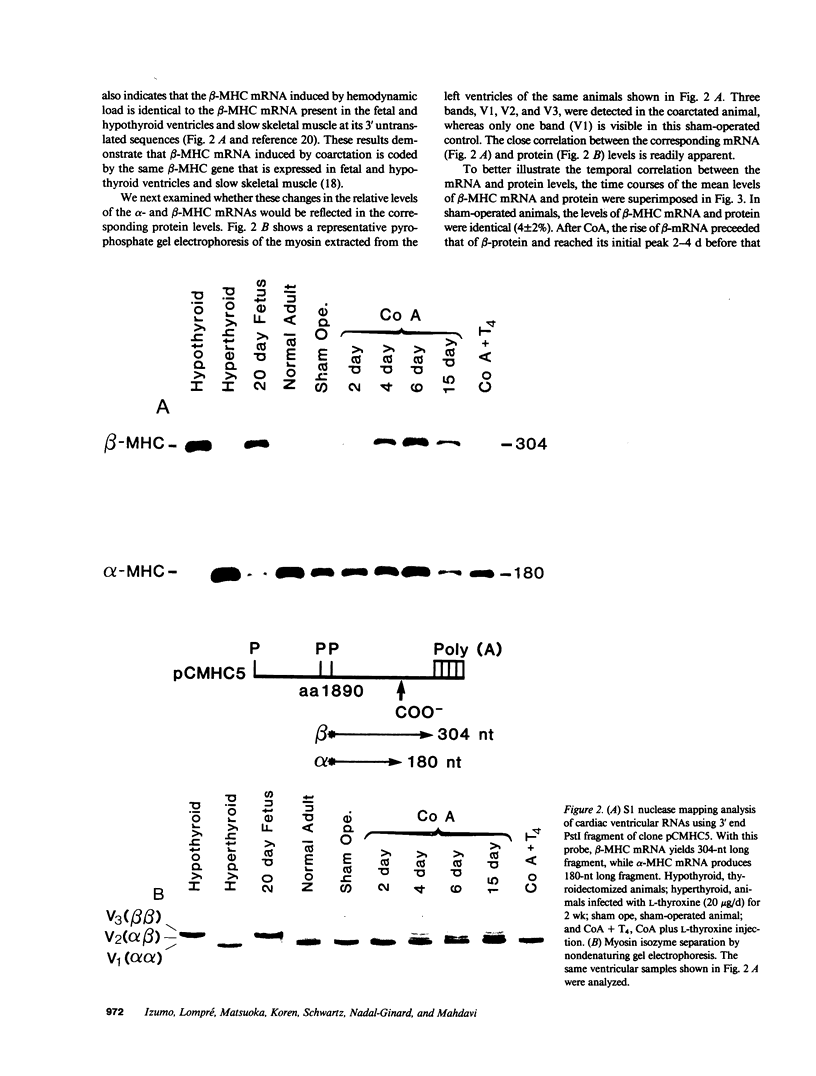
Abstract
Expression of the cardiac myosin isozymes is regulated during development, by hormonal stimuli and hemodynamic load. In this study, the levels of expression of the two isoforms (alpha and beta) of myosin heavy chain (MHC) during cardiac hypertrophy were investigated at the messenger RNA (mRNA) and protein levels. In normal control and sham-operated rats, the alpha-MHC mRNA predominated in the ventricular myocardium. In response to aortic coarctation, there was a rapid induction of the beta-MHC mRNA followed by the appearance of comparable levels of the beta-MHC protein in parallel to an increase in the left ventricular weight. Administration of thyroxine to coarctated animals caused a rapid deinduction of beta-MHC and induction of alpha-MHC, both at the mRNA and protein levels, despite progression of left ventricular hypertrophy. These results suggest that the MHC isozyme transition during hemodynamic overload is mainly regulated by pretranslational mechanisms, and that a complex interplay exists between hemodynamic and hormonal stimuli in MHC gene expression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afflitto J. J., Inchiosa M. A., Jr Decrease in rat cardiac myosin ATPase with aortic constriction: prevention by thyroxine treatment. Life Sci. 1979 Jul 23;25(4):353–364. doi: 10.1016/0024-3205(79)90267-4. [DOI] [PubMed] [Google Scholar]
- Alpert N. R., Mulieri L. A. Increased myothermal economy of isometric force generation in compensated cardiac hypertrophy induced by pulmonary artery constriction in the rabbit. A characterization of heat liberation in normal and hypertrophied right ventricular papillary muscles. Circ Res. 1982 Apr;50(4):491–500. doi: 10.1161/01.res.50.4.491. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bhan A. K., Scheuer J. Effects of physical training on cardiac myosin ATPase activity. Am J Physiol. 1975 Apr;228(4):1178–1182. doi: 10.1152/ajplegacy.1975.228.4.1178. [DOI] [PubMed] [Google Scholar]
- Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967 Jul;50(6 Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzonite R. A., Zak R. Regulation of myosin isoenzyme composition in fetal and neonatal rat ventricle by endogenous thyroid hormones. J Biol Chem. 1984 Oct 25;259(20):12628–12632. [PubMed] [Google Scholar]
- Cooper G., 4th, Kent R. L., Uboh C. E., Thompson E. W., Marino T. A. Hemodynamic versus adrenergic control of cat right ventricular hypertrophy. J Clin Invest. 1985 May;75(5):1403–1414. doi: 10.1172/JCI111842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutilletta A. F. Myosin heavy chain mRNA during the development and regression of myocardial hypertrophy. Eur Heart J. 1984 Dec;5 (Suppl F):193–197. doi: 10.1093/eurheartj/5.suppl_f.193. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H. Diabetes mellitus induces changes in cardiac myosin of the rat. Diabetes. 1980 Jul;29(7):579–582. doi: 10.2337/diab.29.7.579. [DOI] [PubMed] [Google Scholar]
- Everett A. W., Sinha A. M., Umeda P. K., Jakovcic S., Rabinowitz M., Zak R. Regulation of myosin synthesis by thyroid hormone: relative change in the alpha- and beta-myosin heavy chain mRNA levels in rabbit heart. Biochemistry. 1984 Apr 10;23(8):1596–1599. doi: 10.1021/bi00303a002. [DOI] [PubMed] [Google Scholar]
- Friedman D. J., Umeda P. K., Sinha A. M., Hsu H. J., Jakovcic S., Rabinowitz M. Characterization of genomic clones specifying rabbit alpha- and beta-ventricular myosin heavy chains. Proc Natl Acad Sci U S A. 1984 May;81(10):3044–3048. doi: 10.1073/pnas.81.10.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorza L., Mercadier J. J., Schwartz K., Thornell L. E., Sartore S., Schiaffino S. Myosin types in the human heart. An immunofluorescence study of normal and hypertrophied atrial and ventricular myocardium. Circ Res. 1984 Jun;54(6):694–702. doi: 10.1161/01.res.54.6.694. [DOI] [PubMed] [Google Scholar]
- Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980 Oct;69(4):576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- Hirzel H. O., Tuchschmid C. R., Schneider J., Krayenbuehl H. P., Schaub M. C. Relationship between myosin isoenzyme composition, hemodynamics, and myocardial structure in various forms of human cardiac hypertrophy. Circ Res. 1985 Nov;57(5):729–740. doi: 10.1161/01.res.57.5.729. [DOI] [PubMed] [Google Scholar]
- Hoh J. F., McGrath P. A., Hale P. T. Electrophoretic analysis of multiple forms of rat cardiac myosin: effects of hypophysectomy and thyroxine replacement. J Mol Cell Cardiol. 1978 Nov;10(11):1053–1076. doi: 10.1016/0022-2828(78)90401-7. [DOI] [PubMed] [Google Scholar]
- Ingwall J. S., Kramer M. F., Fifer M. A., Lorell B. H., Shemin R., Grossman W., Allen P. D. The creatine kinase system in normal and diseased human myocardium. N Engl J Med. 1985 Oct 24;313(17):1050–1054. doi: 10.1056/NEJM198510243131704. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Kira Y., Kochel P. J., Gordon E. E., Morgan H. E. Aortic perfusion pressure as a determinant of cardiac protein synthesis. Am J Physiol. 1984 Mar;246(3 Pt 1):C247–C258. doi: 10.1152/ajpcell.1984.246.3.C247. [DOI] [PubMed] [Google Scholar]
- Litten R. Z., 3rd, Martin B. J., Low R. B., Alpert N. R. Altered myosin isozyme patterns from pressure-overloaded and thyrotoxic hypertrophied rabbit hearts. Circ Res. 1982 Jun;50(6):856–864. doi: 10.1161/01.res.50.6.856. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Mahdavi V., Chambers A. P., Nadal-Ginard B. Cardiac alpha- and beta-myosin heavy chain genes are organized in tandem. Proc Natl Acad Sci U S A. 1984 May;81(9):2626–2630. doi: 10.1073/pnas.81.9.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdavi V., Periasamy M., Nadal-Ginard B. Molecular characterization of two myosin heavy chain genes expressed in the adult heart. Nature. 1982 Jun 24;297(5868):659–664. doi: 10.1038/297659a0. [DOI] [PubMed] [Google Scholar]
- Malhotra A., Penpargkul S., Fein F. S., Sonnenblick E. H., Scheuer J. The effect of streptozotocin-induced diabetes in rats on cardiac contractile proteins. Circ Res. 1981 Dec;49(6):1243–1250. doi: 10.1161/01.res.49.6.1243. [DOI] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Mercadier J. J., Bouveret P., Gorza L., Schiaffino S., Clark W. A., Zak R., Swynghedauw B., Schwartz K. Myosin isoenzymes in normal and hypertrophied human ventricular myocardium. Circ Res. 1983 Jul;53(1):52–62. doi: 10.1161/01.res.53.1.52. [DOI] [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Wisnewsky C., Samuel J. L., Bercovici J., Swynghedauw B., Schwartz K. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res. 1981 Aug;49(2):525–532. doi: 10.1161/01.res.49.2.525. [DOI] [PubMed] [Google Scholar]
- Morkin E., Flink I. L., Goldman S. Biochemical and physiologic effects of thyroid hormone on cardiac performance. Prog Cardiovasc Dis. 1983 Mar-Apr;25(5):435–464. doi: 10.1016/0033-0620(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Nag A. C., Cheng M. Expression of myosin isoenzymes in cardiac-muscle cells in culture. Biochem J. 1984 Jul 1;221(1):21–26. doi: 10.1042/bj2210021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pope B., Hoh J. F., Weeds A. The ATPase activities of rat cardiac myosin isoenzymes. FEBS Lett. 1980 Sep 8;118(2):205–208. doi: 10.1016/0014-5793(80)80219-5. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M. Protein synthesis and turnover in normal and hypertrophied heart. Am J Cardiol. 1973 Feb;31(2):202–210. doi: 10.1016/0002-9149(73)91033-3. [DOI] [PubMed] [Google Scholar]
- Rupp H. The adaptive changes in the isoenzyme pattern of myosin from hypertrophied rat myocardium as a result of pressure overload and physical training. Basic Res Cardiol. 1981 Jan-Feb;76(1):79–88. doi: 10.1007/BF01908164. [DOI] [PubMed] [Google Scholar]
- Samuel J. L., Rappaport L., Syrovy I., Wisnewsky C., Marotte F., Whalen R. G., Schwartz K. Differential effect of thyroxine on atrial and ventricular isomyosins in rats. Am J Physiol. 1986 Mar;250(3 Pt 2):H333–H341. doi: 10.1152/ajpheart.1986.250.3.H333. [DOI] [PubMed] [Google Scholar]
- Schaible T. F., Malhotra A., Ciambrone G., Scheuer J. The effects of gonadectomy on left ventricular function and cardiac contractile proteins in male and female rats. Circ Res. 1984 Jan;54(1):38–49. doi: 10.1161/01.res.54.1.38. [DOI] [PubMed] [Google Scholar]
- Scheuer J., Bhan A. K. Cardiac contractile proteins. Adenosine triphosphatase activity and physiological function. Circ Res. 1979 Jul;45(1):1–12. doi: 10.1161/01.res.45.1.1. [DOI] [PubMed] [Google Scholar]
- Schreiber S. S., Oratz M., Rothschild M. A., Reff F. Effect of hydrostatic pressure on isolated cardiac nuclei: Stimulation of RNA polymerase II activity. Cardiovasc Res. 1978 May;12(5):265–268. doi: 10.1093/cvr/12.5.265. [DOI] [PubMed] [Google Scholar]
- Schwartz K., Lecarpentier Y., Martin J. L., Lompré A. M., Mercadier J. J., Swynghedauw B. Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol. 1981 Dec;13(12):1071–1075. doi: 10.1016/0022-2828(81)90297-2. [DOI] [PubMed] [Google Scholar]
- Sheer D., Morkin E. Myosin isoenzyme expression in rat ventricle: effects of thyroid hormone analogs, catecholamines, glucocorticoids and high carbohydrate diet. J Pharmacol Exp Ther. 1984 Jun;229(3):872–879. [PubMed] [Google Scholar]
- Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983 Aug;72(2):732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha A. M., Umeda P. K., Kavinsky C. J., Rajamanickam C., Hsu H. J., Jakovcic S., Rabinowitz M. Molecular cloning of mRNA sequences for cardiac alpha- and beta-form myosin heavy chains: expression in ventricles of normal, hypothyroid, and thyrotoxic rabbits. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5847–5851. doi: 10.1073/pnas.79.19.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B., Moalic J. M., Bouveret P., Bercovici J., de la Bastie D., Schwartz K. Messenger RNA content and complexity in normal and overloaded rat heart: a preliminary report. Eur Heart J. 1984 Dec;5 (Suppl F):211–217. doi: 10.1093/eurheartj/5.suppl_f.211. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H., Sugi M., Kuro-o M., Ueda S., Takaku F., Furuta S., Shirai T., Yazaki Y. Isozymic changes in myosin of human atrial myocardium induced by overload. Immunohistochemical study using monoclonal antibodies. J Clin Invest. 1984 Aug;74(2):662–665. doi: 10.1172/JCI111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen R. G., Toutant M., Butler-Browne G. S., Watkins S. C. Hereditary pituitary dwarfism in mice affects skeletal and cardiac myosin isozyme transitions differently. J Cell Biol. 1985 Aug;101(2):603–609. doi: 10.1083/jcb.101.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Parmley W. W., Mason D. T. The cardiac hypertrophy process. Analyses of factors determining pathological vs. physiological development. Circ Res. 1979 Dec;45(6):697–707. doi: 10.1161/01.res.45.6.697. [DOI] [PubMed] [Google Scholar]