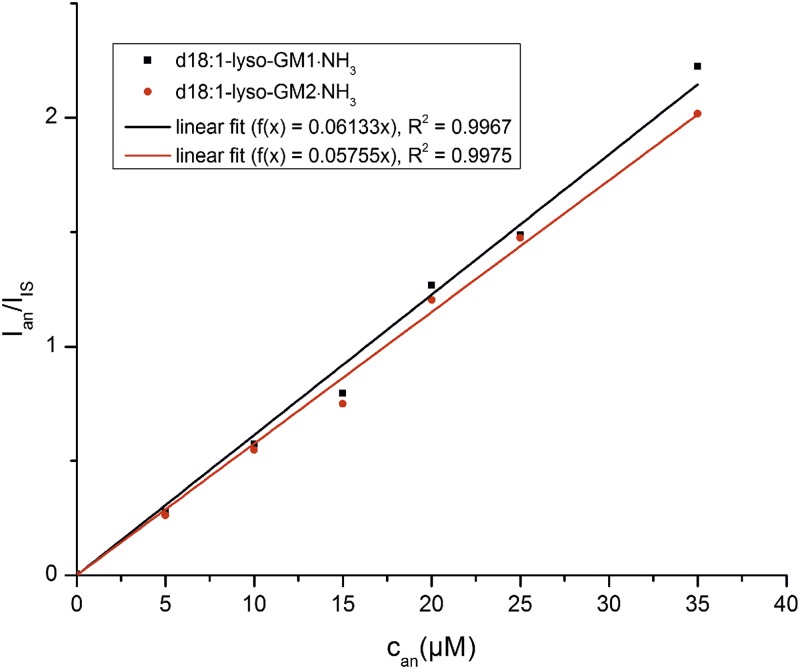
Six different concentrations of pure d18:1-lyso-GM1·NH3 and of pure d18:1-lyso-GM2·NH3 (0, 5, 10, 15, 20, 25, and 35 μM) in MeOH were prepared. The d18:1-lyso-GM1·NH3 solutions were each spiked by 26 to a final concentration of 13.4 μM of the internal standard and the d18:1-lyso-GM2·NH3 solutions were spiked by 27 to a concentration of 14.0 μM of the internal standard. The solutions were measured in the full scan mode and the ratio of the peak height of the most intensive peak ([M + K + H]2+) was plotted against the concentration of the analyte (see Fig. 8 in the Results section).
Fig. 8.
Quantification of pure lyso-GM1·NH3 and lyso-GM2·NH3. A concentration series of pure d18:1-lyso-GM1 and d18:1-lyso-GM2 was quantified by using the internal standards d17:1-lyso-GM1·NH3 (26) and d17:1-lyso-GM2·NH3 (27).
RESULTS
Purification of bovine brain gangliosides to obtain starting materials
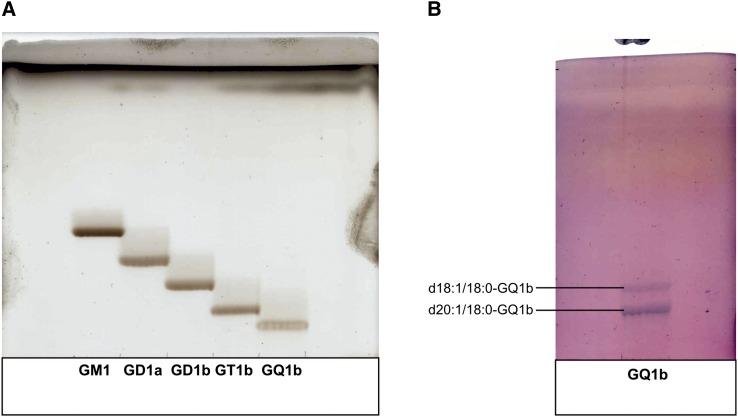
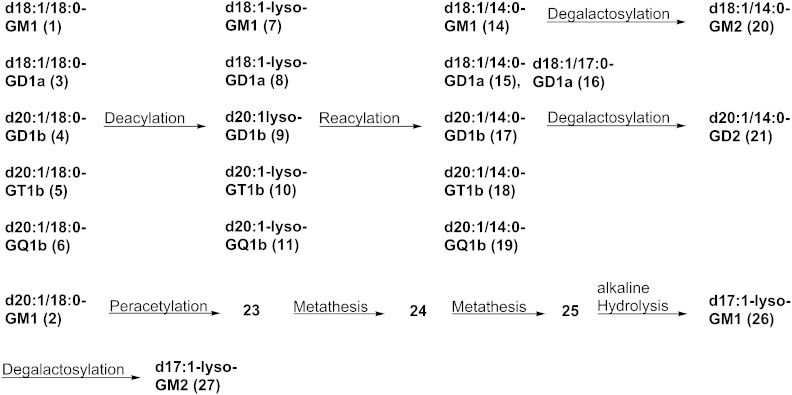
Bovine brain gangliosides (Cronassial®) (5.03 g) were separated into different ganglioside classes by anion-exchange chromatography. Monosialogangliosides were eluted by an ammonium acetate concentration of 0.05 mol/l. Disialogangliosides and trisialogangliosides were successively eluted by an ammonium acetate concentration of 0.15 mol/l and were separated without overlap. GQ1b was eluted by an ammonium acetate concentration of 0.45 mol/l and was already obtained in pure form after desalting. The recovery of the anion-exchange chromatography was 95%. In the next step, the ganglioside classes were purified by NP column chromatography using different techniques and different solvent systems. The disialogangliosides were separated into GD1a and GD1b. The purified gangliosides were analyzed by HPTLC, ESI-MS, and ESI-MS/MS. The NP HPTLC showed only one band for each ganglioside (Fig. 2A). The RP-TLCs showed two distinct bands for each ganglioside (Fig. 2B, given for GQ1b), whereby the upper band corresponds to the d18:1/18:0-lipoform while the lower band corresponds to the d20:1/18:0-lipoform. In the next step, the gangliosides were separated into their lipoforms by RP column chromatography using MeOH/H2O in different compositions as eluent. The purity of the ganglioside lipoforms was demonstrated by RP-TLC and ESI-MS. Their structure was analyzed by ESI-MS/MS and 1H-NMR. CHN analysis demonstrated a purity of 86–95% compared with the theoretical value, which is due to residual H2O content (Table 1). The residual H2O content was 10–15% when the gangliosides were dried by lyophilization and around 5% when the gangliosides were dried in vacuum. An overview about the preparations which were performed in this work is given in Fig. 3.
Fig. 2.
HPTLC analysis of the purified gangliosides. A: NP TLC of GM1, GD1a, GD1b, GT1b, and GQ1b. Solvent system: CHCl3/MeOH/0.2% CaCl2 (50:42:11, v/v/v). B: RP TLC of GQ1b. Solvent system: MeOH/H2O (90:20, v/v).
Fig. 3.
Overview about the preparations performed in this work.
Preparation of lysogangliosides
In this work, one step preparations of lysogangliosides were applied. The d18:1-lyso-GM1·NH3 was prepared by alkaline treatment of d18:1/18:0-GM1·NH3 in a yield of 52%, which is comparable to the yield of 54% reported by Sonnino et al. (31). This method turned out to be not suitable for the regioselective deacylation of oligosialogangliosides: d18:1-lyso-GD1a·2NH3 was obtained in a yield of only 4.8% due to concomitant deacetylation of sialic acid groups. Hence, we applied a biocatalyst to this reaction. The enzyme SCDase (EC 3.5.1.69) catalyzes the hydrolysis of the amide bond within the ceramide moiety of various GSLs, but not ceramides, in a reversible manner (35). We applied the method to d18:1/18:0-GD1a·2NH3 (3), d20:1/18:0-GD1b·2NH3 (4), d20:1/18:0-GT1b·3NH3 (5), and d20:1/18:0-GQ1b·4NH3 (6) (Table 3). Yields were in the range of 44–60%. The ESI-MS spectrum of d18:1-lyso-GD1a showed no impurities of other GD1a lipoforms. By comparing the [M + 2H]2+ peaks, it was found that d20:1-lyso-GD1b·2NH3 contained 3.1% of d18:1-lyso-GD1b·2NH3 due to traces of d18:1/20:0-GD1b·2NH3 in the precursor, which could not be separated by RP column chromatography. Also, d20:1-lyso-GT1b·3NH3 contained 4.7% of its d18:1-lipoform. For d20:1-lyso-GQ1b·4NH3, no other GQ1b-lipoforms were detected.
TABLE 3.
Results of the enzymatic deacylation of gangliosides
| Lysoganglioside | nSCDase/nsubstrate (mU/μmol) | Amount of Lyso-ganglioside (mg) | Yield (%) |
| d18:1-lyso-GD1a·2NH3 (8) | 110 | 0.74 | 47 |
| d20:1-lyso-GD1b·2NH3 (9) | 110 | 1.72 | 53 |
| d20:1-lyso-GT1b·3NH3 (10) | 73.6 | 1.16 | 60 |
| d20:1-lyso-GQ1b·4NH3 (11) | 74.3 | 0.97 | 44 |
The required amount of the enzyme SCDase for a conversion larger than 90% is given in column 2.
Reacylation of lysogangliosides
The lysogangliosides were reacylated by the method of Schwarzmann and Sandhoff (36) using N-hydroxysuccinimide esters of tetradecanoic acid and heptadecanoic acid. The reaction was applied to d18:1-lyso-GM1·NH3 (7), d18:1-lyso-GD1a·2NH3 (8), d20:1-lyso-GD1b·2NH3 (9), d20:1-lyso-GT1b·3NH3 (10), and d20:1-lyso-GQ1b·4NH3 (11). Yields were in the range of 19–86% (Table 4). The ESI-MS of 14, 15, 16 (Fig. 4), and 19 showed no traces of other ganglioside lipoforms than the desired products. The ESI-MS of d20:1/14:0-GD1b·2NH3 (17) showed an impurity of 3.4% of d18:1/14:0-GD1b·2NH3 and the ESI-MS of d20:1/14:0-GT1b·3NH3 (18) showed an impurity of 4.8% of d18:1/14:0-GT1b·3NH3 as it was observed for their precursors.
TABLE 4.
Ganglioside standards that were synthesized by deacylation and reacylation of the fatty acid of gangliosides
| Ganglioside Standard | Amount (mg) | Yield (%) |
| d18:1/14:0-GM1·NH3 (14) | 6.32 | 28 |
| d18:1/14:0-GD1a·2NH3 (15) | 0.88 | 42 |
| d18:1/17:0-GD1a·2NH3 (16) | 0.98 | 86 |
| d20:1/14:0-GD1b·2NH3 (17) | 1.28 | 70 |
| d20:1/14:0-GT1b·3NH3 (18) | 0.40 | 34 |
| d20:1/14:0-GQ1b·4NH3 (19) | 0.19 | 19 |
The yields of the reacylation step are given in column 3.
Fig. 4.
ESI-MS of d18:1/17:0-GD1a·2NH3 (16).
Preparation of ganglioside standards by enzymatic degalactosylation
For the preparation of gangliosides that do not occur in larger amounts in bovine brain, we applied glycosidase-treatment to ganglioside standards already modified in their lipid part. The commercially available enzyme, β-galactosidase (EC 3.2.1.23), from bovine testes can hydrolyze terminal galactosyl residues in β1-3, β1-4, and β1-6 linkage from saccharides, glycosaminoglycans, glycoproteins, and glycolipids (37). The reaction was applied to the preparation of d18:1/14:0-GM2·NH3 (20) from d18:1/14:0-GM1·NH3 (14) and d20:1/14:0-GD2·2NH3 (21) from d20:1/14:0-GD1b·2NH3 (16). The preparations had yields of 77 and 61%, respectively (Table 5). The lower yield for the preparation of GD2 derivatives is due to a slight desialylation of GD1b, which occurs at the applied pH of 4.5. This was demonstrated by applying the reaction conditions described to GD1b in the absence of enzyme. The ESI-MS of 20 showed no traces of other GM2 lipoforms than the desired product. The ESI-MS of 21 showed an impurity of 3.2% of d18:1/14:0-GD2·2NH3 as already observed for the precursor.
TABLE 5.
Ganglioside standards that were prepared by enzymatic degalactosylation
| Product | nβ-galactosidase/nsubstrate (mU/μmol) | Amount (mg) | Yield (%) |
| d18:1/14:0-GM2·NH3 (20) | 300 | 1.84 | 77 |
| d20:1/14:0-GD2·2NH3 (21) | 359 | 0.25 | 61 |
The required amount of the enzyme β-galactosidase for a conversion larger than 90% is given in column 2.
Modification of the sphingosine chain of gangliosides
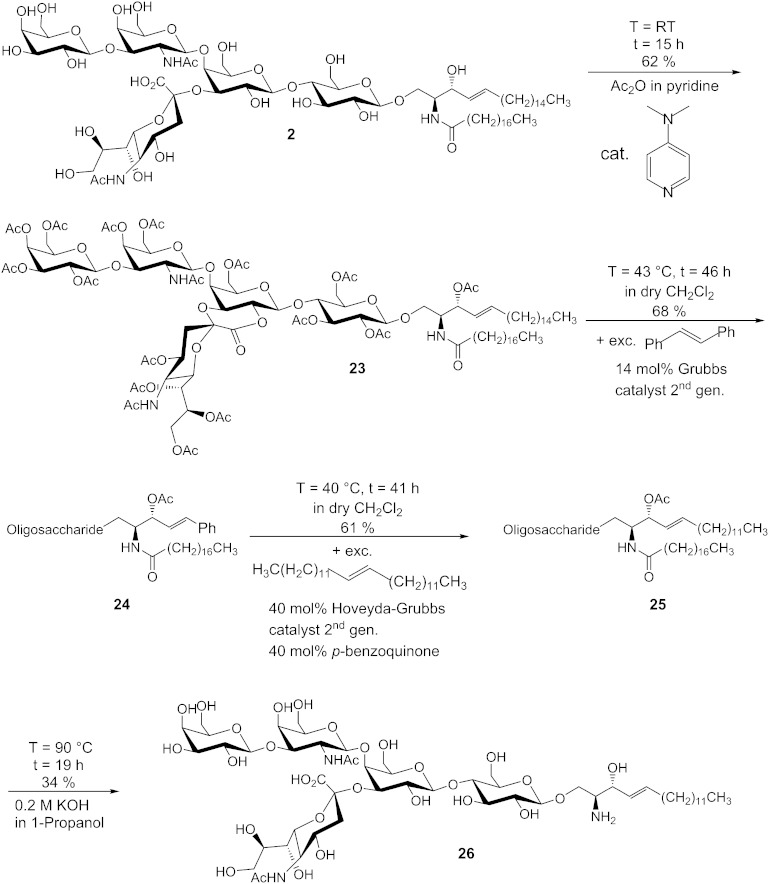
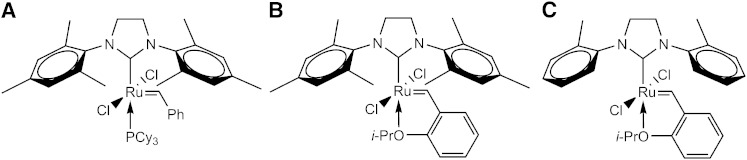
The olefin cross metathesis is a suitable reaction for the modification of the sphingosine chain of gangliosides and GSLs because it tolerates hard nucleophiles and electrophiles and it is performed under neutral conditions. The following synthetic steps were performed for the synthesis of d17:1-lyso-GM1·NH3 (26) from d20:1/18:0-GM1·NH3 (2) (Fig. 5). Our approach includes a stilbenolysis followed by the introduction of a new alkyl chain. We did not choose an ethenolysis because reported yields (tested by the model methyl oleate using standard Ru-based catalysts) are only in the range of 13–57% (38) and 13–24% (39), respectively. In the recent years, more effective catalysts for ethenolysis have been developed, which give yields up to 95% (40) and 80% (41), respectively, but only for (Z)-olefins. These catalysts are much more reactive toward (Z)-olefins than toward (E)-olefins because the residues of the olefin are forced into the same direction in the metallacyclobutane formation by bulky ligands (42). A direct reaction of a peracetylated ganglioside lactone with 13-hexacosene is impracticable because of separation problems. The procedure illustrated in Fig. 5 has three advantages: First, the phenyl group facilitates the chromatographic purification because it is more polar than an alkyl group. Second, the phenyl group serves as an UV probe. Third, stilbene is not prone to isomerizations because the phenyl group stops the migration of the double bond. In the first step, 2 was peracetylated by a modified method of Schwarzmann, Hofmann, and Pütz (43). These reaction conditions led to the formation of lactone 23 in a yield of 62%. In the ESI-MS spectrum, only peaks of the product and the methyl ester of the product, which is formed by opening of the lactone by MeOH, which is used as solvent in the measurement, were found. Compound 23 was converted to 24 by a 10-fold excess of (E)-stilbene and 14 mol% of Grubbs catalyst 2nd generation in a yield of 68%. The 1H-NMR spectrum demonstrated that selectively, the E-isomer was formed due to the bulky residues. (E)-hexacosene (22) was synthesized by an olefin cross metathesis of 1-tetradecene, because it is not commercially available. It was observed that only a statistical mixture of constitutional isomers is obtained if no hydride scavenger is used. The presence of 20 mol% of p-benzoquinone leads to an isomeric purity of 98%, as analyzed by GC-MS. By using 20 mol% of tetrafluoro-1,4-benzoquinone, an isomeric purity of 100% was obtained. In the 1H-NMR and 13C-NMR spectra, only one signal for the olefinic protons was observable, so we concluded that selectively the E-isomer was formed. Then, 24 was converted to 25 by a 20-fold excess of 22 in a yield of 61%. Hereby, also selectively, the E-isomer was formed. To optimize the conditions for the formation of 25, we tested the more effective hydride scavengers, tetrafluoro-1,4-benzoquinone and 2,6-dichloro-1,4-benzoquinone, as well as the Stewart-Grubbs catalyst, which contains N-tolyl groups instead of N-mesityl groups in the N-heterocyclic carbene ligand (Fig. 6). This catalyst is reported to be more active than the Hoveyda-Grubbs catalyst 2nd generation in cross metathesis reactions of sterically challenging olefins (44). The results are given in Table 6. Unexpectedly, the more active hydride scavengers, tetrafluoro-1,4-benzoquinone and 2,6-dichloro-1,4-benzoquinone, suppressed the catalyst activity even when they were added in low amounts. Furthermore, the Stewart-Grubbs catalyst showed no activity toward 24 regardless of the hydride scavenger tested. So the most effective reaction conditions are 20 mol% of Hoveyda-Grubbs catalyst 2nd generation and 20 mol% of p-benzoquinone. In the last step, the protecting groups and also the acyl chain were removed from 25 by using the chemical deacylation method to prepare d17:1-lyso-GM1·NH3 (26). The yield was 34%. The ESI-MS showed that the product contained 4.8% of d16:1-lyso-GM1·NH3, which indicates that isomerizations could not be completely suppressed by p-benzoquinone, but to an extent larger than 95%. Other isomers were not found. The isomer was removed by isocratic RP column chromatography. A proportion of 26 was used for the preparation of d17:1-lyso-GM2·NH3 (27) by the described enzymatic degalactosylation method. The yield was 69% (Fig. 7).
Fig. 5.
Synthetic approach for the modification of the sphingosine part of gangliosides. cat., catalyst; exc., excess; gen., generation; T, temperature; t, time; RT, room temperature.
Fig. 6.
Important achiral olefin metathesis catalysts. A: Grubbs catalyst 2nd generation. B: Hoveyda-Grubbs catalyst 2nd generation. C: Stewart-Grubbs catalyst.
TABLE 6.
Optimization of the reaction conditions for the synthesis of compound 25 from compound 24
| Catalyst (20 mol%) | Hydride Scavenger | Conversion (%) |
| Hoveyda-Grubbs catalyst 2nd generation | p-Benzoquinone (40 mol%) | 30 |
| Hoveyda-Grubbs catalyst 2nd generation | p-Benzoquinone (20 mol%) | 60 |
| Hoveyda-Grubbs catalyst 2nd generation | Tetrafluoro-1,4-bezoquinone (40 mol%) | <5 |
| Hoveyda-Grubbs catalyst 2nd generation | Tetrafluoro-1,4-bezoquinone (20 mol%) | <5 |
| Hoveyda-Grubbs catalyst 2nd generation | Tetrafluoro-1,4-bezoquinone (1 mol%) | <5 |
| Hoveyda-Grubbs catalyst 2nd generation | 2,6-Dichloro-1,4-benzoquinone (20 mol%) | <5 |
| Hoveyda-Grubbs catalyst 2nd generation | 2,6-Dichloro-1,4-benzoquinone (5 mol%) | <5 |
| Hoveyda-Grubbs catalyst 2nd generation | 2,6-Dichloro-1,4-benzoquinone (1 mol%) | <5 |
| Stewart-Grubbs catalyst | p-Benzoquinone (20 mol%) | 0 |
| Stewart-Grubbs catalyst | Tetrafluoro-1,4-bezoquinone (20 mol%) | 0 |
| Stewart-Grubbs catalyst | 2,6-Dichloro-1,4-benzoquinone (20 mol%) | 0 |
The olefin metathesis catalysts tested are given in column 1. They were applied in 20 mol%. (E)-hexacos-13-ene (22) was applied in 10-fold excess. The different hydride scavengers tested are given in column 2. Conversions of 24 to 25, which are given in column 3, were estimated by TLC after 1 day of reaction time.
Fig. 7.
ESI-MS of d17:1-lyso-GM2·NH3 (27).
As a proof of principle, the synthesized lysoganglioside standards 26 and 27 were tested for the quantification of pure natural lysogangliosides (Fig. 8). Good linearity was obtained for both compounds in the applied concentration range. As expected, quantification of pure substances is possible in the full scan mode, but for biological samples MS/MS modes have to be applied.
DISCUSSION
Partial synthesis of nonnatural ganglioside lipoforms
For the synthesis of internal ganglioside standards, we chose a partial synthesis rather than a total synthesis because of the high effort necessary for the latter. Starting materials were obtained by purification of a ganglioside mixture from bovine brain. It was confirmed by ESI-MS that bovine brain gangliosides mainly contain d18:1/18:0- and d20:1/18:0-lipoforms. Usually, the purity of gangliosides is determined by fluorimetric (45) or photometric (28, 29) sialic acid determination, sphingoid base determination by HPLC (46), GC-flame ionization detection (30), or densitometry (47). We analyzed the purified ganglioside lipoforms by elemental analysis and used these substances as external standards for fluorimetric and photometric sialic acid determination. The results of the CHN analysis demonstrated that there is residual H2O content of 10–15% in the gangliosides after lyophilization. By drying in vacuum, it can be reduced to 5%. For modification of the fatty acid acyl chain, we followed a chemical and an enzymatic deacylation approach. The results of the chemical deacylation demonstrated that this method is appropriate for the selective deacylation of GM1, but inappropriate for the preparation of oligosialolysogangliosides. The enzymatic deacylation turned out to be more appropriate: yields were in the range of 44–60% for the preparation of lyso-GD1a (8), lyso-GD1b (9), lyso-GT1b (10), and lyso-GQ1b (11). This is comparable to the results of Ando et al. (48), who reported yields of 62% resp. 52% for the preparation of lyso-GM1 and lyso-GM2. Due to the occurrence of traces of d18:1/20:0-lipoforms in the starting material, which could not be separated from the d20:1/18:0-lipoforms by RP column chromatography, the prepared d20:1-lyso-GD1b·2NH3 (9) and d20:1-lyso-GT1b·3NH3 (10) contained 3.1% and 4.7%, respectively, of their d18:1-lipoform. Because they do not disturb the mass spectrometric application of the standards, we decided not to remove these minor byproducts. The reacylation of the lysogangliosides was carried out in yields in the range of 19–86%. In general, the reaction works accurately for monosialolysogangliosides and disialolysogangliosides with yields up to 86%. For lyso-GT1b and lyso-GQ1b, the yields drop to 34 and 19%, respectively. An optimization of the reaction conditions for these substrates should be performed in the future.
For the enzymatic degalactosylation of d18:1/14:0-GM1·NH3 (7), a yield of 77% was obtained, which is higher than the yield of 54% obtained by Larsson et al. (49) for a fluorescence-labeled GM1 substrate. For the degradation of d17:1-lyso-GM1·NH3 (26) to d17:1-lyso-GM2·NH3 (27), a comparable yield of 69% was obtained. The method is also applicable to GD1b derivatives, although we observed a slight degradation to GM1 due to the low pH value. Hereby, a sufficient yield of 61% was obtained for the degradation of d20:1/14:0-GD1b·2NH3 (17) to d20:1/14:0-GD2·2NH3 (21). As far as we know, this method has not been applied to lyso-GM1 and GD1b derivatives in the literature.
Development of a new synthetic method to modify the sphingosine chain of gangliosides
We developed a synthetic method based on the olefin cross metathesis for the preparation of gangliosides and lysogangliosides with homogenous and, if required, artificial sphingoid bases. Advantages of this method are a simplified chromatographic separation by introduction of a phenyl group, the opportunity to use gangliosides heterogeneous in their sphingosine part as educts, and the applicability of the method for the synthesis of lysoganglioside standards. A drawback of the method is that isomerizations can only be suppressed to less than 5%. A direct modification of lysogangliosides by an olefin metathesis is impracticable because the reaction does not tolerate amino groups.
We are aware of only two methods for the partial synthesis of ganglioside lipoforms as MS standards in the literature (Fig. 9). The method by Neuenhofer and colleagues (36, 50) has a total yield of approximately 29% for the synthesis of, for example, d18:1/17:0-GM1 from d18:1/18:0-GM1. It includes a total deacylation of the ganglioside except the N-acetylgalactosamine residue followed by a regioselective protection of the amino group in the sphingosine part. Subsequently, the remaining amino groups are reacetylated and the protecting group is removed. The enzymatic deacylation (32, 51) followed by chemical reacylation has the highest overall yield of approximately 42%. The olefin metathesis method for the synthesis of, for example, d17:1/18:0-GM1 from GM1 has a total yield of approximately 23% if the yield for the removal of the protecting groups is estimated by 90%. But in contrast to our method the other methods require homologous pure gangliosides as starting materials. Current experiments in our group indicate that the method is applicable to peracetylated disialogangliosides, but eventually not to trisialogangliosides because they might be too unreactive in olefin metathesis reactions. Furthermore, an application to neutral GSLs and ceramides should be possible with even higher yields because they are more reactive in olefin metathesis reactions.
Fig. 9.
Comparison of published methods for the partial synthesis of ganglioside lipoforms to our new method. On the left the method of Neuenhofer et al. is shown. The first three steps can be replaced by the enzymatic deacylation method, which is shown in the center. The olefin metathesis method developed by us is shown on the right. cat., catalyst; exc., excess; gen., generation.
CONCLUSION
Analytical and functional studies of ganglioside lipoforms is an emerging area of research (1, 14, 15). We provide a protocol for the preparation of artificial ganglioside and lysoganglioside lipoforms starting from a ganglioside mixture. It relies on selective chemical and enzymatic deacylation steps, reacylation of the sphingosine part, and also selective degalactosylation. These methods were used for the preparation of new internal standards for GM1, GM2, GD2, GD1a, GD1b, GT1b, and GQ1b. We also developed a new olefin metathesis method, which was used for the synthesis of lyso-GM1 and lyso-GM2 standards. These compounds are suitable as calibrator substances for the mass spectrometric determination of gangliosides and lysogangliosides, and can also be applied for functional studies.
Until now, no method was reported for the modification of the sphingosine part of gangliosides. The method should also be suited for isotope labeling of the sphingosine part of sphingolipids and gangliosides because the phenyl group in intermediates of the metathesis reaction facilitates the chromatographic separation of educts and products. Furthermore, this method may also be used to incorporate fluorescent dyes into the sphingosine part.
Acknowledgments
The authors are thankful for the scholarship from the North Rhine-Westphalia International Graduate Research School Life & Medical Sciences Chemical Biology. They also thank graduate engineer Heike Hupfer for the MS measurements.
Footnotes
Abbreviations:
- CHN
- carbon-hydrogen-nitrogen
- GSL
- glycosphingolipid
- H2O
- water
- HPTLC
- high-performance TLC
- MeOH
- methanol
- NP
- normal phase, RP, reversed phase
- SCDase
- sphingolipid ceramide N-deacylase
Financial support for this work was provided by the European Community (7th framework program “Lipidomic Net”, proposal number 202272).
REFERENCES
- 1.Kolter T. 2012. Ganglioside biochemistry. ISRN Biochemistry. 2012: 36 http://dx.doi.org/10.5402/2012/506160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang B., Brand-Miller J. 2003. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 57: 1351–1369. [DOI] [PubMed] [Google Scholar]
- 3.Schnaar R. L., Gerardy-Schahn R., Hildebrandt H. 2014. Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94: 461–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sonnino S., Chigorno V. 2000. Ganglioside molecular species containing C18- and C20-sphingosine in mammalian nervous tissues and neuronal cell cultures. Biochim. Biophys. Acta. 1469: 63–77. [DOI] [PubMed] [Google Scholar]
- 5.Sugiura Y., Shimma S., Konishi Y., Yamada M. K., Setou M. 2008. Imaging mass spectrometry technology and application on ganglioside study; visualization of age-dependent accumulation of C20-ganglioside molecular species in the mouse hippocampus. PLoS ONE. 3: e3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu R. K., Nakatani Y., Yanagisawa M. 2009. The role of glycosphingolipid metabolism in the developing brain. J. Lipid Res. 50(Suppl): S440–S445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell T. D. 2007. Cholera toxin, LT-I, LT-IIa and LT-IIb: the critical role of ganglioside binding in immunomodulation by type I and type II heat-labile enterotoxins. Expert Rev. Vaccines. 6: 821–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schnaar R. L. 2010. Brain gangliosides in axon-myelin stability and axon regeneration. FEBS Lett. 584: 1741–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kabayama K., Sato T., Saito K., Loberto N., Prinetti A., Sonnino S., Kinjo M., Igarashi Y., Inokuchi J. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA. 104: 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coskun Ü., Grzybek M., Drechsel D., Simons K. 2011. Regulation of human EGF receptor by lipids. Proc. Natl. Acad. Sci. USA. 108: 9044–9048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukherjee P., Faber A. C., Shelton L. M., Baek R. C., Chiles T. C., Seyfried T. N. 2008. Thematic review series: sphingolipids. Ganglioside GM3 suppresses the proangiogenic effects of vascular endothelial growth factor and ganglioside GD1a. J. Lipid Res. 49: 929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraĉun I., Rösner H., Cosović C., Stavljenić A. 1984. Topographical atlas of the gangliosides of the adult human brain. J. Neurochem. 43: 979–989. [DOI] [PubMed] [Google Scholar]
- 13.Müthing J. 1996. High-resolution thin-layer chromatography of gangliosides. J. Chromatogr. A. 720: 3–25. [DOI] [PubMed] [Google Scholar]
- 14.Farwanah H., Kolter T. 2012. Lipidomics of glycosphingolipids. Metabolites. 2: 134–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levery S. B. 2005. Glycosphingolipid structural analysis and glycosphingolipidomics. Methods Enzymol. 405: 300–369. [DOI] [PubMed] [Google Scholar]
- 16.Fong B., Norris C., Lowe E., McJarrow P. 2009. Liquid chromatography-high-resolution mass spectrometry for quantitative analysis of gangliosides. Lipids. 44: 867–874. [DOI] [PubMed] [Google Scholar]
- 17.Fong B., Norris C., McJarrow P. 2011. Liquid chromatography-high-resolution electrostatic ion-trap mass spectrometric analysis of GD3 ganglioside in dairy products. Int. Dairy J. 21: 42–47. [Google Scholar]
- 18.Lee H., German J. B., Kjelden R., Lebrilla C. B., Barile D. 2013. Quantitative analysis of gangliosides in bovine milk and colostrum-based dairy products by ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 61: 9689–9696. [DOI] [PubMed] [Google Scholar]
- 19.Gu J., Tifft C. J., Soldin S. J. 2008. Simultaneous quantification of GM1 and GM2 gangliosides by isotope dilution tandem mass spectrometry. Clin. Biochem. 41: 413–417. [DOI] [PubMed] [Google Scholar]
- 20.Pirman D. A., Reich R. F., Kiss A., Heeren R. M., Yost R. A. 2013. Quantitative MALDI tandem mass spectrometric imaging of cocaine from brain tissue with a deuterated internal standard. Anal. Chem. 85: 1081–1089. [DOI] [PubMed] [Google Scholar]
- 21.Neuenhofer S., Conzelmann E., Schwarzmann G., Egge H., Sandhoff K. 1986. Occurrence of lysoganglioside lyso-GM2 (II3-Neu5Ac-gangliotriaosylsphingosine) in GM2 gangliosidosis brain. Biol. Chem. Hoppe Seyler. 367: 241–244. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi T., Goto I., Okada S., Orii T., Ohno K., Nakano T. 1992. Accumulation of lysosphingolipids in tissues from patients with GM1 and GM2 gangliosidoses. J. Neurochem. 59: 1452–1458. [DOI] [PubMed] [Google Scholar]
- 23.Kodama T., Togawa T., Tsukimura T., Kawashima I., Matsuoka K., Kitakaze K., Tsuji D., Itoh K., Ishida Y., Suzuki M., et al. 2011. Lyso-GM2 ganglioside: a possible biomarker of Tay-Sachs disease and Sandhoff disease. PLoS ONE. 6: e29074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cuadros-Rodríguez L., Bagur-González M. G., Sánchez-Viñas M., González-Casado A., Gómez-Sáez A. M. 2007. Principles of analytical calibration/quantification for the separation sciences. J. Chromatogr. A. 1158: 33–46. [DOI] [PubMed] [Google Scholar]
- 25.Zamfir A., Vukelic Z., Peter-Katalinic J. 2002. A capillary electrophoresis and off-line capillary electrophoresis/electrospray ionization-quadrupole time of flight-tandem mass spectrometry approach for ganglioside analysis. Electrophoresis. 23: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 26.Williams M. A., McCluer R. H. 1980. The use of Sep-Pak C18 cartridges during the isolation of gangliosides. J. Neurochem. 35: 266–269. [DOI] [PubMed] [Google Scholar]
- 27.Domon B., Costello C. 1988. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconj. J. 5: 397–409. [Google Scholar]
- 28.Svennerholm L. 1957. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim. Biophys. Acta. 24: 604–611. [DOI] [PubMed] [Google Scholar]
- 29.Miettinen T., Takkiluukkainen I. T. 1959. Use of butyl acetate in determination of sialic acid. Acta Chem. Scand. 13: 856–858. [Google Scholar]
- 30.Momoi T., Ando S., Magai Y. 1976. High resolution preparative column chromatographic system for gangliosides using DEAE-Sephadex and a new porus silica, Iatrobeads. Biochim. Biophys. Acta. 441: 488–497. [PubMed] [Google Scholar]
- 31.Sonnino S., Acquotti D., Kirschner G., Uguaglianza A., Zecca L., Rubino F., Tettamanti G. 1992. Preparation of lyso-GM1 (II3Neu5AcGgOse4-long chain bases) by a one-pot reaction. J. Lipid Res. 33: 1221–1226. [PubMed] [Google Scholar]
- 32.Ito M., Kita K., Kurita T., Sueyoshi N., Izu H. 2000. Enzymatic N-deacylation of sphingolipids. Methods Enzymol. 311: 297–303. [DOI] [PubMed] [Google Scholar]
- 33.Tagawa Y., Laroy W., Nimrichter L., Fromholt S. E., Moser A. B., Moser H. W., Schnaar R. L. 2002. Anti-ganglioside antibodies bind with enhanced affinity to gangliosides containing very long chain fatty acids. Neurochem. Res. 27: 847–855. [DOI] [PubMed] [Google Scholar]
- 34.Lapidot Y., Rappoport S., Wolman Y. 1967. Use of esters of N-hydroxysuccinimide in the synthesis of N-acylamino acids. J. Lipid Res. 8: 142–145. [PubMed] [Google Scholar]
- 35.Kita K., Kurita T., Ito M. 2001. Characterization of the reversible nature of the reaction catalyzed by sphingolipid ceramide N-deacylase. A novel form of reverse hydrolysis reaction. Eur. J. Biochem. 268: 592–602. [DOI] [PubMed] [Google Scholar]
- 36.Schwarzmann G., Sandhoff K. 1987. Lysogangliosides: synthesis and use in preparing labeled gangliosides. Methods Enzymol. 138: 319–341. [DOI] [PubMed] [Google Scholar]
- 37.Distler J. J., Jourdian G. W. 1973. The purification and properties of beta-galactosidase from bovine testes. J. Biol. Chem. 248: 6772–6780. [PubMed] [Google Scholar]
- 38.Anderson D. R., Ung T., Mkrtumyan G., Bertrand G., Grubbs R. H., Schrodi Y. 2008. Kinetic Selectivity of olefin metathesis catalysts bearing cyclic (alkyl)(amino)carbenes. Organometallics. 27: 563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nickel A., Ung T., Mkrtumyan G., Uy J., Lee C., Stoianova D., Papazian J., Wei W-H., Mallari A., Schrodi Y., et al. 2012. A highly efficient olefin metathesis process for the synthesis of terminal alkenes from fatty acid esters. Top. Catal. 55: 518–523. [Google Scholar]
- 40.Marinescu S. C., Levine D. S., Zhao Y., Schrock R. R., Hoveyda A. H. 2011. Isolation of pure disubstituted E olefins through Mo-catalyzed Z-selective ethenolysis of stereoisomeric mixtures. J. Am. Chem. Soc. 133: 11512–11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyazaki H., Herbert M. B., Liu P., Dong X., Xu X., Keitz B. K., Ung T., Mkrtumyan G., Houk K. N., Grubbs R. H. 2013. Z-selective ethenolysis with a ruthenium metathesis catalyst: experiment and theory. J. Am. Chem. Soc. 135: 5848–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fürstner A. 2013. Teaching metathesis “simple” stereochemistry. Science. 341: 1229713. [DOI] [PubMed] [Google Scholar]
- 43.Schwarzmann G., Hofmann P., Putz U. 1997. Synthesis of ganglioside GM1 containing a thioglycosidic bond to its labeled ceramide(s). A facile synthesis starting from natural gangliosides. Carbohydr. Res. 304: 43–52. [DOI] [PubMed] [Google Scholar]
- 44.Stewart I. C., Douglas C. J., Grubbs R. H. 2008. Increased efficiency in cross-metathesis reactions of sterically hindered olefins. Org. Lett. 10: 441–444. [DOI] [PubMed] [Google Scholar]
- 45.Sugahara K., Sugimoto K., Nomura O., Usui T. 1980. Enzymatic assay of serum sialic acid. Clin. Chim. Acta. 108: 493–498. [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi T., Goto I. 1991. A sensitive assay of lysogangliosides using high-performance liquid chromatography. Biochim. Biophys. Acta. 1081: 159–166. [DOI] [PubMed] [Google Scholar]
- 47.Chigorno V., Sonnino S., Ghidoni R., Tettamanti G. 1982. Densitometric quantification of brain gangliosides separated by two-dimensional thin layer chromatography. Neurochem. Int. 4: 397–404. [DOI] [PubMed] [Google Scholar]
- 48.Ando T., Li S. C., Ito M., Li Y. T. 2005. Facile method for the preparation of lyso-GM1 and lyso-GM2. J. Chromatogr. A. 1078: 193–195. [DOI] [PubMed] [Google Scholar]
- 49.Larsson E. A., Olsson U., Whitmore C. D., Martins R., Tettamanti G., Schnaar R. L., Dovichi N. J., Palcic M. M., Hindsgaul O. 2007. Synthesis of reference standards to enable single cell metabolomic studies of tetramethylrhodamine-labeled ganglioside GM1. Carbohydr. Res. 342: 482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Neuenhofer S., Schwarzmann G., Egge H., Sandhoff K. 1985. Synthesis of lysogangliosides. Biochemistry. 24: 525–532. [DOI] [PubMed] [Google Scholar]
- 51.Kurita T., Izu H., Sano M., Ito M., Kato I. 2000. Enhancement of hydrolytic activity of sphingolipid ceramide N-deacylase in the aqueous-organic biphasic system. J. Lipid Res. 41: 846–851. [PubMed] [Google Scholar]