Abstract
Objective
To examine if the genes encoding the pleckstrin homology domain–containing protein gene (PLEKHA1), hypothetical LOC387715/ARMS2 gene, and HtrA serine peptidase 1 gene (HTRA1) located on the long arm of chromosome 10 (10q26 region) confer risk for neovascular age-related macular degeneration (AMD) in an independent or interactive manner when controlling for complement factor H gene (CFH) genotype and smoking exposure.
Design
Retrospective matched-pair case– control study.
Participants
Hospital clinic-based sample of 134 unrelated patients with neovascular AMD who have a sibling with normal maculae (268 subjects).
Methods
Disease status was ascertained by at least 2 investigators by review of fundus photographs and/or fluorescein angiography according to the Age-Related Eye Disease Study grading scale. If necessary, a home retinal examination was performed (n = 6). A combination of direct sequencing and analysis of 8 highly polymorphic microsatellite markers was used to genotype 33 megabases of the 10q26 region on leukocyte DNA. Smoking history was obtained via a standardized questionnaire and measured in pack-years. The family-based association test, haplotype analysis, multiple conditional logistic regression, and linkage analysis were used to determine significant associations.
Main Outcome Measure
Neovascular AMD status.
Results
Of the 23 variants we identified in the 10q26 region, 6 were significant. Four of the 6 were novel and included 2 genotypes that reduced risk of AMD. Many single-nucleotide polymorphisms (SNPs), including the previously reported variants rs10490924 (hypothetical LOC387715/ARMS2) and rs11200638 (HTRA1), defined 2 significant haplotypes associated with increased risk of neovascular AMD. The coding HTRA1 SNP rs2293870, not part of the significant haplotypes containing rs10490924 and rs11200638, showed as strong an association with increased susceptibility to neovascular AMD. Linkage analysis supported our findings of SNP association (P<10−15). No significant interactions were found between any of the SNPs in the 10q26 and smoking or between these SNPs and CFH genotype.
Conclusions
Independent of CFH genotype or smoking history, an individual’s risk of AMD could be increased or decreased, depending on their genotype or haplotype in the 10q26 region.
Neovascular age-related macular degeneration (AMD) is characterized by the growth of abnormal new blood vessels beneath the retina that can cause severe and rapid vision loss due to hemorrhage and exudation. It is this more advanced form that is responsible for the majority of debilitating vision loss due to AMD. In the United States, it is predicted that about 3 million individuals over the age of 50 will have advanced AMD in at least one eye by 2020.1
Current diagnostic methods focus on the detection of neovascular AMD, because available treatments are directed against this advanced stage of the disease. Although the newest treatments offer some chance of visual improvement, they require invasive delivery methods, cannot prevent disease, and have limited ability to reverse vision loss. Assessments of an individual’s risk of developing advanced AMD are based on ocular findings in those who already have the early stages. Methods have yet to be discovered that determine which individuals are at highest risk of vision loss due to AMD prior to the development of any signs of the disease. The identification of genetic variants that could be used as biomarkers would help to predict risk of the more advanced stages of AMD. Moreover, discovering precisely which genes and environmental factors contribute to the pathophysiology of AMD and elucidating their modes of interaction could provide new targets for therapeutic or behavioral intervention, thereby reducing or preventing the incidence of this disease.
Although the complement factor H gene (CFH) Y402H variant on 1q32 appears to be the genetic risk factor most consistently associated with AMD,2–6 the 10q26 region, where the pleckstrin homology domain– containing protein gene (PLEKHA1), hypothetical LOC387715/ARMS2 gene, and HtrA serine peptidase 1 gene (HTRA1) reside adjacent to one another (Table 1 [available at http://aaojournal.org), appears to be the most strongly associated overall with AMD susceptibility.7 Although many reports have demonstrated that variant rs10490924 in hypothetical LOC387715/ ARMS2 gene is associated with all types of AMD,8–11 it was recently shown that the single-nucleotide polymorphism (SNP) rs11200638 in the HTRA1 promoter, in linkage disequilibrium (LD) with rs10490924, is likely the causal variant.12–14 Moreover, data from the Age-Related Eye Disease Study showed a significant association between the SNP rs1045216 in PLEKHA1 and increased risk of neovascular AMD.11
Table 1.
Location of Microsatellite Markers and Single-Nucleotide Polymorphisms (SNPs)
| Marker/SNP | Physical Location (Base Pairs) |
|---|---|
| D10S1690 | 95984772 |
| D10S1230 | 122732682 |
| D10S1483 | 123273561 |
| PLEKHA1 rs1045216 | 124179187 |
| LOC387715/ARMS2 rs10490923 | 124204241 |
| LOC387715/ARMS2 rs2736911 | 124204345 |
| LOC387715/ARMS2 rs10490924 | 124204438 |
| LOC387715/ARMS2 rs10664316 | 124206376 |
| LOC387715/ARMS2 rs7088128 | 124206400 |
| HTRA1 rs11200638 | 124210534 |
| HTRA1 −602 from ATG* G>A | 124210557 |
| HTRA1 −502 from ATG* C>T | 124210657 |
| HTRA1 rs2672598 | 124210672 |
| HTRA1 rs1049331 | 124211260 |
| HTRA1 rs2293870 | 124211266 |
| HTRA1 rs12267142 | 124238232 |
| HTRA1 rs2239586 | 124239225 |
| HTRA1 rs2672582 | 124256490 |
| HTRA1 intron 5 (del GTTT) | 124256969–124256981 |
| HTRA1 rs2672583 | 124257077 |
| HTRA1 rs3013206 | 124257163 |
| HTRA1 rs2672585 | 124258391 |
| HTRA1 rs11538140 | 124261518 |
| HTRA1 rs2272599 | 124261585 |
| HTRA1 rs28689095 | 124261715 |
| HTRA1 rs2293871 | 124263661 |
| D10S587 | 125178595 |
| D10S1213 | 125356627 |
| D10S1723 | 125650839 |
| D10S1656 | 126091772 |
| D10S1222 | 129150739 |
The physical location of each microsatellite marker was determined using Map-O-Mat (http://compgen.rutgers.edu/mapomat), and the physical location of each SNP was determined using Ensembl (http://www.ensembl.org/Homo_sapiens/index.html).
First coding ATG.
In the study presented here, we genotyped 33 megabases of the 10q26 region (Table 1) in samples from unrelated patients with neovascular AMD who had one sibling with normal maculae and was past the age at which the affected sibling was diagnosed with neovascular AMD. Our phenotypically well-defined cohort of 268 subjects comprised 134 extremely discordant sibpairs—that is, pairs with one member in the upper 10% of disease severity (affected sibling) and the other member in the bottom 10% to 30% of disease severity (unaffected sibling).15,16 We have previously demonstrated that such types of sibpairs can be powerful in identifying the contribution that genes and environmental factors make simultaneously to AMD susceptibility.17 We sought to validate previous findings and possibly identify novel variants in the 10q26 region using a combination of direct sequencing and analysis of highly polymorphic microsatellite markers tightly linked to the genes of interest. For each subject, we directly sequenced exon 12 of PLEKHA1, the entire putative coding region of LOC387715/ARMS2, and the promoter region and entire coding region of HTRA1 to identify the contribution that allelic risk factors such as those reported in the 10q26 region make independently and in combination with the factors most consistently associated with AMD susceptibility, CFH Y402H genotype2–6,18 and smoking.19
Materials and Methods
Patient Population
The protocol was reviewed and approved by the institutional review boards at the Massachusetts Eye and Ear Infirmary, Boston, Massachusetts, and William Beaumont Hospital, Royal Oak, Michigan, and conforms to the tenets of the Declaration of Helsinki. Eligible patients were enrolled in this study after they gave informed consent in person, over the phone, or through the mail before answering a standardized questionnaire and donating 10 to 50 ml of venous blood. In most cases, the donation of venous blood from both patients and their siblings was made on the same day that fundus photographs and/or fluorescein angiographs were obtained.
Details of the recruitment of patients and their siblings are described elsewhere.20 In brief, index patients with neovascular AMD were recruited from the Retina Service of the Massachusetts Eye and Ear Infirmary and Associated Retina Consultants at the Beaumont Hospital. All index patients were 50 years or older and had the neovascular form of AMD in at least one eye, defined by subretinal hemorrhage, fibrosis, or fluorescein angiographic presence of neovascularization documented at the time of or before enrollment in the study. Patients whose only exudative finding was a retinal pigment epithelium detachment were excluded because this finding may not represent definite neovascular AMD and, therefore, the severe phenotype we sought. Patients with signs of pathologic myopia, presumed ocular histoplasmosis syndrome, angioid streaks, choroidal rupture, any hereditary retinal diseases other than AMD, and previous laser treatment due to retinal conditions other than AMD were also excluded.
The unaffected siblings had normal maculae at an age older than that at which the index patient was first diagnosed with neovascular AMD. Normal maculae (defined as the zone centered at the foveola and extending 2 disc diameters [3000 μm] in radius) fulfilled the criteria of 0 to 5 small drusen (all <63 μm in diameter), no pigment abnormalities, no geographic atrophy, and no neovascularization (as defined previously21,22; AMD level 1 on the Age-Related Eye Disease Study scale). Disease status of every participant was confirmed by at least 2 of the investigators (IKK, JWM, TPD) by evaluation of fundus photographs or fluorescein angiograms, except when one of the investigators directly examined an unaffected sibling during a home visit (n = 6 cases). Subject characteristics of our extremely discordant sibpair population are presented in Table 2 (available at http://aaojournal.org).
Table 2.
Subject Characteristics
| Population | Mean Age | Range | Standard Deviation | % Male (n/134) |
|---|---|---|---|---|
| Affected siblings | 71.28 | 48.97–86.38 | 8.26 | 45.5% (61) |
| Unaffected siblings | 72.77 | 41.32–90.86 | 8.97 | 39.6% (53) |
Ascertainment of Smoking Exposure
We administered a standardized questionnaire to all eligible participants in person or over the phone to ascertain smoking exposure, with the age of the index patient at the time of the fundus photographs as our cutoff reference age for smoking exposure for all members in the sibpair. In most cases, the diagnosis of AMD was made simultaneously with the diagnosis of neovascular AMD. If a participant ever smoked, we recorded the age when they started smoking, age when they quit (if they did quit), and number of packs of cigarettes smoked per day, on average. Based on the responses, the number of pack-years of cigarettes smoked was calculated for each smoker. Participants who smoked <100 cigarettes during their lifetime (i.e., <1/73 of a pack-year) were categorized as having never smoked. A pack-year was defined as 1 pack of cigarettes per day for 1 year, with 1 pack defined as 20 cigarettes. For our statistical analysis (see below), the reference cutoff for smoking was defined as ≥10 pack-years, versus <10 pack-years. With this cutoff, our subjects were divided into 2 approximately equal groups.17
Genotyping Analysis
Leukocyte DNA was purified by using either the standard phenol-chloroform or the DNAzol (Invitrogen Corp., Carlsbad, CA) extraction protocol. Previously reported oligonucleotide primers were used to amplify the coding region and flanking intronic sequences of exon 12 for PLEKHA1,9 exon 9 of CFH,4 and the promoter sequence for HTRA1.12 For the putative LOC387715/ ARMS2 gene region (including both exons) and the 9 exons of HTRA1, oligonucleotide primers were selected using the Primer3 program (http://primer3.sourceforge.net) to encompass the entire coding region and flanking intronic sequences (Table 3 [available at http://aaojournal.org]).
Table 3.
Primers Used
| Gene | Region | Forward Primer* | Reverse Primer* |
|---|---|---|---|
| PLEKHA1 | Exon 12 | CTGACCGTGTCTGACTGCC | CCCCTTATCATCTTTGGCTA |
| Putative LOC387715/ARMS2 | Exon 1 | TTGTGTGACGGGAAAAGACA | AAGCACCTGAAGGCTGGTTA |
| Putative LOC387715/ARMS2 | Exon 2 | TTGTTACAAAAGGAATGGAATGTC | GGAATGCAGTGACAGAGAGGA |
| HTRA1 | Promoter a | ATGCCACCCACAACAACTTT | GGTTCTCTCGCTGAGATTCG |
| HTRA1 | Promoter b | CGGATGCACCAAAGATTCTCC | TTCGCGTCCTTCAAACTAATGG |
| HTRA1 | Exon 1a | GAGGCCCTCCTGCACTCT | CAGGTTGGCGTAGGTGTTG |
| HTRA1 | Exon 1b | GAGTCGCCATGCAGATCC | CGAGCTGGGATGGAGAGA |
| HTRA1 | Exon 2 | AAACAAACTTGGGCCATCAG | TTGCTAGTGGCGGTGAAAG |
| HTRA1 | Exon 3 | TAGGTGTGTGTGGCTGTTGC | AAGTTTTCCTGAGCCCCTTC |
| HTRA1 | Exon 4 | CGCAGCAAAGGGATGTTAGT | GAATCCACATGGCTTGGTCT |
| HTRA1 | Exon 5 | CCAGGCAGGGACATAGATTG | TCAGCAGCCCAGGAGATTTA |
| HTRA1 | Exon 6 | GGTGTCCTGATGCCTCTCTC | TGCCATGATCAGAGGACAAA |
| HTRA1 | Exon 7 | GTCCAGACCAGGATTTGAGC | CCAAGGCTAATGACCTGTCC |
| HTRA1 | Exon 8 | AGGAGAAGACGGGAACTGGT | CTCGTGGAGCAAGGACTTTT |
| HTRA1 | Exon 9/3′-UTR | CTGACCCACTGATGGTTTGA | CTATTCCAGCAGCCCAGAGT |
Primers are written in the 5′ to 3′ direction and were chosen to encompass the entire coding region and flanking intronic sequences using the Primer3 program (http://primer3.sourceforge.net).
For all 4 genes, polymerase chain reaction (PCR) was used to amplify genomic DNA fragments from 20 ng of leukocyte DNA in a solution of × 10 PCR buffer containing 25 mmol/l of MgCl2; 0.2 mmol/l each of deoxyadenosine triphosphate, deoxythymidine triphosphate, deoxyguanosine triphosphate, and deoxycytidine triphosphate; and 0.5 U of Taq DNA polymerase (USB Corp., Cleveland, OH). For the PLEKHA1 and HTRA1 genes, 5 mol/l of (carboxymethyl) trimethylammonium inner salt was added to each PCR (Sigma-Aldrich, St. Louis, MO). The temperatures used during the PCR were as follows: for PLEKHA1 and HTRA1, 95° C for 5 minutes, followed by 35 cycles of 60° C for 30 seconds, 72° C for 30 seconds, and 95° C for 30 seconds, with a final annealing at 60° C for 1.5 minutes and extension of 72° C for 5 minutes; for LOC387715/ARMS2, 95° C for 5 minutes, followed by 35 cycles of 62° C for 30 seconds, 72° C for 30 seconds, and 95° C for 30 seconds, with a final annealing at 62° C for 1.5 minutes and extension of 72° C for 5 minutes; for CFH, 95° C for 5 minutes, followed by 35 cycles of 56° C for 30 seconds, 72° C for 30 seconds, and 95° C for 30 seconds, with a final annealing at 56° C for 1.5 minutes and extension of 72° C for 5 minutes. For sequencing reactions, PCR products were digested according to the manufacturer’s protocol with exonuclease I and shrimp alkaline phosphatase (USB), then were subjected to a cycle sequencing reaction using the Big Dye Terminator 3.1 Cycle Sequencing kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Products were purified with Performa DTR Ultra 96-well plates (Edge Biosystems, Gaithersburg, MD) to remove excess dye terminators. Samples were sequenced on an ABI Prism 3100 DNA sequencer (Applied Biosystems). Electropherograms generated from the ABI Prism 3100 were analyzed using the Lasergene DNA and protein analysis software (DNASTAR, Inc., Madison, WI). Electropherograms were read by 2 independent evaluators without knowledge of the subject’s disease status. All patients were sequenced in the forward direction (5′–3′) unless variants, polymorphisms, or mutations were identified, in which case confirmation was obtained in some cases by sequencing in the reverse direction.
Genotyping of Microsatellite Markers
We analyzed 8 highly heterozygous microsatellite markers spanning 33 megabases of the 10q26 region (Table 1 [available at http://aaojournal.orgx]). These markers included several that were tightly linked to PLEKHA1, LOC387715/ARMS2, and HTRA1 (Tables 3, 4 [available at http://aaojournal.org]). All markers were fluorescently labeled with either 5-carboxyfluorescein or 6-carboxyfluorescein on the 5′ end of the reverse primer, and an additional sequence of CTGTCTT was added to the 5′ of the forward primer. The PCR was used to amplify genomic DNA fragments from 20 ng of leukocyte DNA in a solution of ×10 PCR buffer containing 25 mmol/l of MgCl2; 0.2 mmol/l each of deoxyadenosine triphosphate, deoxythymidine triphosphate, deoxyguanosine triphosphate, and deoxycytidine triphosphate; and 0.5 U of Taq DNA polymerase (USB). The temperatures used during the PCR were 95° C for 5 minutes, followed by 35 cycles of 54° to 60° C (specific to primer pair) for 30 seconds, 72° C for 30 seconds, and 95° C for 30 seconds, with a final annealing at 54° to 60° C (specific to primer pair) for 1.5 minutes and extension of 72° C for 5 minutes. Polymerase chain reaction products were diluted 1:20 for markers labeled with FAM and 1:10 for markers labeled with HEX. Samples were pooled according to product size and denatured before being genotyped on the ABI 3730xl DNA Analyzer (Applied Biosystems). Data were then analyzed using ABI’s Genemapper 3.7 software for analysis, which interrogates the quality of the size standard and makes the appropriate genotype calls based on size. For quality control purposes, all genotypes were then evaluated manually.
Table 4.
Description of Single-Nucleotide Polymorphisms (SNPs) Analyzed
| rs No. | Gene | Region | Nucleotide Change | Amino Acid Change (If Applicable) | Physical Location (Base Pairs) | MAF (Unaffected) | Frequency in Affected |
|---|---|---|---|---|---|---|---|
| 1045216 | PLEKHA1 | Exon 12 | G>A | Thr320Ala | 124179187 | A = 0.291 | A = 0.254 |
| 10490923 | LOC387715/ARMS2 | Exon 1 | G>A | Arg3His | 124204241 | A = 0.108 | A = 0.060 |
| 2736911 | LOC387715/ARMS2 | Exon 1 | T>C | Arg38End | 124204345 | C = 0.097 | C = 0.086 |
| 10490924 | LOC387715/ARMS2 | Exon 1 | G>T | Ala69Ser | 124204438 | T = 0.328 | T = 0.526 |
| 10664316 | LOC387715/ARMS2 | Intron 1 | del AT | 124206375^124206376 | − = 0.414 | −= 0.269 | |
| 7088128 | LOC387715/ARMS2 | Intron 1 | A>G | 124206400 | G = 0.119 | G = 0.067 | |
| 11200638* | HTRA1 | Promoter | G>A | 124210534 | A = 0.317 | A = 0.522 | |
| HTRA1 | Promoter | G>A | 124210557 | A = 0.022 | A = 0.015 | ||
| HTRA1 | Promoter | C>T | 124210657 | T = 0.060 | T = 0.049 | ||
| 2672598 | HTRA1 | Promoter | C>T | 124210672 | T = 0.496 | T = 0.321 | |
| 1049331 | HTRA1 | Exon 1 | C>T | Ala34Ala | 124211260 | T = 0.317 | T = 0.526 |
| 2293870 | HTRA1 | Exon 1 | G>C† | Gly36Gly | 124211266 | C = 0.090 | C = 0.086 |
| G>T | Gly36Gly | 124211266 | T = 0.317 | T = 0.522 | |||
| 12267142* | HTRA1 | Intron 1 | C>G | 124238232 | G = 0.022 | G = 0.022 | |
| 2239586 | HTRA1 | Intron 3 | C>T | 124239225 | T = 0.108 | T = 0.078 | |
| 2239587 | HTRA1 | Intron 3 | G>A | 124239299 | A = 0.116 | A = 0.086 | |
| 2672582 | HTRA1 | Intron 4 | C>T | 124256490 | T = 0.429 | T = 0.507 | |
| HTRA1 | Intron 5 | del GTTT | 124256973^124256976 | −= 0.392 | −= 0.351 | ||
| 2672583 | HTRA1 | Intron 5 | G>A | 124257077 | A = 0.422 | A = 0.515 | |
| 3013206* | HTRA1 | Intron 5 | A>T | 124257163 | T = 0.045 | T = 0.022 | |
| 2672585 | HTRA1 | Intron 6 | C>G | 124258391 | G = 0.425 | G = 0.507 | |
| 11538140* | HTRA1 | Exon 8 | C>T | Asp407Asp | 124261518 | T = 0.004 | T = 0.004 |
| 2272599 | HTRA1 | Intron 8 | A>G | 124261585 | G = 0.474 | G= 0.545 | |
| 2293871 | HTRA1 | Intron 8 | C>T | 124263661 | T = 0.138 | T = 0.101 |
MAF = minor allele frequency.
The physical location of each SNP was determined using Ensembl (http://www.ensembl.org/Homo_sapiens/index.html).
Variants/SNPs excluded from statistical analysis because MAF did not meet the criteria of ≥5% in both affected and unaffected siblings combined.
The most minor of the 3 alleles
Statistical Analyses
The program FBAT (http://biosun1.harvard.edu/~fbat/fbat.htm), which tests for family-based association, was used to evaluate the effect of each SNP individually on risk of AMD.23 Single-nucleotide polymorphisms were included for analysis in FBAT only if the minor allele frequency in the affected and unaffected siblings combined was ≥5% and the number of informative families was at least 4. A Bonferroni correction was applied to the pointwise P values that were calculated for each allele of the 14 SNPs that met these criteria.
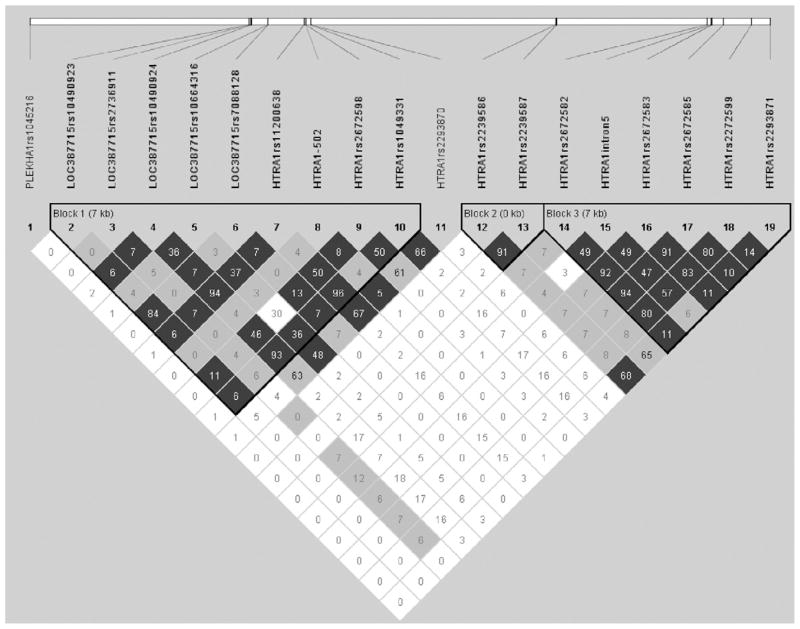
Haploview (http://www.broad.mit.edu/mpg/haploview) was used to generate the LD plot (Fig 1 [available at http://aaojournal.org]) among the 19 identified SNPs that had a minor allele frequency ≥ 5%.24 Linkage disequilibrium (r2) between each of the 19 SNPs is depicted in Figure 1. The haplotype blocks were constructed by Haploview using the method proposed by Gabriel et al.25 Individual haplotypes were inferred and tested for association with AMD using FBAT.23
Figure 1.
Ensembl single-nucleotide polymorphisms (SNPs) are shown along the 10q26 region encompassing the pleckstrin homology domain– containing protein gene, LOC387715/ARMS2, and HtrA serine peptidase 1 gene, illustrating the 3 distinct haplotype blocks that were defined by the confidence intervals, an algorithm proposed by Gabriel et al using Haploview. The linkage disequilibrium (LD) (r2) between any 2 SNPs is listed in the cross cell. The darker the color, the higher the LD between any 2 SNPs. kb = kilobase.
Conditional logistic regression (CLR) (SAS 9.1 [http://www.sas.com]) was performed to identify factors associated with wet AMD. Potential risk factors of interest, as defined above, were evaluated initially one at a time. A multiple CLR model for each significant SNP in the 10q26 region was built using those factors from the single factor model that appeared to be associated with neovascular AMD with P≤0.1. The CFH Y402H CT genotype was kept in the models, although it has P>0.1, to adjust the effect of CFH more precisely. For each significant SNP, the minor allele (in unaffected siblings) in both the homozygous and heterozygous states versus the common allele in the homozygous state was examined in the model.
Genotype and allele frequencies for all SNPs identified as significant were calculated in the affected and separately in unaffected siblings (Table 5 [available at http://aaojournal.org]). Deviation from Hardy–Weinberg equilibrium was tested on each SNP using the chi-square test. Population-attributable risk (PAR) (θ × [RR–1]/RR) was calculated for the significant SNPs that were identified from FBAT and CLR analysis for our 134 matched discordant sibpair data, where the relative risk (RR) is approximated by the odds ratio (OR)26 and θ is the proportion of cases exposed to the factor for each significant SNP in the 10q26 region.
Table 5.
Genotype and Allele Frequencies
| Affected Siblings (Index Cases)
|
Unaffected Siblings
|
|||
|---|---|---|---|---|
| Frequency (%) | N | Frequency (%) | N | |
| LOC387715/ARMS2 | ||||
| rs10490924 | ||||
| Genotype | ||||
| TT | 33.58 | 45 | 16.42 | 22 |
| TG | 38.06 | 51 | 32.84 | 44 |
| GG | 28.36 | 38 | 50.75 | 68 |
| Total | 134 | 134 | ||
| Allele | ||||
| T | 52.61 | 141 | 32.84 | 88 |
| G | 47.39 | 127 | 67.16 | 180 |
| Total | 268 | 268 | ||
| rs10664316 | ||||
| Genotype | ||||
| -- | 11.19 | 15 | 18.66 | 25 |
| --AT | 31.34 | 42 | 45.52 | 61 |
| AT | 57.46 | 77 | 35.82 | 48 |
| Total | 134 | 134 | ||
| Allele | ||||
| -- | 26.87 | 72 | 41.42 | 111 |
| AT | 73.13 | 196 | 58.58 | 157 |
| Total | 268 | 268 | ||
| HTRA1 | ||||
| rs11200638 | ||||
| Genotype | ||||
| AA | 32.09 | 43 | 15.67 | 21 |
| AG | 40.30 | 54 | 32.09 | 43 |
| GG | 27.61 | 37 | 52.2 | 70 |
| Total | 134 | 134 | ||
| Allele | ||||
| A | 52.24 | 140 | 31.72 | 85 |
| G | 47.76 | 128 | 68.28 | 183 |
| Total | 268 | 268 | ||
| rs2672598 | ||||
| Genotype | ||||
| TT | 11.94 | 16 | 26.12 | 35 |
| TC | 40.30 | 54 | 47.01 | 63 |
| CC | 47.76 | 64 | 26.87 | 36 |
| Total | 134 | 134 | ||
| Allele | ||||
| T | 32.09 | 86 | 49.63 | 133 |
| C | 67.91 | 182 | 50.37 | 135 |
| Total | 268 | 268 | ||
| rs1049331 | ||||
| Genotype | ||||
| TT | 32.84 | 44 | 15.67 | 21 |
| TC | 39.55 | 53 | 32.09 | 43 |
| CC | 27.61 | 37 | 52.24 | 70 |
| Total | 134 | 134 | ||
| Allele | ||||
| T | 52.61 | 141 | 31.72 | 85 |
| C | 47.39 | 127 | 68.2 | 183 |
| Total | 268 | 268 | ||
| rs2293870 | ||||
| Genotype | ||||
| CC | 0.75 | 1 | 2.24 | 3 |
| TT | 31.34 | 42 | 15.67 | 21 |
| CG | 6.72 | 9 | 9.70 | 13 |
| TG | 32.84 | 44 | 28.36 | 38 |
| CT | 8.96 | 12 | 3.73 | 5 |
| GG | 19.41 | 26 | 40.30 | 54 |
| Total | 134 | 134 | ||
| Allele | ||||
| C | 8.58 | 23 | 8.96 | 24 |
| T | 52.23 | 140 | 31.72 | 85 |
| G | 39.18 | 105 | 59.33 | 159 |
| Total | 268 | 268 | ||
For linkage analysis of the 8 microsatellite markers, identity by state (IBS) scores were calculated from the number of alleles (0, 1, or 2) shared between each pair, the index, and the discordant sibling, for each of the 8 markers. Using heterozygosities for each marker obtained from Map-O-Mat (http://compgen.rutgers.edu/mapomat), the expected IBS (null hypothesis of no linkage) was calculated and then compared with the observed IBS values. A goodness-of-fit test was applied to assess the significance of the difference between the observed and expected distributions. Bonferroni correction was applied to the P values of the association tests on microsatellite markers and AMD risk.
Results
We identified 23 variants including deletions. Only the SNPs that had a minor allele frequency of ≥5% in the affected and unaffected siblings combined were used for statistical analysis (n = 19) (Table 4 [available at http://aaojournal.org]). Using FBAT, 6 SNPs showed significant association with AMD risk after applying a Bonferroni correction (Table 6 [available at http://aaojournal.org]). Genotype and allele frequencies for each of these SNPs are given in Table 5 (available at http://aaojournal.org). No significant deviations from Hardy–Weinberg equilibrium for any of the variants studied were observed in either affected or unaffected siblings, indicating unlikely contamination of our dataset. FBAT demonstrated that the variant most strongly associated with AMD risk was a previously unreported synonymous change in exon 1 of HTRA1, triallelic SNP rs2293870 (P<10−4). Additionally, we identified novel significant associations with AMD susceptibility for an intronic deletion in hypothetical locus LOC387715/ARMS2, SNP rs10664316 (P<10−3); the HTRA1 promoter SNP rs2672598 (P<10−2); and another synonymous HTRA1 SNP, rs1049331 (P<10−3) (Table 4). In agreement with others, FBAT demonstrated that SNPs rs10490924 (P<10−3) and rs11200638 (P<10−3) were significantly associated with AMD risk, whereas no significant association was observed between neovascular AMD and PLEKHA1.12,13 Except for SNP rs2293870, all significant SNPs were part of the same haplotype block as depicted in the LD (r2) plot in Figure 1 (available at http://aaojournal.org). The SNP rs10490924 was in high LD with HTRA1 SNPs rs11200638 and rs1049331 (r2>0.90). The intronic SNP rs10664316 was not in high LD with any other SNPs (r2>0.50), although we found this small portion of the hypothetical LOC387715/ARMS2 gene to be conserved in the mouse (http://www.ensembl.org/Homo_sapiens/geneview?gene=ENSG00000166033). Linkage analysis supported our findings of SNP association, as IBS scores calculated for each of the 8 highly heterozygous microsatellite markers analyzed in this region demonstrated that D10S1656 was significantly associated with neovascular AMD (P<10−15) (see “Materials and Methods” and Table 7 [the latter available at http://aaojournal.org]).
Table 6.
Single-Marker Analysis Results from the FBAT Program Assuming an Additive Genetic Model
| SNP | No. of Informative Families | Variance (S) | Z Value | Pointwise P Value | Familywise P Value* |
|---|---|---|---|---|---|
| PLEKHA1 rs1045216 | 16 | 16.000 | 0.500 | 0.6171 | 1.0 |
| LOC387715/ARMS2 rs10490923 | 3 | † | † | † | † |
| LOC387715/ARMS2 rs2736911 | 2 | † | † | † | † |
| LOC387715/ARMS2 rs10490924 | 31 | 31.000 | 4.131 | 0.000036 | 0.001 |
| LOC387715/ARMS2 rs10664316 | 47 | 47.000 | 4.230 | 0.000023 | 0.0007 |
| LOC387715/ARMS2 rs7088128 | 25 | 25.000 | 2.600 | 0.009322 | 0.27 |
| HTRA1 rs11200638 | 28 | 28.000 | 4.158 | 0.000032 | 0.0009 |
| HTRA1 −502 (from ATG‡) | 2 | † | † | † | † |
| HTRA1 rs2672598 | 27 | 27.000 | 3.657 | 0.000256 | 0.007 |
| HTRA1 rs1049331 | 29 | 29.000 | 4.271 | 0.000019 | 0.0006 |
| HTRA1 rs2293870 (T vs. G/C) | 61 | 26.500 | 4.662 | 3.13×10−6 | 0.00009 |
| HTRA1 rs2293870 (G vs. T/C) | 65 | 26.000 | 5.295 | 1.19×10−6 | 0.00003 |
| HTRA1 rs2239586 | 2 | † | † | † | † |
| HTRA1 rs2239587 | 2 | † | † | † | † |
| HTRA1 rs2672582 | 29 | 29.000 | 1.671 | 0.0947 | 1.0 |
| HTRA1 intron 5 (del GTTT) | 41 | 41.000 | 0.781 | 0.4349 | 1.0 |
| HTRA1 rs2672583 | 29 | 29.000 | 2.043 | 0.0411 | 1.0 |
| HTRA1 rs2672585 | 60 | 19.500 | 2.491 | 0.0127 | 0.37 |
| HTRA1 rs2272599 | 35 | 35.000 | 1.859 | 0.0630 | 1.83 |
| HTRA1 rs2293871 | 4 | 4.000 | 2.000 | 0.0455 | 1.32 |
SNP = single-nucleotide polymorphism.
Minor allele frequency is ≥ 5% in both affected and unaffected siblings combined.
Applied Bonferroni correction on 29 tests.
No. of informative families is less than 4, no statistics are available
First coding.
Table 7.
Microsatellite Marker Results
| D10S1213 | D10S1656* | D10S1723 | D10S587 | D10S1690 | D10S1230 | D10S1483 | D10S1222 | |
|---|---|---|---|---|---|---|---|---|
| No. of 0s† | 21 | 51 | 21 | 20 | 4 | 10 | 10 | 7 |
| No. of 1s† | 67 | 55 | 71 | 60 | 37 | 44 | 48 | 59 |
| No. of 2s† | 42 | 24 | 41 | 51 | 30 | 32 | 38 | 35 |
| Total | 130 | 130 | 133 | 131 | 71 | 86 | 96 | 101 |
| No. of NAs | 4 | 4 | 1 | 3 | 63 | 48 | 38 | 33 |
| Heterozygocity | 0.827 | 0.750 | 0.884 | 0.793 | 0.650 | 0.740 | 0.830 | 0.730 |
| χ2 | 0.480 | 73.261 | 1.403 | 0.360 | 2.902 | 0.781 | 3.429 | 7.072 |
| P value | 0.787 | 1.23×10−16 | 0.496 | 0.835 | 0.234 | 0.677 | 0.180 | 0.029 |
| P value‡ | 1 | 9.88×10−16 | 1 | 1 | 1 | 1 | 1 | 0.223 |
NA = not applicable.
Located 1.8 megabases from the end of the HtrA serine peptidase 1 gene.
Number of alleles (0, 1, or 2) shared between the sibling pair.
Adjusted values using the Bonferroni correction on 8 tests.
FBAT demonstrated that 2 haplotypes (h2 and h6) in the 10q26 region were significantly associated with AMD risk (Table 8 [available at http://aaojournal.org]). The SNPs rs10490924, rs10664316, rs11200638, rs2672598, and rs1049331 were more strongly associated with increased AMD risk as part of the most significant haplotype, h2 (P<10−4) than when examined individually in FBAT analysis (Table 6).
Table 8.
Haplotype Analysis Results from the FBAT
| Order |
LOC387715/ARMS2 rs10490923 |
LOC387715/ARMS2 rs2736911 |
LOC387715/ARMS2 rs10490924 |
LOC387715/ARMS2 rs10664316 |
LOC387715/ARMS2 rs7088128 |
HTRA1 rs11200638 |
HTRA1−502 (from ATG*) C>T |
HTRA1 rs2672598 |
HTRA1 rs1049331 |
No. of Informative Families |
Frequency | P Value† |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| h1 | G | C | G | — | A | G | C | C | C | 47 | 0.347 | 0.1113 |
| h2 | G | C | T | AT | A | A | C | C | T | 27 | 0.235 | 0.00002 |
| h3 | G | C | G | AT | A | G | C | C | C | 24 | 0.104 | 0.2304 |
| h4 | G | C | G | — | A | G | C | T | C | 16 | 0.104 | 0.1433 |
| h5 | G | C | G | AT | G | G | C | C | C | 15 | 0.078 | 0.4555 |
| h6 | G | C | G | — | G | G | C | T | C | 10 | 0.060 | 0.0010 |
hn = haplotype n.
Estimated haplotypes with allele frequency > 0.05 were listed and tested for association. When considering all possible haplotypes together, the resulting
First coding ATG.
Results based on 100 000 permutations.
P value from 100 000 permutations was 0.00006.
Multiple CLR analyses were conducted to determine how each of the 6 significant SNPs contributed to the risk of neovascular AMD while adjusting for CFH 402H genotype and smoking and, moreover, to examine if interactions existed between each SNP, CFH, and/or smoking (Table 9). For each significant SNP, models were created to examine the minor allele in unaffected siblings in both the homozygous and heterozygous states versus having the common allele in the homozygous state. When we included smoking and CFH in each model with any of the significant variants, the significance and effect size were not modified with respect to AMD risk. Multiple CLR demonstrated that the most significantly associated variants with increased AMD risk were HTRA1 SNPs rs11200638 (AA vs. GG: OR, 98.41; confidence interval [CI], 13.45–720.08; P<10−5) (AG vs. GG: OR, 6.05; CI, 2.13–17.21; P<10−3) and rs1049331 (TT vs. CC: OR, 105.52; CI, 14.64–760.5; P<10−5) (TC vs. CC: OR, 5.97; CI, 2.10–16.99; P<10−3). The HTRA1 SNP rs2672598 conferred a 33-fold reduced risk of AMD homozygously (P<10−4) and 8-fold heterozygously (P<10−3). The minor alleles in the homozygous state, when compared with the common alleles in the homozygous state for SNPs rs10490924, rs11200638, rs1049331, rs2672598, and rs2293870, more strongly influenced AMD risk than the CFH Y402H C allele in the homozygous state (vs. TT). As we previously reported17 and can be seen in our expanded population in Table 9, one C allele for CFH Y402H was not significantly associated with neovascular AMD risk (P>0.2).
Table 9.
Results of Multiple Conditional Logistic Regression Analysis Showing Significant Single-Nucleotide Polymorphisms
| Risk Factor | Referent | Odds Ratio (95% CI) | P Value |
|---|---|---|---|
| LOC387715/ARMS2 rs10490924 “T” is rare allele | |||
| rs10490924 TT | GG | 61.91 (10.89–352.01) | 0.0000033 |
| rs10490924 TG | GG | 5.32 (1.82–15.52) | 0.0022 |
| Smoking ≥ 10 pack-years | <10 pack-years | 3.81 (1.84–7.91) | 0.0003 |
| CFH CC | TT | 18.13 (4.20–78.22) | 0.0001 |
| CFH TC | TT | 1.78 (0.72–4.40) | 0.2089 |
| LOC387715/ARMS2 rs10664316 “del AT” is rare allele | |||
| rs10664316 del AT/del AT | AT/AT | 0.09 (0.02–0.36) | 0.0007 |
| rs10664316 del AT/AT | AT/AT | 0.30 (0.13–0.72) | 0.0069 |
| Smoking ≥ 10 pack-years | <10 pack-years | 3.00 (1.58–5.70) | 0.0008 |
| CFH CC | TT | 12.64 (3.26–49.04) | 0.0002 |
| CFH TC | TT | 1.56 (0.68–3.58) | 0.2998 |
| HTRA1 rs11200638 “A” is rare allele | |||
| rs11200638 AA | GG | 98.41 (13.45–720.08) | 0.0000062 |
| rs11200638 AG | GG | 6.05 (2.13–17.21) | 0.0007 |
| Smoking ≥ 10 pack-years | <10 pack-years | 4.08 (1.93–8.63) | 0.0002 |
| CFH CC | TT | 19.59 (4.36–88.03) | 0.0001 |
| CFH TC | TT | 1.69 (0.67–4.26) | 0.2687 |
| HTRA1 rs2672598 “T” is rare allele | |||
| rs2672598 TT | CC | 0.03 (0.01–0.14) | 0.000013 |
| rs2672598 TC | CC | 0.12 (0.04–0.39) | 0.0004 |
| Smoking ≥ 10 pack-years | <10 pack-years | 3.48 (1.73–7.01) | 0.0005 |
| CFH CC | TT | 10.34 (2.76–38.67) | 0.0005 |
| CFH TC | TT | 1.69 (0.70–4.06) | 0.2428 |
| HTRA1 rs1049331 “T” is rare allele | |||
| rs1049331 TT | CC | 105.52 (14.64–760.50) | 0.0000038 |
| rs1049331 TC | CC | 5.97 (2.10–16.99) | 0.0008 |
| Smoking ≥ 10 pack-years | <10 pack-years | 4.09 (1.93–8.66) | 0.0002 |
| CFH CC | TT | 19.29 (4.28–86.94) | 0.0001 |
| CFH TC | TT | 1.67 (0.66–4.20) | 0.2754 |
| HTRA1 rs2293870 “T” and “C” are rare alleles | |||
| rs2293870 CC, CT or TT | GG | 25.97 (6.32–106.66) | 0.0000062 |
| rs2293870 CG or TG | GG | 5.89 (1.96–17.71) | 0.0016 |
| Smoking ≥ 10 pack-years | <10 pack-years | 3.31 (1.66–6.60) | 0.0006 |
| CFH CC | TT | 13.94 (3.37–57.67) | 0.0003 |
| CFH TC | TT | 1.58 (0.64–3.90) | 0.3227 |
CFH = complement factor H gene; CI = confidence interval.
For each of our significant SNPs in the 10q26 region, there were no interactions between the homozygous or heterozygous genotypes and smoking or between the homozygous and heterozygous genotypes and CFH CC genotype.
Another way of looking at the influence of these genetic variants on AMD risk is by looking at the PAR, an estimate of what percentage of cases can be attributed to a particular risk factor. For example, Table 10 shows the PAR for the 6 SNPs significantly associated with risk of neovascular AMD. Using rs11200638 as an example, it is the A allele that is associated with increased AMD risk (G>A). The presence of 2 A alleles (2 risk alleles) explains 32% of the risk in the entire population (adjusted PAR = 0.32). This is similar in the instance of having at least 1 A allele: 34% of the risk in the entire population is explained (adjusted PAR = 0.34). However, when examining the presence of at least 1 A allele in combination with established risk factors (i.e., CFH and smoking) the adjusted PAR % is 82% (adjusted PAR = 0.82). Applying the above to each of the 5 other significantly associated LOC387715/ARMS2/HTRA1 SNPs (the presence of 1 risk allele for any of these SNPs) shows that the PAR % explains 71% to 82% of risk in the total population when combined with CFH and smoking.
Table 10.
Population-Attributable Risk (PAR) Table
| Exposure | Relative Risk | Frequency in Cases, θ | Adjusted PAR (95% Confidence Interval) |
|---|---|---|---|
| LOC387715/ARMS2 rs10490924 | |||
| TT versus GG | 61.9 | 0.34 | 0.33 (0.23–0.42) |
| TG versus GG | 5.3 | 0.39 | 0.32 (0.21–0.41) |
| Smoking (<10 vs. ≥10 pack-years) | 3.8 | 0.54 | 0.40 (0.30–0.48) |
| Smoking ≥ 10 pack-years or T allele* | 4.0 | 0.87 | 0.65 (0.60–0.70) |
| CFH CC versus TT | 18.1 | 0.34 | 0.32 (0.22–0.41) |
| Smoking ≥ 10 pack-years or T allele or CFH CC* | 10.3 | 0.90 | 0.81 (0.78–0.84) |
| LOC387715/ARMS2 rs10664316 | |||
| AT/AT versus del AT/del AT | 11.5 | 0.57 | 0.52 (0.45–0.59) |
| AT/AT versus del AT/AT | 3.5 | 0.31 | 0.22 (0.10–0.33) |
| Smoking (<10 vs. ≥10 pack-years) | 3.0 | 0.54 | 0.36 (0.26–0.45) |
| Smoking ≥ 10 pack-years or AT allele* | 2.5 | 0.94 | 0.56 (0.50–0.62) |
| CFH CC versus TT | 12.6 | 0.34 | 0.31 (0.21–0.41) |
| Smoking ≥ 10 pack-years or AT/AT (no del allele) or CFH CC* | 6.0 | 0.85 | 0.71 (0.66–0.75) |
| HTRA1 rs11200638 | |||
| AA versus GG | 98.4 | 0.32 | 0.32 (0.21–0.41) |
| AG versus GG | 6.1 | 0.41 | 0.34 (0.24–0.43) |
| Smoking (<10 vs. ≥10 pack-years) | 4.1 | 0.54 | 0.41 (0.32–0.49) |
| Smoking ≥ 10 pack-years or A allele* | 4.1 | 0.87 | 0.66 (0.60–0.70) |
| CFH CC versus TT | 19.6 | 0.34 | 0.32 (0.22–0.41) |
| Smoking ≥ 10 pack-years or A allele or CFH CC* | 10.7 | 0.91 | 0.82 (0.80–0.85) |
| HTRA1 rs2672598 | |||
| CC versus TT | 36.5 | 0.48 | 0.46 (0.38–0.54) |
| TC versus TT | 4.3 | 0.41 | 0.32 (0.21–0.41) |
| Smoking (<10 vs. ≥10 pack-years) | 3.5 | 0.54 | 0.39 (0.29–0.47) |
| Smoking ≥ 10 pack-years or C allele* | 3.5 | 0.94 | 0.67 (0.62–0.72) |
| CFH CC versus TT | 10.3 | 0.34 | 0.30 (0.20–0.40) |
| Smoking ≥ 10 pack-years or CC (no T allele) or CFH CC* | 8.0 | 0.86 | 0.75 (0.71–0.79) |
| HTRA1 rs1049331 | |||
| TT versus CC | 105.5 | 0.33 | 0.33 (0.22–0.42) |
| TC versus CC | 6.0 | 0.40 | 0.33 (0.23–0.42) |
| Smoking (<10 vs. ≥10 pack-years) | 4.1 | 0.54 | 0.41 (0.32–0.49) |
| Smoking ≥ 10 pack-years or T allele* | 4.1 | 0.87 | 0.66 (0.60–0.70) |
| CFH CC versus TT | 19.3 | 0.34 | 0.32 (0.22–0.41) |
| Smoking ≥ 10 pack-years or T allele or CFH CC* | 10.7 | 0.91 | 0.82 (0.80–0.85) |
| HTRA1 rs2293870 | |||
| CC, CT, or TT versus GG | 26.0 | 0.41 | 0.39 (0.30–0.48) |
| CG or TG versus GG | 5.9 | 0.40 | 0.33 (0.23–0.42) |
| Smoking (<10 vs. ≥10 pack-years) | 3.3 | 0.54 | 0.38 (0.28–0.46) |
| Smoking ≥ 10 pack-years or C or T allele* | 3.4 | 0.90 | 0.64 (0.58–0.68) |
| CFH CC versus TT | 13.9 | 0.34 | 0.31 (0.21–0.41) |
| Smoking ≥ 10 pack-years or C or T allele or CFH CC* | 8.0 | 0.94 | 0.82 (0.78–0.86) |
CFH = complement factor H gene.
Relative risk is estimated by conditional logistic regression adjusting for other factors.
This value is less than the sum of the adjusted PARs, because these risk factors are not mutually exclusive, and the relative risk used here is not adjusting for other factors.
We wanted to assess the potential functional effect of the unreported SNPs newly identified as significantly associated with AMD susceptibility. Given that the SNP rs10664316 is located in an intron 4782 base pairs upstream of the first HTRA1 coding ATG and is weakly conserved in mice, we focused our attention on the highly conserved novel SNPs we identified in the promoter region and exon 1 of HTRA1 (http://www.ensembl.org/Homo_sapiens/geneview?gene=ENSG00000166033). Using the computer program MapInspector (www.genomatrix.de), we found that the protective allele for the SNP rs2672598 created a binding site for the transcription factor ELK-1. We also found that the HTRA1 triallelic SNP rs2293870 was located in a binding domain for insulinlike growth factor (IGF) (http://smart.embl-heidelberg.de and http://www.ensembl.org/Homo_sapiens/geneview?gene=ENSG00000166033).
The synonymous variants rs1049331 and rs2293870 identified in exon 1 of HTRA1 did not appear to alter a splice site (http://www.fruitfly.org/seq_tools/splice-instrucs.html).
Discussion
Two of the 4 novel variants that we identified in HTRA1 have predicted functional effects on the pathophysiology of neovascular AMD. Variant rs2672598 created a binding site for the transcription factor ELK-1, whose activity was reported to be impaired in a patient with a premature form of aging and insulin resistance.27 We hypothesize, then, that if the risk allele of the promoter SNP rs11200638 results in increased expression of HTRA1,12,13 then the minor allele of rs2672598 might exert a protective effect by enabling the binding of ELK-1, which could downregulate the expression of HTRA1. On the other hand, the SNP rs2293870 is located in one of the HTRA1 binding domains for IGFs.28 The replacement of a purine with a pyrimidine (G > C or T) may conceivably alter the proper binding of HTRA1 for IGF. Further, IGF has been implicated in other ocular conditions characterized by neovascularization, such as diabetic retinopathy and retinopathy of prematurity.29 Therefore, the potential effect of rs2293870 on a regulatory pathway involving HTRA1 and IGFs has biologic plausibility for AMD. Both variants merit further investigation both in vivo and in vitro. Although we identified 2 other SNPs, rs10664316 and rs1049331 (associated with reduced and increased AMD risk, respectively), that do not appear to have predicted functional effects, this does not preclude them from being biomarkers that in fact could be functionally associated with AMD susceptibility.30,31
In summary, in a population of extremely discordant sibling pairs, we identified unreported variants in the HTRA1 region that both increase and decrease risk of neovascular AMD. These findings validate previous reports showing that HTRA1 is the likely candidate gene in the 10q26 region.
Although we identified another novel hypothetical LOC387715/ARMS2 gene variant, rs10664316, that may ultimately play a role in AMD susceptibility,30,31 our newly described variants in HTRA1 have postulated functional regulatory effects, suggesting possible etiologic mechanisms. Our findings validate that the 10q26 region is more strongly associated with neovascular AMD risk than the 1q32 region in which CFH resides.12,13,32 Moreover, our results demonstrate that HTRA1 variants influence risk independent of CFH genotype and smoking, supporting the role for HTRA1 in a distinct pathway underlying AMD pathogenesis.
Acknowledgments
Supported by grants from the Ruth and Milton Steinbach Fund, New York, New York; Lincy Foundation, Beverly Hills, California; Massachusetts Lions, New Bedford, Massachusetts; Friends of the Massachusetts Eye and Ear Infirmary (MEEI), Boston, Massachusetts; Genetics of Age-Related Macular Degeneration Fund, MEEI, Boston, Massachusetts; Research to Prevent Blindness, New York, New York; Natural Science Foundation of China, Beijing, China (project no. 30730057); and National Institutes of Health, Bethesda, Maryland (nos. EY014458, EY14104, MH44292).
Footnotes
The authors have no conflict of interest in any materials mentioned in the article.
References
- 1.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–74. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 2.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–9. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards AO, Ritter R, III, Abel KJ, et al. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–4. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- 4.Haines JL, Hauser MA, Schmidt S, et al. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–21. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- 5.Zareparsi S, Branham KE, Li M, et al. Strong association of the Y402H variant in complement factor H at 1q32 with susceptibility to age-related macular degeneration. Am J Hum Genet. 2005;77:149–53. doi: 10.1086/431426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/ CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher SA, Abecasis GR, Yashar BM, et al. Meta-analysis of genome scans of age-related macular degeneration. Hum Mol Genet. 2005;14:2257–64. doi: 10.1093/hmg/ddi230. [DOI] [PubMed] [Google Scholar]
- 8.Jakobsdottir J, Conley YP, Weeks DE, et al. Susceptibility genes for age-related maculopathy on chromosome 10q26. Am J Hum Genet. 2005;77:389– 407. doi: 10.1086/444437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rivera A, Fisher SA, Fritsche LG, et al. Hypothetical LOC387715 is a second major susceptibility gene for age-related macular degeneration, contributing independently of complement factor H to disease risk. Hum Mol Genet. 2005;14:3227–36. doi: 10.1093/hmg/ddi353. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt S, Hauser MA, Scott WK, et al. Cigarette smoking strongly modifies the association of LOC387715 and age-related macular degeneration. Am J Hum Genet. 2006;78:852–64. doi: 10.1086/503822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conley YP, Jakobsdottir J, Mah T, et al. CFH, ELOVL4, PLEKHA1 and LOC387715 genes and susceptibility to age-related maculopathy: AREDS and CHS cohorts and meta-analyses. Hum Mol Genet. 2006;15:3206–18. doi: 10.1093/hmg/ddl396. [DOI] [PubMed] [Google Scholar]
- 12.DeWan A, Liu M, Hartman S, et al. HTRA1 promoter polymorphism in wet age-related macular degeneration. Science. 2006;314:989–92. doi: 10.1126/science.1133807. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Camp NJ, Sun H, et al. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314:992–3. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 14.Cameron DJ, Yang Z, Gibbs D, et al. HTRA1 variant confers similar risks to geographic atrophy and neovascular age-related macular degeneration. Cell Cycle. 2007;6:1122–5. doi: 10.4161/cc.6.9.4157. [DOI] [PubMed] [Google Scholar]
- 15.Risch N, Zhang H. Extreme discordant sib pairs for mapping quantitative trait loci in humans. Science. 1995;268:1584–9. doi: 10.1126/science.7777857. [DOI] [PubMed] [Google Scholar]
- 16.Risch NJ, Zhang H. Mapping quantitative trait loci with extreme discordant sib pairs: sampling considerations. Am J Hum Genet. 1996;58:836– 43. [PMC free article] [PubMed] [Google Scholar]
- 17.DeAngelis MM, Ji F, Kim IK, et al. Cigarette smoking, CFH, APOE, ELOVL4, and risk of neovascular age-related macular degeneration. Arch Ophthalmol. 2007;125:49–54. doi: 10.1001/archopht.125.1.49. [DOI] [PubMed] [Google Scholar]
- 18.Thakkinstian A, Han P, McEvoy M, et al. Systematic review and meta-analysis of the association between complementary factor H Y402H polymorphisms and age-related macular degeneration. Hum Mol Genet. 2006;15:2784–90. doi: 10.1093/hmg/ddl220. [DOI] [PubMed] [Google Scholar]
- 19.Thornton J, Edwards R, Mitchell P, et al. Smoking and age-related macular degeneration: a review of association. Eye. 2005;19:935–44. doi: 10.1038/sj.eye.6701978. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis MM, Lane AM, Shah CP, et al. Extremely discordant sib-pair study design to determine risk factors for neovascular age-related macular degeneration. Arch Ophthalmol. 2004;122:575–80. doi: 10.1001/archopht.122.4.575. [DOI] [PubMed] [Google Scholar]
- 21.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study system for classifying age-related macular degeneration from stereoscopic color fundus photographs: the Age-Related Eye Disease Study report number 6. Am J Ophthalmol. 2001;132:668– 81. doi: 10.1016/s0002-9394(01)01218-1. [DOI] [PubMed] [Google Scholar]
- 22.Age-Related Eye Disease Study Research Group. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS report no. 17. Arch Ophthalmol. 2005;123:1484–98. doi: 10.1001/archopht.123.11.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horvath S, Xu X, Lake SL, et al. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26:61–9. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- 24.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 25.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 26.Armitage P, Berry G. Statistical Methods in Medical Research. 2. Oxford, United Kingdom: Blackwell Scientific; 1987. [Google Scholar]
- 27.Knebel B, Avci H, Bullmann C, et al. Reduced phosphorylation of transcription factor Elk-1 in cultured fibroblasts of a patient with premature aging syndrome and insulin resistance. Exp Clin Endocrinol Diabetes. 2005;113:94–101. doi: 10.1055/s-2004-830554. [DOI] [PubMed] [Google Scholar]
- 28.Clausen T, Southan C, Ehrmann M. The HtrA family of proteases: implications for protein composition and cell fate. Mol Cell. 2002;10:443–55. doi: 10.1016/s1097-2765(02)00658-5. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10:133–40. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 30.Greally JM. Genomics: encyclopaedia of humble DNA. Nature. 2007;447:782–3. doi: 10.1038/447782a. [DOI] [PubMed] [Google Scholar]
- 31.ENCODE Project Consortium. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature. 2007;447:799– 816. doi: 10.1038/nature05874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shuler RK, Jr, Hauser MA, Caldwell J, et al. Neovascular age-related macular degeneration and its association with LOC387715 and complement factor H polymorphism. Arch Ophthalmol. 2007;125:63–7. doi: 10.1001/archopht.125.1.63. [DOI] [PubMed] [Google Scholar]