Abstract
Background
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial found no difference in the primary outcome between warfarin and aspirin in 2305 patients with reduced left ventricular ejection fraction in sinus rhythm. However, it is unknown whether any subgroups benefit from warfarin or aspirin.
Methods and Results
We used a Cox model stepwise selection procedure to identify subgroups that may benefit from warfarin or aspirin on the WARCEF primary outcome. A secondary analysis added major hemorrhage to the outcome. The primary efficacy outcome was time to the first to occur of ischemic stroke, intracerebral hemorrhage, or death. Only age group was a significant treatment effect modifier (P for interaction, 0.003). Younger patients benefited from warfarin over aspirin on the primary outcome (4.81 versus 6.76 events per 100 patient-years: hazard ratio, 0.63; 95% confidence interval, 0.48–0.84; P=0.001). In older patients, therapies did not differ (9.91 versus 9.01 events per 100 patient-years: hazard ratio, 1.09; 95% confidence interval, 0.88–1.35; P=0.44). With major hemorrhage added, in younger patients the event rate remained lower for warfarin than aspirin (5.41 versus 7.25 per 100 patient-years: hazard ratio, 0.68; 95% confidence interval, 0.52–0.89; P=0.005), but in older patients it became significantly higher for warfarin (11.80 versus 9.35 per 100 patient-years: hazard ratio, 1.25; 95% confidence interval, 1.02–1.53; P=0.03).
Conclusions
In patients <60 years, warfarin improved outcomes over aspirin with or without inclusion of major hemorrhage. In patients ≥60 years, there was no treatment difference, but the aspirin group had significantly better outcomes when major hemorrhage was included.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00041938.
Keywords: aspirin, heart failure, sinus rhythm, stroke, warfarin
Chronic heart failure (HF) is a major cause of mortality and morbidity. HF is associated with a hypercoagulable state, left ventricular thrombus formation, and cerebral embolism.1,2 It is also associated with both sudden death and death resulting from progressive HF that may be caused by unrecognized atherothrombotic events.3 This provides a rationale for the use of oral anticoagulants to treat patients with chronic HF.
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial found no significant difference between warfarin and aspirin among patients with HF for the primary end point of first to occur of ischemic stroke, intracerebral hemorrhage (ICH), or death, although there was a large reduction in ischemic stroke in the warfarin group.4 Patients with HF are diverse in terms of demographics, etiology of HF, symptomatology, and many other factors.4–6 Patients in various groups may respond differently to warfarin or aspirin, and there is a great interest in this issue.7,8 As such, we sought to explore whether there are readily identifiable subgroups for whom warfarin or aspirin is preferable, without compromise from increased risk of major bleeding. We chose to consider multiple baseline clinical factors, both individually and in combination because many of them are closely interrelated and cannot be considered in isolation. To our knowledge, this is the first study able to assess this issue with a large and comprehensive database.
Methods
Patients
In the randomized, double-blind WARCEF trial, patients with left ventricular ejection fraction ≤35% in sinus rhythm were randomly assigned to warfarin (target International Normalized Ratio [INR] 2.75, with acceptable target range of 2.0–3.5) or aspirin (325 mg/d). Patients were enrolled at 168 centers in 11 countries between October 2002 and January 2010. The study was approved by institutional review boards at the coordinating centers of all sites, and all subjects gave informed consent. The median follow-up time was 3.4 years (Q1, 2.0; Q3, 5.0). Left ventricular ejection fraction was assessed by quantitative echocardiography (or a wall-motion index of ≤1.2) or radionuclide contrast ventriculography within 3 months before randomization. Patients who had a clear indication for warfarin or aspirin were not eligible. Additional eligibility criteria were a modified Rankin score of ≤4 (on a scale of 0–6, with higher scores indicating more severe disability) and planned treatment with a β-blocker, an angiotensin-converting enzyme inhibitor (or, if the side-effect profile with angiotensin-converting enzyme inhibitors was unacceptable, with an angiotensin-receptor blocker), or hydralazine and nitrates. Patients were ineligible if they had a condition that conferred a high risk of cardiac embolism, such as atrial fibrillation, a mechanical cardiac valve, endocarditis, or an intracardiac mobile or pedunculated thrombus.
Randomization and Outcome Events
Patients were randomly assigned (1:1) to warfarin or aspirin by a 24-hour central computerized system. Randomization was stratified according to whether or not patients had an ischemic stroke or transient ischemic attack within 12 months before randomization, New York Heart Association classification (I versus II, III, or IV), and clinical site. Participants, investigators, and the sponsor were masked to individual participant treatment assignments. The Statistical Analysis Center fabricated clinically plausible INR results for patients in the aspirin group and provided these results to the sites, along with the actual INR results for the patients in the warfarin group. All patients were treated as if they were receiving active warfarin.
The primary efficacy outcome was time to the first to occur of ischemic stroke, ICH, or death. Ischemic stroke was defined as a clinically relevant new lesion detected by computer tomography or MRI or, in the absence of new lesion, clinical findings consistent with the occurrence of clinical stroke that lasted for >24 hours. A total of 622 events occurred: 302 in warfarin and 320 in aspirin arm. The primary safety outcome was major hemorrhage, defined as intracerebral, epidural, subdural, subarachnoid, spinal intramedullary, retinal hemorrhage, or any other bleeding with >2 g hemoglobin decline in 48 hours, requiring ≥2 units of transfusion or requiring hospitalization or surgical intervention. Minor hemorrhage included all other hemorrhages. An independent end point adjudication committee unaware of the treatment assignments adjudicated all efficacy outcomes and major hemorrhages.
Statistical Analysis
Subgroup Candidates
We purposefully included both prespecified and other variables known to affect HF patient outcome.9,10 Prespecified criteria were sex, race ethnicity, left ventricular ejection fraction, New York Heart Association class, and etiology of HF. Other variables were age, body mass index, education, country, diabetes mellitus, hypertension, smoking, alcohol use, 6-minute walk distance, prior stroke or transient ischemic attack, systolic blood pressure, diastolic blood pressure, atrial fibrillation, myocardial infarction, defibrillator or pacemaker use, coronary artery disease, peripheral vascular disease, statin use, prerandomization use of warfarin, prerandomization use of aspirin or other antiplatelets, mini-mental status examination, blood urea nitrogen, estimated glomerular filtration rate, white blood cell count, serum sodium, hematocrit, and hemoglobin. We used a 2-stage approach, with no adjustments for multiple testing because the analyses were exploratory.
In stage 1, we created 32 individual stratified Cox models, each assessing the impact of 1 candidate variable on the treatment effect for the WARCEF primary outcome, without taking account of the impact of the other candidates. Each of these models included terms for treatment (warfarin or aspirin), 1 candidate variable, and the interaction of that candidate variable with treatment. The candidate variable was considered to be an effect modifier if its interaction with treatment was significant at level α=0.05 2-sided. In this stage we dichotomized age at <60 versus ≥60 years because 60 was the closest age to the sample mean and median for age in 5-year increments. Body mass index was scored low (<25 kg/m2), medium (25–30 kg/m2), or high (>30 kg/m2).11 Ejection fraction was scored low (≤20%), medium (>20% and <30%), or high (≥30%). Other continuous variables were dichotomized at the median. Countries with low event rates were combined.
Stage 2 used a stepwise selection procedure to develop a multivariable Cox model identifying the subgroups likely to benefit from 1 of the 2 treatments, when accounting for the impact of all other selected variables. All candidate variables were eligible for selection. In this stage, age and body mass index were retained as categorical variables but other continuous variables were not dichotomized. An interaction term was included in the final model only if its P values and the corresponding main effect term both met the 0.05 selection criterion or if the combination of the main effect and the interaction term was jointly significant at that level using a type 3 test. Treatment was forced into the final model. All statistical analyses were performed with SAS (version 9.2).
Results
In the stage 1 models, which considered each candidate variable separately, only age and country interacted significantly with treatment (P=0.001 and 0.02, respectively; Table 1). Among younger patients (<60 years), the rate of the primary end point was 4.81 events per 100 patient-years in the warfarin group and significantly higher in the aspirin group at 6.76 events per 100 patient-years (unadjusted hazard ratio [HR], 0.65; 95% confidence interval [CI], 0.49–0.86; P=0.002). Among older patients (≥60 years), the primary end point rates did not differ by treatment (9.91 events per 100 patient-years in the warfarin group compared with 9.01 events per 100 patient-years in the aspirin group; unadjusted HR for warfarin versus aspirin 1.16; 95% CI, 0.94–1.43; P=0.16). In Poland there was a statistically significant benefit for warfarin. The rate of primary end point was 7.70 events per 100 patient-years in the warfarin group, compared with 12.38 events per 100 patient-years in the aspirin group (unadjusted HR 0.61; 95% CI, 0.40–0.95; P=0.03). There were nonsignificant trends toward a benefit for warfarin in the United States (P=0.07) and for aspirin in Canada (P=0.06; Table 1).
Table 1.
Hazard Ratios for the Primary End Point for Individual Cox Models*
| Warfarin |
Aspirin |
Hazard Ratio (95% CI) for Warfarin vs Aspirin |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Events/Patients | Rate† | Events/Patients | Rate† | For HR‡ | For Interaction§ | ||
| Age group, y | |||||||
| <60 | 93/508 | 4.81 | 130/522 | 6.76 | 0.65 (0.49–0.86) | 0.002 | 0.001 |
| ≥60 | 209/634 | 9.91 | 190/641 | 9.01 | 1.16 (0.94–1.43) | 0.16 | |
| Country | |||||||
| Poland | 34/129 | 7.70 | 50/134 | 12.38 | 0.61 (0.40–0.95) | 0.03 | |
| Ukraine | 20/57 | 9.68 | 28/58 | 13.75 | 0.78 (0.43–1.43) | 0.43 | |
| United States | 115/463 | 6.06 | 130/440 | 7.55 | 0.79 (0.61–1.02) | 0.07 | 0.02 |
| Argentina and other European | 68/308 | 7.75 | 67/348 | 6.57 | 1.18 (0.84–1.67) | 0.34 | |
| Hungary | 30/75 | 13.06 | 22/77 | 9.34 | 1.29 (0.73–2.28) | 0.38 | |
| Canada | 35/110 | 8.90 | 23/106 | 5.12 | 1.69 (0.98–2.90) | 0.06 | |
| Sex | |||||||
| Men | 240/906 | 7.55 | 281/939 | 8.71 | 0.88 (0.73–1.05) | 0.16 | 0.07 |
| Women | 62/236 | 7.17 | 39/224 | 4.83 | 1.34 (0.88–2.03) | 0.17 | |
| Smoking | |||||||
| Current | 46/213 | 5.96 | 63/195 | 9.30 | 0.62 (0.41–0.92) | 0.02 | |
| Former | 173/585 | 8.29 | 178/604 | 8.50 | 0.98 (0.78–1.22) | 0.84 | 0.08 |
| Never | 83/344 | 6.99 | 79/364 | 6.26 | 1.10 (0.80–1.53) | 0.55 | |
| NYHA class | |||||||
| NYHA 3 or 4 | 116/368 | 9.12 | 131/347 | 11.72 | 0.78 (0.60–1.01) | 0.06 | 0.11 |
| NYHA 1 or 2 | 186/774 | 6.71 | 189/816 | 6.48 | 1.03 (0.83–1.26) | 0.82 | |
| Prior stroke or TIA | 45/155 | 8.39 | 56/139 | 12.56 | 0.72 (0.47–1.11) | 0.14 | 0.21 |
| No prior stroke or TIA | 257/987 | 7.33 | 264/1024 | 7.36 | 0.98 (0.82–1.17) | 0.79 | |
| Statin | |||||||
| No | 21/137 | 6.80 | 29/147 | 9.42 | 0.77 (0.42–1.39) | 0.39 | |
| Unknown | 92/315 | 6.79 | 112/312 | 8.58 | 0.78 (0.59–1.04) | 0.09 | 0.22 |
| Yes | 189/690 | 7.94 | 179/704 | 7.40 | 1.06 (0.85–1.31) | 0.61 | |
| Hemoglobin | |||||||
| Low | 180/613 | 8.31 | 184/586 | 9.25 | 0.84 (0.67–1.04) | 0.11 | 0.23 |
| High | 122/529 | 6.50 | 136/577 | 6.65 | 1.03 (0.80–1.33) | 0.81 | |
| 6-minute walk, m | |||||||
| High | 97/499 | 5.49 | 126/550 | 6.60 | 0.81 (0.61–1.06) | 0.12 | 0.24 |
| Low | 205/643 | 9.00 | 194/613 | 9.14 | 0.99 (0.81–1.22) | 0.94 | |
| Diabetes mellitus | |||||||
| No | 177/771 | 6.37 | 201/812 | 7.06 | 0.86 (0.70–1.06) | 0.16 | 0.25 |
| Yes | 125/371 | 9.86 | 119/351 | 10.05 | 1.06 (0.81–1.39) | 0.67 | |
| Aspirin or other antiplatelet, prior use | |||||||
| Unknown | 88/304 | 6.77 | 99/288 | 8.34 | 0.75 (0.55–1.02) | 0.06 | |
| No | 38/221 | 7.67 | 36/233 | 6.97 | 1.01 (0.63–1.62) | 0.96 | 0.25 |
| Yes | 176/617 | 7.83 | 185/642 | 7.94 | 1.03 (0.83–1.27) | 0.82 | |
| BUN | |||||||
| Low | 126/555 | 6.03 | 151/598 | 6.95 | 0.83 (0.65–1.07) | 0.15 | 0.26 |
| High | 176/587 | 9.00 | 169/565 | 9.08 | 1.01 (0.81–1.26) | 0.93 | |
| BMI category | |||||||
| >30 | 89/415 | 5.75 | 106/428 | 6.95 | 0.82 (0.61–1.10) | 0.18 | |
| <25 | 91/294 | 9.08 | 92/265 | 10.52 | 0.84 (0.61–1.14) | 0.26 | 0.27 |
| 25–30 | 122/433 | 8.17 | 122/470 | 7.47 | 1.10 (0.84–1.44) | 0.47 | |
| Warfarin, prior use | |||||||
| Yes | 26/90 | 7.72 | 27/89 | 8.62 | 0.69 (0.38–1.23) | 0.21 | 0.29 |
| No | 276/1052 | 7.44 | 293/1074 | 7.88 | 0.96 (0.81–1.13) | 0.60 | |
| Device | |||||||
| No | 236/870 | 7.61 | 268/911 | 8.35 | 0.89 (0.75–1.07) | 0.23 | 0.32 |
| Yes | 66/272 | 7.00 | 52/252 | 6.33 | 1.11 (0.76, 1.63) | 0.59 | |
| eGFR | |||||||
| High | 115/549 | 5.71 | 141/591 | 6.62 | 0.83 (0.65–1.08) | 0.17 | 0.36 |
| Low | 187/593 | 9.22 | 179/572 | 9.41 | 0.98 (0.79–1.22) | 0.84 | |
| WBC | |||||||
| High | 144/590 | 6.76 | 166/596 | 8.28 | 0.87 (0.69–1.10) | 0.23 | 0.40 |
| Low | 158/552 | 8.25 | 154/567 | 7.59 | 1.00 (0.79–1.27) | 0.98 | |
| Myocardial infarction | |||||||
| No | 144/593 | 6.73 | 155/600 | 7.30 | 0.87 (0.69–1.11) | 0.26 | 0.43 |
| Yes | 158/549 | 8.30 | 165/563 | 8.64 | 1.00 (0.79–1.26) | 0.998 | |
| Education | |||||||
| College grad | 32/163 | 4.96 | 52/193 | 6.99 | 0.72 (0.45–1.14) | 0.16 | |
| High School grad | 134/487 | 7.36 | 130/460 | 8.04 | 0.92 (0.71–1.19) | 0.52 | 0.45 |
| Less than High School | 136/492 | 8.62 | 138/510 | 8.25 | 1.01 (0.79–1.29) | 0.96 | |
| Alcohol use | |||||||
| Current | 63/279 | 6.23 | 67/293 | 6.73 | 0.80 (0.56–1.15) | 0.23 | |
| Former | 73/250 | 7.85 | 87/256 | 9.31 | 0.87 (0.62–1.22) | 0.42 | 0.49 |
| Never | 166/613 | 7.89 | 166/614 | 7.89 | 1.02 (0.82–1.28) | 0.85 | |
| Sodium | |||||||
| High | 160/611 | 7.30 | 167/608 | 7.82 | 0.89 (0.71–1.12) | 0.32 | 0.55 |
| Low | 142/531 | 7.66 | 153/555 | 8.06 | 0.99 (0.78–1.25) | 0.92 | |
| Mini-mental status examination | |||||||
| Low | 121/397 | 8.91 | 127/415 | 8.93 | 0.88 (0.67–1.14) | 0.33 | 0.58 |
| High | 181/745 | 6.74 | 193/748 | 7.40 | 0.97 (0.78–1.19) | 0.75 | |
| Coronary artery disease | |||||||
| No | 109/491 | 5.96 | 112/480 | 6.39 | 0.88 (0.67–1.18) | 0.41 | 0.62 |
| Yes | 193/651 | 8.71 | 208/683 | 9.13 | 0.97 (0.79–1.20) | 0.80 | |
| Atrial fibrillation | |||||||
| Yes | 14/44 | 8.91 | 13/42 | 9.93 | 0.78 (0.33–1.80) | 0.55 | 0.67 |
| No | 288/1098 | 7.41 | 307/1121 | 7.87 | 0.94 (0.79–1.11) | 0.45 | |
| Diastolic blood pressure | |||||||
| High | 133/577 | 6.38 | 142/587 | 6.92 | 0.89 (0.70–1.14) | 0.38 | 0.67 |
| Low | 169/565 | 8.62 | 178/576 | 8.98 | 0.96 (0.77–1.20) | 0.74 | |
| PVD | |||||||
| No | 158/560 | 7.87 | 171/575 | 8.54 | 0.92 (0.76–1.09) | 0.33 | 0.68 |
| Yes | 144/582 | 7.07 | 149/588 | 7.34 | 1.01 (0.67–1.52) | 0.97 | |
| Ischemic cardiomyopathy | |||||||
| No | 147/654 | 6.01 | 155/660 | 6.39 | 0.91 (0.72–1.15) | 0.43 | 0.70 |
| Yes | 155/488 | 9.68 | 165/503 | 10.28 | 0.97 (0.77–1.23) | 0.82 | |
| Race/ethnicity | |||||||
| Hispanic | 17/85 | 6.62 | 18/81 | 8.42 | 0.67 (0.33–1.33) | 0.25 | |
| Black | 48/166 | 7.37 | 47/166 | 7.63 | 0.92 (0.59–1.41) | 0.69 | 0.79 |
| White | 229/859 | 7.56 | 245/879 | 7.98 | 0.96 (0.80–1.16) | 0.67 | |
| Other | 8/32 | 7.51 | 10/37 | 7.44 | 0.98 (0.37–2.61) | 0.97 | |
| Hypertension | |||||||
| Unknown | 12/38 | 7.75 | 13/35 | 9.52 | 0.82 (0.34–1.95) | 0.65 | |
| Yes | 167/671 | 7.15 | 190/696 | 8.06 | 0.90 (0.72–1.12) | 0.33 | 0.83 |
| No | 123/433 | 7.91 | 117/432 | 7.60 | 0.99 (0.76–1.29) | 0.93 | |
| Hematocrit | |||||||
| High | 128/552 | 6.6 | 143/577 | 7.07 | 0.87 (0.52–1.47) | 0.61 | 0.83 |
| Low | 174/590 | 8.27 | 177/586 | 8.80 | 0.91 (0.73–1.13) | 0.38 | |
| Systolic blood pressure | |||||||
| Low | 158/560 | 7.87 | 171/575 | 8.54 | 0.92 (0.73–1.16) | 0.48 | 0.88 |
| High | 144/582 | 7.07 | 149/588 | 7.34 | 0.94 (0.74–1.20) | 0.64 | |
| Ejection fraction | |||||||
| Middle | 83/283 | 8.04 | 76/269 | 8.00 | 0.90 (0.71–1.15) | 0.41 | |
| High | 59/261 | 6.09 | 81/272 | 8.26 | 0.93 (0.65–1.33) | 0.70 | 0.97 |
| Low | 100/305 | 9.48 | 89/298 | 8.80 | 0.95 (0.70–1.28) | 0.72 | |
BMI indicates body mass index; BUN, blood urea nitrogen; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; NYHA, New York Heart Association; PVD, peripheral vascular disease; TIA, transient ischemic attack; and WBC, white blood cell.
Each model includes terms for treatment, baseline variable, and treatment×baseline variable interaction. Variables are presented in increasing order of P value for the interaction term. Within variables, subgroups are in increasing order of the HR.
Rates are per 100 patient-years.
P value for the HR.
P value for the test for treatment×baseline variable interaction in a Cox model stratified by site, prior stroke status, and NYHA classification.
Primary Outcome
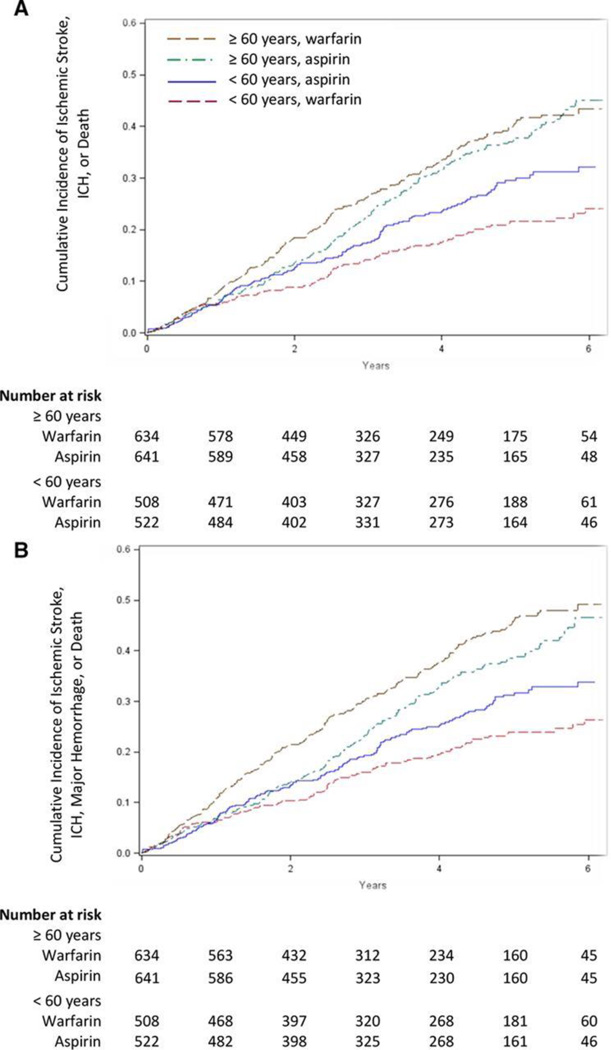
The final stage 2 multiple regression model included terms for treatment, sex, blood urea nitrogen, left ventricular ejection fraction, 6-minute walk, diabetes mellitus, hemoglobin, peripheral vascular disease, diastolic blood pressure, body mass index category, age group, and age group-by-treatment interaction, all of which affected the prognosis (Table I in the online-only Data Supplement). After adjustment for other predictors of outcome, only age group was a significant warfarin or aspirin treatment effect modifier with respect to the primary composite end point (P for interaction, 0.003; Table 2). Among younger patients, there was a statistically significant benefit for warfarin (adjusted HR for warfarin versus aspirin 0.63; 95% CI, 0.48–0.84; P=0.001). However, among older patients, there was no difference between warfarin and aspirin (adjusted HR, 1.09; 95% CI, 0.88–1.35; P=0.44; Table 2). Figure 1A shows the unstratified cumulative incidence curves for the primary end point for the younger and older age groups, respectively.
Table 2.
Adjusted Hazard Ratios for the Primary End Point and Its Components for Final Cox Regression Model
| Warfarin |
Aspirin |
Adjusted Hazard Ratio (95%) CI) for Warfarin vs Aspirin |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Events, % | Rate* | Events, % | Rate* | For HR† | For Interaction | ||
| <60 y | n=508 | 1935.0 pt-years | n=522 | 1924.2 pt-years | |||
| Primary composite | 93 (18.3) | 4.81 | 130 (24.9) | 6.76 | 0.63 (0.48–0.84) | 0.001 | 0.003 |
| Ischemic stroke | 12 (2.4) | 0.62 | 26 (5.0) | 1.35 | 0.51 (0.32–0.81) | 0.005 | 0.64‡ |
| Intracerebral hemorrhage | 2 (0.4) | 0.10 | 0 (0.0) | 0.00 | 2.35 (0.44–12.48) | 0.32 | …§ |
| Death | 79 (15.6) | 4.08 | 104 (19.9) | 5.40 | 0.65 (0.48–0.89) | 0.007 | 0.003 |
| ≥60 y | n=634 | 2109.7 pt-years | n=641 | 2108.6 pt-years | |||
| Primary composite | 209 (33.0) | 9.91 | 190 (29.6) | 9.01 | 1.09 (0.88–1.35) | 0.44 | 0.003 |
| Ischemic stroke | 17 (2.7) | 0.81 | 29 (4.5) | 1.38 | 0.51 (0.32–0.81) | 0.005 | 0.64‡ |
| Intracerebral hemorrhage | 3 (0.5) | 0.14 | 2 (0.3) | 0.09 | 2.35 (0.44–12.48) | 0.32 | …§ |
| Death | 189 (29.8) | 8.96 | 159 (24.8) | 7.54 | 1.18 (0.94–1.49) | 0.16 | 0.003 |
6MW indicates 6-minute walk; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; DBP, diastolic blood pressure; HR, hazard ratio; ICH, intracerebral hemorrhage; NYHA, New York Heart Association; and PVD, peripheral vascular disease.
Rates are per 100 patient-years.
From Cox models stratified by site, prior stroke status, and NYHA classification. HRs for composite end point, death, and stroke are adjusted for age category, sex, diabetes mellitus, PVD, BMI, BUN, ejection fraction, DBP, 6MW, and hemoglobin. HRs for ICH adjusted for age category. Treatment-by-age category interaction terms are included as applicable.
Hazard ratios are from models without treatment×age category interaction term for ischemic stroke, given no evidence of treatment×age category interaction.
Not enough events to test for interaction. HR is from the model without a treatment×age category term.
Figure 1.
Kaplan–Meier estimates in patients ≥60 and <60 years for the primary end point (A) and the primary end point or major hemorrhage (B). Plots show the Kaplan–Meier estimated probability of an event. ICH indicates intracerebral hemorrhage.
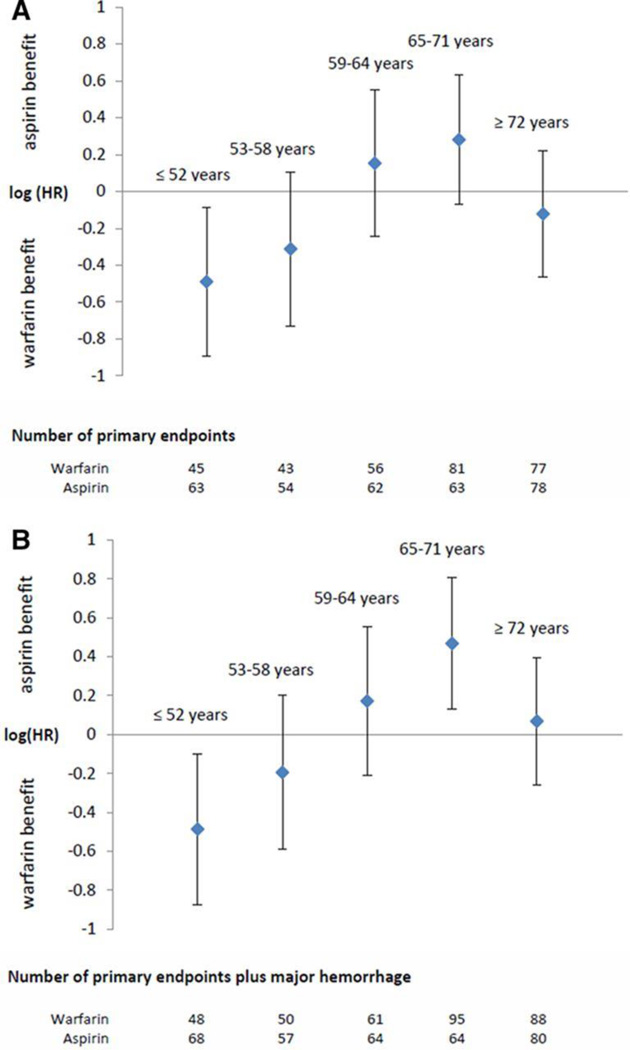
We evaluated our age cutoff (<60 years) by modeling age as a continuous variable and by comparing warfarin and aspirin by age quintile. There was a significant interaction between treatment and continuous age as a linear effect on the log HR (P=0.04 when adjusting for covariates from the final selected model; table not presented). According to this model, there was a significant benefit for warfarin among younger patients. The upper limit of the 95% CI crossed 1.0 at 59.4 years, and there was no significant treatment effect among patients >60 years. When warfarin and aspirin were compared in age quintiles, there was a statistically significant interaction between treatment and age quintile (P=0.04). Figure 2A presents the HRs for treatment effect by quintile and supports 60 years as a reasonable cutoff.
Figure 2.
Natural logarithm of the hazard ratio (HR) by quintile of age for the comparison of warfarin versus aspirin on the primary end point (A) and the primary end point or major hemorrhage (B).
Components of the Primary Outcome
The ischemic stroke rates in younger and older patients were similar, and in both age groups those assigned to warfarin achieved a substantial reduction in ischemic stroke compared with aspirin (HR, 0.51; 95% CI, 0.32–0.81; P for HR, 0.005; P for interaction, 0.64; Table 2). Warfarin reduced death in the younger group (4.08 per 100 patient-years for warfarin versus 5.40 for aspirin; adjusted HR, 0.65; 95% CI, 0.48–0.89; P=0.007), but not in the older group, whose death rate was higher (8.96 per 100 patient-years for warfarin versus 7.54 for aspirin; adjusted HR, 1.18; 95% CI, 0.94–1.49; P=0.16). Because few patients experienced ICH, it was not possible to test for a differential treatment effect by age group. There was no significant difference overall between warfarin and aspirin with respect to ICH (adjusted HR, 2.35; 95% CI, 0.44–12.48; P=0.32; Table 2).
Hemorrhage
In the younger age group, there was no significant difference between warfarin and aspirin in the rate of major hemorrhages (odds ratio, 1.30; 95% CI, 0.56–3.07; P=0.64). However, in the older age group, significantly more major hemorrhages occurred in those receiving warfarin (odds ratio, 2.73; 95% CI, 1.56–4.97; P<0.001; Table 3). When the time to first to occur of primary outcome or major hemorrhage was analyzed, there was a statistically significant treatment-by-age group interaction with respect to this composite outcome (P<0.001; Table 4). Among the younger patients, those randomized to warfarin had a lower rate of combined events compared with aspirin patients (5.41 versus 7.25% per 100 patient-years; adjusted HR, 0.68; 95% CI, 0.52–0.89; P=0.005), whereas older patients in the warfarin arm experienced a significantly higher rate of events than those in the aspirin arm (11.8 versus 9.35% per 100 patient-years; adjusted HR, 1.25; 95% CI, 1.02–1.53; P=0.03; Table 4). Figure 1B presents the unstratified cumulative incidence curves and Figure 2B the HRs by age quintile, both by treatment, when major hemorrhage is included in the outcome.
Table 3.
Odds Ratios for Major and Minor Hemorrhage
| Warfarin |
Aspirin |
Odds Ratio (95% CI)‡ for Warfarin vs Aspirin |
P Value | |||
|---|---|---|---|---|---|---|
| Patients, % | Events* (Rate†) | Patients, % | Events* (Rate†) | |||
| <60 y | n=508 | 1935.0 pt-years | n=522 | 1924.2pt-years | ||
| Major hemorrhage | 15 (3.0) | 16 (0.83) | 12 (2.3) | 14 (0.73) | 1.30 (0.56–3.07) | 0.64 |
| Minor hemorrhage | 129 (25.4) | 201 (10.39) | 79 (15.1) | 129 (6.70) | 1.95 (1.41–2.71) | <0.001 |
| ≥60 y | n=634 | 2109.7 pt-years | n=641 | 2108.6 pt-years | ||
| Major hemorrhage | 51 (8.0) | 56 (2.65) | 19 (3.0) | 21 (1.00) | 2.73 (1.56–4.97) | <0.001 |
| Minor hemorrhage | 151 (23.8) | 267 (12.66) | 110 (17.2) | 167 (7.92) | 1.43 (1.07–1.92) | 0.014 |
CI indicates confidence interval.
Numbers of events refect multiple hemorrhages in some patients.
Rates are per 100 patient-years.
Odds ratios were calculated by the exact test of 2 independent proportions, stratified by continent.
Table 4.
Adjusted Hazard Ratios for Primary End point or Major Hemorrhage and Components, According to Age Subgroup
| Warfarin |
Aspirin |
Adjusted Hazard Ratio (95% CI) for Warfarin vs Aspirin |
P Value |
||||
|---|---|---|---|---|---|---|---|
| Events, % | Rate* | Events, % | Rate* | For HR† | For Interaction‡ | ||
| <60 y | n=508 | 1903.7 pt-years | n=522 | 1904.1 pt-years | |||
| Primary plus hemorrhage | 103 (20.3) | 5.41 | 138 (26.4) | 7.25 | 0.68 (0.52–0.89) | 0.005 | <0.001 |
| Major hemorrhage | 13 (2.6) | 0.68 | 12 (2.3) | 0.63 | 0.98 (0.42–2.27) | 0.96 | 0.06 |
| Ischemic stroke | 12 (2.4) | 0.63 | 25 (4.8) | 1.31 | 0.50 (0.31–0.81) | 0.005 | 0.68§ |
| Intracerebral hemorrhage | 2 (0.4) | 0.11 | 0 (0.0) | 0.00 | 2.05 (0.36–11.52) | 0.42 | …∥ |
| Death | 76 (15.0) | 3.99 | 101 (19.3) | 5.30 | 0.66 (0.48–0.90) | 0.009 | 0.002 |
| ≥60 y | n=634 | 2026.2 pt-years | n=641 | 2085.9 pt-years | |||
| Primary plus hemorrhage | 239 (37.7) | 11.8 | 195 (30.4) | 9.35 | 1.25 (1.02–1.53) | 0.03 | <0.001 |
| Major hemorrhage | 48 (7.6) | 2.37 | 17 (2.7) | 0.82 | 2.67 (1.48–4.81) | 0.001 | 0.06 |
| Ischemic stroke | 16 (2.5) | 0.79 | 29 (4.5) | 1.39 | 0.50 (0.31–0.81) | 0.005 | 0.68§ |
| Intracerebral hemorrhage | 2 (0.3) | 0.10 | 2 (0.3) | 0.10 | 2.05 (0.36–11.52) | 0.42 | …∥ |
| Death | 173 (27.3) | 8.54 | 147 (22.9) | 7.05 | 1.22 (0.96–1.56) | 0.009 | 0.002 |
6MW indicates 6-minute walk; BMI, body mass index; BUN, blood urea nitrogen; CI, confidence interval; DBP, diastolic blood pressure; HR, hazard ratio; ICH, intracerebral hemorrhage; NYHA, New York Heart Association; and PVD, peripheral vascular disease.
Rates are per 100 patient-years.
From Cox models stratified by site, prior stroke status, and NYHA classification. HRs for composite end point, death, stroke, and hemorrhage are adjusted for age category, sex, diabetes mellitus, PVD, BMI, BUN, ejection fraction, DBP, 6MW, and hemoglobin. HRs for ICH are adjusted for age category.
Treatment-by-age category interaction terms are included as applicable.
Hazard ratios presented are from models without treatment×age category interaction term for ischemic stroke, because there was no evidence of treatment×age category interaction.
Not enough information to test for interaction. The HR presented is from the model without a treatment×age category term.
Patient Characteristics by Age
Because randomization was not stratified by age group, we compared the warfarin and aspirin arms in each age group in terms of baseline characteristics. Among the younger patients, only education level was significantly different between the warfarin and aspirin groups (P=0.03; Table IIA in the online-only Data Supplement). Among the older patients, the differences between warfarin and aspirin were significant for 6-minute walk distance (P=0.02) and nitrate use (P=0.01; Table IIB in the online-only Data Supplement). Adjusting the analyses of time to primary composite end point and time to primary composite end point plus major hemorrhage for education and nitrate use did not materially change the results.
The younger warfarin patients had statistically significantly lower mean INR values than the older warfarin patients (2.36±0.63 versus 2.51±0.56, respectively; P<0.001, with patients weighted equally. When patients were weighted by total INR days of follow-up, the mean INR values in the 2 age groups were statistically different in same direction; P<0.001). At the same time, mean percentage of time in therapeutic range (TTR) in younger patients was significantly lower than in older patients (52.8±28.5% versus 60.4±28.0%; P<0.001). Compared with older patients, younger patients had a significantly longer time spent with INR below the therapeutic range (37.4±29.8% versus 28.4±27.6%; P<0.0001). However, the time with INR above the therapeutic range was similar between the 2 groups (9.7±12.5% versus 11.2±13.2%; P>0.06). The 2 age groups did not differ in terms of the mean proportion of follow-up time spent on interruption of study therapy (28.7% for younger versus 30.3% for older; P=0.30).
Discussion
WARCEF, with >4× the number of patient-years of follow-up compared with the next largest trial, was the largest trial to compare the efficacy of warfarin and aspirin in patients with HF in sinus rhythm.12 It found no significant difference between them on the composite primary end point of ischemic stroke, ICH, or death.4 However, the primary aim of WARCEF was to compare warfarin and aspirin in the general HF population, and not among different subgroups. The causes of HF are multifactorial, and warfarin or aspirin may benefit some specific groups but not others.7,13 We sought to identify such groups using an automated stepwise selection procedure to adjust for subgroup variability in baseline characteristics and identify treatment-by-variable interactions for multiple variables that may covary.
Country of recruitment significantly affected the efficacy of warfarin or aspirin, but this was because of country differences in age distribution. After adjustment for all factors considered, age group was the only modifier of treatment effect. Warfarin patients <60 years experienced a significant reduction in the rate of composite primary end point, and also in its separate components of ischemic stroke and death, without an increase in major bleeding. Older patients, in contrast, experienced no difference between warfarin and aspirin in the composite primary end point. Warfarin did reduce their ischemic stroke risk, but it was also associated with a nonsignificant increase in death; and when major bleeding was added to the composite outcome, the overall risk of a poor outcome became significantly greater for the older warfarin patients. The reduced benefit of warfarin in older patients compared with younger patients is not attributable to lower INRs or a lower TTR. In fact, the older group demonstrated significantly higher TTR and less time spent above therapeutic INR while having similar time duration spent in interruption of study therapy compared with the younger group.
There are ≈5.7 million patients with HF in the United States and 25% are aged <60 years.14,15 Of this group, ≤60% or 855 000 are thought to have systolic rather than diastolic HF.16,17 Among this group, since the prevalence of atrial fibrillation is smaller in younger patients with HF, 90% or 769 500 patients are in sinus rhythm.18 In our study, in the younger population, the absolute yearly risk reduction was 1.95% (relative risk reduction of 28.8%), which would mean that ≈15 005 net events (5617 strokes and 10 157 deaths at a cost of 769 ICHs) may possibly be avoided by the use of warfarin in younger patients. However, in the older population, because warfarin resulted in increased bleeding risk while not affecting the primary end point, unnecessary bleeding may be avoided by the use of aspirin. We saw a longer time on interruption of study therapy among older patients assigned to warfarin. This may have blunted any possible benefit of warfarin and points to difficulty of warfarin use in older patients.19,20 Although our findings may have a large public health impact, they require confirmation in a future trial. Also, given the possible benefit of warfarin in the younger population, the role of new anticoagulants needs to be established. Younger patients may benefit from the ease of use of these agents, and older patients may have a lower bleeding risk while maintaining the benefit for stroke reduction.21,22
In WARCEF, patients were double-blindly randomized, lost to follow-up rate was low, and core echocardiography laboratory and centralized adjudication process were used to achieve high data quality. However, there are important limitations. First, candidate variables included those that were not prespecified, although they are well known or thought to affect the outcome in patients with HF. Second, no correction for the number of variables examined was made. However, under a simple Bonferroni correction for the 32 variables considered in stage 1, the effect modification for age was significant. Third, no placebo group was included. Therefore, the comparison is strictly between those receiving warfarin and aspirin. Finally, although we can point to potential mechanisms, we have no clear biological explanation for our results.
Conclusions
In our exploratory analysis, patients with HF in sinus rhythm aged <60 years benefited from warfarin compared with aspirin on the combined outcome of ischemic stroke, ICH, or death, whereas older patients did not. When major hemorrhages are also considered, the warfarin benefit for the younger patients persisted, but older patients on warfarin had more adverse outcomes than those on aspirin. A pivotal trial to confirm the possible benefit of warfarin for younger patients is warranted given the potentially large impact on treatment of patients with HF.
Supplementary Material
CLINICAL PERSPECTIVE.
The Warfarin versus Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) Trial found no difference between warfarin and aspirin for the primary end point of ischemic stroke, intracerebral hemorrhage, or death in patients with heart failure and reduced systolic function. There was a benefit for warfarin over aspirin for ischemic stroke alone, but it was offset by a higher rate of major hemorrhages in the warfarin patients. This study asks whether there are easily identifiable groups of patients who may benefit from warfarin without increased risk of bleeding. We found that, among patients <60 years, compared with the aspirin patients, the warfarin patients had a significant reduction in the overall primary end point without a higher risk of bleeding. The same was true when we considered ischemic stroke and death separately. In patients >60 years, however, although the ischemic stroke rate was lower for the warfarin patients, the risk of bleeding was significantly higher than among the aspirin patients. In summary, this post hoc analysis found that, compared with aspirin, warfarin may be beneficial for younger patients with heart failure in sinus rhythm, but not for older patients.
Acknowledgments
Sources of Funding
The WARCEF trial was supported by grant nos. U01-NS-043975 to Dr Homma and U01-NS-039143 to Dr Thompson from the National Institute of Neurological Diseases and Stroke. Warfarin and warfarin placebo were provided by Taro Pharmaceuticals USA, and aspirin and aspirin placebo by Bayer HealthCare.
Disclosures
Dr Homma reports receiving payment from AGA Medical (now St. Jude Medical) for his work as a member of a data and safety monitoring board and consulting fees from Boehringer Ingelheim; Dr Levin, receiving consulting fees from United Healthcare; Dr Teerlink, receiving consulting fees from Amgen and grant support from Amgen, Cytokinetics, and Novartis on behalf of himself and from NovaCardia/Merck on behalf of himself and his institution; Dr Graham, owning stock in March Pharmaceuticals, Medtronic, and Pfizer; Dr Labovitz, receiving grant support from Boehringer Ingelheim on behalf of his institution, lecture fees from Boehringer Ingelheim, and fees for the development of educational presentations from the American College of Cardiology; Dr Anker, receiving consulting fees from Amgen, Bosch Healthcare, GlaxoSmithKline, Helsinn, LoneStar Heart, Novartis, Professional Dietetics, PsiOxus, Relypsa, SHL Telemedicine, and Thermo Fisher, grant support from Vifor Pharma, and lecture fees from Novartis, holding patents with Brahms AG and Charité Berlin, and receiving royalties from Imperial College; Dr Ponikowski, receiving consulting fees from Bayer, Boehringer Ingelheim, Coridea, Corthera, Johnson & Johnson, Pfizer, Respicardia, and Vifor Pharma, grant support from Vifor Pharma on behalf of himself and his institution, and lecture fees from Abbott, Boehringer Ingelheim, Merck Serono, Pfizer, Respicardia, Sanofi-Aventis, Servier, and Vifor Pharma; and Dr Lip, receiving consulting fees from Astellas, AstraZeneca, Bayer, Biotronik, Boehringer Inhelheim, Bristol-Myers Squibb, Pfizer, Merck, Portola, and Sanofi-Aventis, speakers bureau fees from Bayer, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, and Sanofi-Aventis, and payment for the development of educational presentations from Bayer, Boehringer Ingelheim, and Merck.
Footnotes
A list of all WARCEF Investigators is given in the Appendix in the online-only Data Supplement.
The online-only Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.113.000372/-/DC1.
The other authors report no conflicts.
References
- 1.Pullicino PM, Halperin JL, Thompson JL. Stroke in patients with heart failure and reduced left ventricular ejection fraction. Neurology. 2000;54:288–294. doi: 10.1212/wnl.54.2.288. [DOI] [PubMed] [Google Scholar]
- 2.Lip GY, Gibbs CR. Does heart failure confer a hypercoagulable state? Virchow’s triad revisited. J Am Coll Cardiol. 1999;33:1424–1426. doi: 10.1016/s0735-1097(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 3.Uretsky BF, Thygesen K, Armstrong PW, Cleland JG, Horowitz JD, Massie BM, Packer M, Poole-Wilson PA, Ryden L. Acute coronary findings at autopsy in heart failure patients with sudden death: results from the assessment of treatment with lisinopril and survival (ATLAS) trial. Circulation. 2000;102:611–616. doi: 10.1161/01.cir.102.6.611. [DOI] [PubMed] [Google Scholar]
- 4.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, Ammon SE, Graham S, Sacco RL, Mann DL, Mohr JP, Massie BM, Labovitz AJ, Anker SD, Lok DJ, Ponikowski P, Estol CJ, Lip GY, Di Tullio MR, Sanford AR, Mejia V, Gabriel AP, del Valle ML, Buchsbaum R WARCEF Investigators. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velagaleti RS, Vasan RS. Epidemiology of heart failure. In: Mann DL, editor. Heart Failure, a Companion to Braunwald’s Heart Disease. 2nd ed. St. Louis, MO: Elsevier Saunders; 2011. pp. 339–350. [Google Scholar]
- 6.Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 8.Shah S, Parra D, Rosenstein R. Warfarin versus aspirin in heart failure and sinus rhythm [letter] N Engl J Med. 2012;367:771. doi: 10.1056/NEJMc1207385. [DOI] [PubMed] [Google Scholar]
- 9.Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, Anand I, Maggioni A, Burton P, Sullivan MD, Pitt B, Poole-Wilson PA, Mann DL, Packer M. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 10.Pocock SJ, Ariti CA, McMurray JJ, Maggioni A, Køber L, Squire IB, Swedberg K, Dobson J, Poppe KK, Whalley GA, Doughty RN Meta-Analysis Global Group in Chronic Heart Failure. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 2013;34:1404–1413. doi: 10.1093/eurheartj/ehs337. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD American Heart Association Strategic Planning Task Force and Statistics Committee. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 12.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, Jafri SM, Krol WF, O’Connor CM, Schulman KA, Teo K, Warren SR WATCH Trial Investigators. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 13.Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002;347:969–974. doi: 10.1056/NEJMoa020496. [DOI] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howden L, Meyer J. [Accessed October 8, 2012];United States Census Bureau: 2010 Census Summary Files. http://www.census.gov/prod/cen2010/briefs/c2010br-03.pdf.
- 16.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–327. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee K, Massie B. Systolic and Diastolic Heart Failure. J Card Fail. 2007;13:569–576. doi: 10.1016/j.cardfail.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 18.De Ferrari GM, Klersy C, Ferrero P, Fantoni C, Salerno-Uriarte D, Manca L, Devecchi P, Molon G, Revera M, Curnis A, Sarzi Braga S, Accardi F, Salerno-Uriarte JA ALPHA Study Group. Atrial fibrillation in heart failure patients: prevalence in daily practice and effect on the severity of symptoms. Data from the ALPHA study registry. Eur J Heart Fail. 2007;9:502–509. doi: 10.1016/j.ejheart.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Torn M, Bollen WL, van Meerder FJ, van der Wall EE, Rosendaal FR. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med. 2005;165:1527–1532. doi: 10.1001/archinte.165.13.1527. [DOI] [PubMed] [Google Scholar]
- 20.Froom P, Miron E, Barak M. Oral anticoagulants in the elderly. Br J Haematol. 2003;120:526–528. doi: 10.1046/j.1365-2141.2003.04110.x. [DOI] [PubMed] [Google Scholar]
- 21.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 22.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.