Abstract
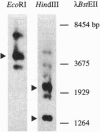
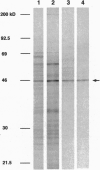
A cDNA encoding a cytochrome P450-dependent oxidase, berbamunine synthase (EC 1.1.3.34; CYP80), from cell suspension cultures of the higher plant Berberis stolonifera Koehne and Wolf (barberry) has been isolated and heterologously expressed in functional form in insect cell culture using a baculovirus-based expression system. This cytochrome P450-dependent enzyme is unusual in that it catalyzes the regio- and stereoselective formation of a C--O phenol couple in bisbenzylisoquinoline alkaloid biosynthesis without concomitant incorporation of activated oxygen into the product. Consistent with the function of an oxidase rather than a monooxygenase, an essential glycine residue in the distal helix, which forms the oxygen-binding pocket in the well-studied bacterial enzyme P-450cam, is replaced by proline at the equivalent position in berbamunine synthase. This oxidase was accumulated in an active form in insect cell microsomes and accepted electrons from the endogenous NADPH-cytochrome P450 reductase. The heterologously expressed enzyme oxidatively couples either two molecules of (R)-N-methylcoclaurine to form the (R,R) dimer guattegaumerine or one molecule each of (R)- and (S)-N-methylcoclaurine to form the (R,S) dimer berbamunine. The ratio of the two bisbenzylisoquinolines formed could be altered by reductase source or by varying the enantiomer composition of the substrates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asseffa A., Smith S. J., Nagata K., Gillette J., Gelboin H. V., Gonzalez F. J. Novel exogenous heme-dependent expression of mammalian cytochrome P450 using baculovirus. Arch Biochem Biophys. 1989 Nov 1;274(2):481–490. doi: 10.1016/0003-9861(89)90461-x. [DOI] [PubMed] [Google Scholar]
- Bozak K. R., Yu H., Sirevåg R., Christoffersen R. E. Sequence analysis of ripening-related cytochrome P-450 cDNAs from avocado fruit. Proc Natl Acad Sci U S A. 1990 May;87(10):3904–3908. doi: 10.1073/pnas.87.10.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Tamura S., Gelboin H. V. Expression of mammalian cytochrome P450 using baculovirus. Methods Enzymol. 1991;206:93–99. doi: 10.1016/0076-6879(91)06080-m. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Holton T. A., Brugliera F., Lester D. R., Tanaka Y., Hyland C. D., Menting J. G., Lu C. Y., Farcy E., Stevenson T. W., Cornish E. C. Cloning and expression of cytochrome P450 genes controlling flower colour. Nature. 1993 Nov 18;366(6452):276–279. doi: 10.1038/366276a0. [DOI] [PubMed] [Google Scholar]
- Imai M., Shimada H., Watanabe Y., Matsushima-Hibiya Y., Makino R., Koga H., Horiuchi T., Ishimura Y. Uncoupling of the cytochrome P-450cam monooxygenase reaction by a single mutation, threonine-252 to alanine or valine: possible role of the hydroxy amino acid in oxygen activation. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7823–7827. doi: 10.1073/pnas.86.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutchan T. M., Bock A., Dittrich H. Heterologous expression of the plant proteins strictosidine synthase and berberine bridge enzyme in insect cell culture. Phytochemistry. 1994 Jan;35(2):353–360. doi: 10.1016/s0031-9422(00)94763-0. [DOI] [PubMed] [Google Scholar]
- Kutchan T. M., Hampp N., Lottspeich F., Beyreuther K., Zenk M. H. The cDNA clone for strictosidine synthase from Rauvolfia serpentina. DNA sequence determination and expression in Escherichia coli. FEBS Lett. 1988 Sep 12;237(1-2):40–44. doi: 10.1016/0014-5793(88)80167-4. [DOI] [PubMed] [Google Scholar]
- Köhrer K., Kutchan T. M., Domdey H. Specific oligodeoxynucleotide probes obtained through RNA sequencing. DNA. 1989 Mar;8(2):143–147. doi: 10.1089/dna.1.1989.8.143. [DOI] [PubMed] [Google Scholar]
- Miller L. K. Insect baculoviruses: powerful gene expression vectors. Bioessays. 1989 Oct;11(4):91–95. doi: 10.1002/bies.950110404. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Ward E., DiMaio J., Ohta D., Ryals J., Sato R. Molecular cloning and sequencing of a cDNA encoding mung bean cytochrome P450 (P450C4H) possessing cinnamate 4-hydroxylase activity. Biochem Biophys Res Commun. 1993 Feb 15;190(3):875–880. doi: 10.1006/bbrc.1993.1130. [DOI] [PubMed] [Google Scholar]
- O'keefe D. P., Leto K. J. Cytochrome P-450 from the Mesocarp of Avocado (Persea americana). Plant Physiol. 1989 Apr;89(4):1141–1149. doi: 10.1104/pp.89.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos T. L., Finzel B. C., Gunsalus I. C., Wagner G. C., Kraut J. The 2.6-A crystal structure of Pseudomonas putida cytochrome P-450. J Biol Chem. 1985 Dec 25;260(30):16122–16130. [PubMed] [Google Scholar]
- Shet M. S., Sathasivan K., Arlotto M. A., Mehdy M. C., Estabrook R. W. Purification, characterization, and cDNA cloning of an NADPH-cytochrome P450 reductase from mung bean. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2890–2894. doi: 10.1073/pnas.90.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W. C., Funk C. D., Brash A. R. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8519–8523. doi: 10.1073/pnas.90.18.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler R., Zenk M. H. The purification and characterization of a unique cytochrome P-450 enzyme from Berberis stolonifera plant cell cultures. J Biol Chem. 1993 Jan 15;268(2):823–831. [PubMed] [Google Scholar]
- Strittmatter P., Fleming P., Connors M., Corcoran D. Purification of cytochrome b5. Methods Enzymol. 1978;52:97–101. doi: 10.1016/s0076-6879(78)52010-7. [DOI] [PubMed] [Google Scholar]
- Teutsch H. G., Hasenfratz M. P., Lesot A., Stoltz C., Garnier J. M., Jeltsch J. M., Durst F., Werck-Reichhart D. Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate 4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4102–4106. doi: 10.1073/pnas.90.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toguri T., Tokugawa K. Cloning of eggplant hypocotyl cDNAs encoding cytochromes P450 belonging to a novel family (CYP77). FEBS Lett. 1994 Feb 7;338(3):290–294. doi: 10.1016/0014-5793(94)80286-6. [DOI] [PubMed] [Google Scholar]
- Toguri T., Umemoto N., Kobayashi O., Ohtani T. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol Biol. 1993 Dec;23(5):933–946. doi: 10.1007/BF00021810. [DOI] [PubMed] [Google Scholar]
- Umemoto N., Kobayashi O., Ishizaki-Nishizawa O., Toguri T. cDNAs sequences encoding cytochrome P450 (CYP71 family) from eggplant seedlings. FEBS Lett. 1993 Sep 13;330(2):169–173. doi: 10.1016/0014-5793(93)80266-w. [DOI] [PubMed] [Google Scholar]
- Vaughn J. L., Goodwin R. H., Tompkins G. J., McCawley P. The establishment of two cell lines from the insect Spodoptera frugiperda (Lepidoptera; Noctuidae). In Vitro. 1977 Apr;13(4):213–217. doi: 10.1007/BF02615077. [DOI] [PubMed] [Google Scholar]
- Vetter H. P., Mangold U., Schröder G., Marner F. J., Werck-Reichhart D., Schröder J. Molecular Analysis and Heterologous Expression of an Inducible Cytochrome P-450 Protein from Periwinkle (Catharanthus roseus L.). Plant Physiol. 1992 Oct;100(2):998–1007. doi: 10.1104/pp.100.2.998. [DOI] [PMC free article] [PubMed] [Google Scholar]