Abstract
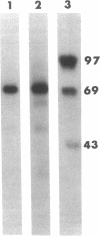
In previous investigations, we have found that the liver appears to be the major source of cholesterol in the human fetus, and, in particular, a principal source of circulating low density lipo-protein-cholesterol (LDL-C). LDL-C plasma levels are low in the normal fetus, most likely due to the rapid uptake and metabolism by the fetal adrenal as precursor for steroid hormone biosynthesis. In contrast, in the anencephalic fetus the adrenals are atrophic, the rate of estrogen and glucocorticoid production is low, and the levels of LDL-C in fetal plasma are high. The purpose of the present investigation was to determine the activity of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the primary rate-limiting enzyme of cholesterol biosynthesis, in anencephalic liver and normal fetal liver. We found that the specific activity of HMG-CoA reductase in normal fetal liver microsomes was 0.428 +/- 0.054 nmol mevalonate formed times mg-1 protein X min-1 (mean +/- SE, n = 9). The rate of HMG-CoA reductase in anencephalic liver microsome preparations was 10-fold less (0.040 +/- 0.003) (mean +/- SE, n = 7) P less than 0.001. Furthermore, we detected HMG-CoA reductase (97,000-mol wt protein) in normal human fetal liver after SDS PAGE and immunoblotting by using a monoclonal antibody directed against HMG-CoA reductase. We were unable to detect any significant quantity of HMG-CoA reductase protein in anencephalic fetal liver, which indicates that low reductase activity was due to low amounts of enzyme protein rather than inactive enzyme. In summary, we conclude that the low levels of cholesterol synthesis observed in anencephalic fetal liver are probably due to both the high levels of LDL-C in fetal plasma as well as the presence of low circulating levels of estrogens and glucocorticoids and that these factors regulate cholesterol synthesis both in vivo and in vitro in fetal liver. This occurs most probably by the modulation of the amount of HMG-CoA reductase, a primary rate-limiting and regulatory enzyme of the cholesterol biosynthetic sequence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENIRSCHKE K. Adrenals in anencephaly and hydrocephaly. Obstet Gynecol. 1956 Oct;8(4):412–425. [PubMed] [Google Scholar]
- Beaufay H., Amar-Costesec A., Feytmans E., Thinès-Sempoux D., Wibo M., Robbi M., Berthet J. Analytical study of microsomes and isolated subcellular membranes from rat liver. I. Biochemical methods. J Cell Biol. 1974 Apr;61(1):188–200. doi: 10.1083/jcb.61.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Human hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: evidence for the regulation of enzymic activity by a bicyclic phosphorylation cascade. Biochem Biophys Res Commun. 1984 Mar 15;119(2):488–498. doi: 10.1016/s0006-291x(84)80275-2. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carr B. R., MacDonald P. C., Simpson E. R. The regulation of de novo synthesis of cholesterol in the human fetal adrenal gland by low density lipoprotein and adrenocorticotropin. Endocrinology. 1980 Oct;107(4):1000–1006. doi: 10.1210/endo-107-4-1000. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Ohashi M., MacDonald P. C., Simpson E. R. Human anencephalic adrenal tissue: low density lipoprotein metabolism and cholesterol synthesis. J Clin Endocrinol Metab. 1981 Aug;53(2):406–411. doi: 10.1210/jcem-53-2-406. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Cholesterol synthesis by human fetal hepatocytes: effect of lipoproteins. Am J Obstet Gynecol. 1984 Nov 1;150(5 Pt 1):551–557. doi: 10.1016/s0002-9378(84)90438-1. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Cholesterol synthesis by human fetal hepatocytes: effects of hormones. J Clin Endocrinol Metab. 1984 Jun;58(6):1111–1116. doi: 10.1210/jcem-58-6-1111. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Cholesterol synthesis in human fetal tissues. J Clin Endocrinol Metab. 1982 Sep;55(3):447–452. doi: 10.1210/jcem-55-3-447. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland. Endocr Rev. 1981 Summer;2(3):306–326. doi: 10.1210/edrv-2-3-306. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Synthesis of cholesterol in the human fetus: 3-hydroxy-3-methylglutaryl coenzyme A reductase activity of liver microsomes. J Clin Endocrinol Metab. 1981 Oct;53(4):810–812. doi: 10.1210/jcem-53-4-810. [DOI] [PubMed] [Google Scholar]
- DAVIS M. E., GOULD R. G., LEROY G. V., PLOTZ E. J., WERBIN H. Hormones in human reproduction. I. Metabolism of progesterone. Am J Obstet Gynecol. 1956 Oct;72(4):740–755. doi: 10.1016/0002-9378(56)90168-5. [DOI] [PubMed] [Google Scholar]
- Das S. K., Foster H. W., Adhikary P. K., Mody B. B., Bhattacharyya D. K. Gestational variation of fatty acid composition of human amniotic fluid lipids. Obstet Gynecol. 1975 Apr;45(4):425–432. [PubMed] [Google Scholar]
- Eisenberg S., Levy R. I. Lipoprotein metabolism. Adv Lipid Res. 1975;13:1–89. [PubMed] [Google Scholar]
- Gray E. S., Abramovich D. R. Morphologic features of the anencephalic adrenal gland in early pregnancy. Am J Obstet Gynecol. 1980 Jun 15;137(4):491–495. doi: 10.1016/0002-9378(80)91134-5. [DOI] [PubMed] [Google Scholar]
- Hellig H., Gattereau D., Lefebvre Y., Bolté E. Steroid production from plasma cholesterol. I. Conversion of plasma cholesterol to placental progesterone in humans. J Clin Endocrinol Metab. 1970 May;30(5):624–631. doi: 10.1210/jcem-30-5-624. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Elder J. H. Antibody directed to determinants of a Moloney virus derived MCF GP70 recognizes a thymic differentiation antigen. J Exp Med. 1983 Nov 1;158(5):1751–1756. doi: 10.1084/jem.158.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin D. S., Pitkin R. M., Connor W. E. Placental transfer of cholesterol into the human fetus. Am J Obstet Gynecol. 1977 Aug 1;128(7):735–739. doi: 10.1016/0002-9378(77)90713-x. [DOI] [PubMed] [Google Scholar]
- Liscum L., Cummings R. D., Anderson R. G., DeMartino G. N., Goldstein J. L., Brown M. S. 3-Hydroxy-3-methylglutaryl-CoA reductase: a transmembrane glycoprotein of the endoplasmic reticulum with N-linked "high-mannose" oligosaccharides. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7165–7169. doi: 10.1073/pnas.80.23.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi M., Carr B. R., Simpson E. R. Low density lipoprotein receptors in adrenal tissue of a human anencephalic fetus. Early Hum Dev. 1982 Nov;7(2):149–154. doi: 10.1016/0378-3782(82)90133-5. [DOI] [PubMed] [Google Scholar]
- Parker C. R., Jr, Carr B. R., Simpson E. R., MacDonald P. C. Decline in the concentration of low-density lipoprotein-cholesterol in human fetal plasma near term. Metabolism. 1983 Sep;32(9):919–923. doi: 10.1016/0026-0495(83)90207-x. [DOI] [PubMed] [Google Scholar]
- Parker C. R., Jr, Carr B. R., Winkel C. A., Casey M. L., Simpson E. R., MacDonald P. C. Hypercholesterolemia due to elevated low density lipoprotein-cholesterol in newborns with anencephaly and adrenal atrophy. J Clin Endocrinol Metab. 1983 Jul;57(1):37–43. doi: 10.1210/jcem-57-1-37. [DOI] [PubMed] [Google Scholar]
- Parker C. R., Jr, Simpson E. R., Bilheimer D. W., Leveno K., Carr B. R., MacDonald P. C. Inverse relation between low-density lipoprotein-cholesterol and dehydroisoandrosterone sulfate in human fetal plasma. Science. 1980 May 2;208(4443):512–514. doi: 10.1126/science.6445079. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Kurnit D. M., Breslow J. L. Hepatic apo-A-I and apo-E and intestinal apo-A-I are synthesized in precursor isoprotein forms by organ cultures of human fetal tissues. J Biol Chem. 1982 Jan 10;257(1):536–544. [PubMed] [Google Scholar]