Abstract
Learned associations between drugs and the places they are used are critical to the development of drug addiction. Contextual conditioning has long been studied in animals as an indirect measure of drug reward, but little is known about the process in humans. Here, we investigated de novo contextual conditioning with d-amphetamine in healthy humans (n = 34). Volunteers underwent four conditioning sessions conducted in two testing rooms with double-blind, alternating d-amphetamine (20 mg) and placebo administration. Before conditioning procedures began, they rated the two rooms to examine pre-existing preferences. One group (Paired, n = 19) always received d-amphetamine in their least preferred room and placebo in the other during conditioning sessions. Another group (Unpaired, n = 15) received d-amphetamine and placebo in both rooms. Subjective drug effects were monitored at repeated times. At a separate re-exposure test, preference ratings for the drug-associated room were increased among the Paired group only, and more subjects in the Paired than the Unpaired group switched their preference to their initially least preferred room. Also, ratings of d-amphetamine drug liking independently predicted room liking at test among the Paired group only. Further, Paired group subjects reported greater stimulation and drug craving after d-amphetamine on the second administration, relative to the first. This study supports preliminary findings that humans, like animals, develop a preference for a place associated with d-amphetamine that is related to its subjective effects. These findings also suggest that experiencing d-amphetamine in a consistent environment produces context-dependent changes in its subjective effects, including an enhanced rewarding efficacy and abuse potential.
Keywords: Conditioned place preference, contextual conditioning, d-amphetamine, humans, sensitization, subjective effects
INTRODUCTION
Learned associations between psychoactive drug effects and the places where they are experienced are believed to play a critical role in drug addiction. Major theories posit that such associations, called contextual conditioning, produce robust, long-lasting changes in motivational and emotional systems that contribute to compulsive drug use and relapse in addiction (Ludwig 1986; Robinson & Berridge 1993; O’Brien et al. 1998; Kelley 2004; Hyman, Malenka & Nestler 2006). Although there is an extensive literature on contextual conditioning in animals (Tzschentke 2007), few studies have examined de novo contextual conditioning in humans. This is surprising because human drug users report greater craving in response to drug-associated places than specific, drug-related objects, i.e. discrete cues (Ludwig 1986; Lee et al. 2007). Conditioning of contexts can be distinguished from conditioning with discrete cues, and recent preclinical research indicates that the two forms of conditioning may involve different neural circuits (Lee et al. 2007; Janak & Chaudhri 2010). Although many studies have examined responses to discrete cues in human drug users, conditioning with contexts, or drug-related places, has not been studied. An understanding of contextual conditioning will help us understand how drugs come to control behavior in addicted individuals.
The conditioned place preference (CPP) procedure is a widely used animal model of drug reward that is based upon contextual conditioning (Tzschentke 2007). In the model, two distinct environments are paired with drug and placebo administration. After several conditioning sessions, in which drug is administered in one environment and placebo in another, the organism is given access to both places during a choice session without drug. Laboratory animals will approach and spend more time in the place where they previously received a drug known to produce rewarding effects in humans, i.e. they exhibit a CPP for the drug-paired context. The extensive literature using this procedure provides strong evidence for conditioning between drug effects and contexts in non-humans. CPP is thought to be based on Classical or Pavlovian conditioning. That is, after repeated associations with the drug, the initially neutral environment (unconditioned stimulus) acquires secondary incentive motivational properties and becomes a conditioned stimulus capable of producing a conditioned response and influencing behavior in absence of the drug, in this case conditioned approach to the drug-paired context. It is often assumed that a CPP in animals is formed through contextual associations with interoceptive drug effects analogous to subjectively rewarding drug effects in humans.
Recently, we (Childs & de Wit 2009) developed a method to study contextual conditioning in humans. Using a model based on the animal place preference procedure, we reported that healthy volunteers who consistently received d-amphetamine in one room rated that room more favorably than another room where they consistently received placebo. In contrast, volunteers who received d-amphetamine and placebo in both rooms rated the rooms equally. Moreover, the acute subjective effects of amphetamine during the conditioning sessions predicted the degree of place liking. Specifically, amphetamine-related liking positively predicted, and amphetamine-related anxiety negatively predicted, room liking scores at test. This pilot study demonstrated the feasibility of measuring drug contextual conditioning in the human laboratory. Further, the findings provided initial support for the theory that contextual conditioning in laboratory animals may be established through drug effects that are analogous to subjective drug effects in humans.
In this study, we aimed to extend our preliminary findings of d-amphetamine CPP in humans using procedures more similar to those used in animal studies. First, we used a biased procedure for assignment of drug- or placebo-associated rooms, based on subjects’ room preference ratings before conditioning. That is, the Paired group always received amphetamine in their least preferred room during the conditioning sessions. We chose to use a biased procedure for stimulus assignment because the results of our first study (Childs & de Wit 2009) suggested that some subjects had a mild preference for one room over the other, even in the absence of conditioning. In addition, preclinical studies indicate that the unbiased method of drug and placebo assignment can produce problems with ceiling effects when the drug is assigned to an already preferred environment (Cunningham, Ferree & Howard 2003). Second, in the present study, participants rated the two testing rooms during a separate re-exposure test session, conducted after the conditioning sessions were completed. During this re-exposure test subjects rated their liking of the rooms while they were physically in each room and not from memory as in our previous study (Childs & de Wit 2009).
We hypothesized that, at test, a greater proportion of participants in the Paired group would switch their preference to the initially non-preferred room than the Unpaired group. We also hypothesized that subjective preference and liking ratings for the drug-paired (initially non-preferred) room would be increased among the Paired group but not the Unpaired group. A secondary aim of the study was to investigate whether administration of amphetamine in a consistent context would induce context-dependent changes in the acute effects of the drug. We hypothesized that the acute stimulant effects of d-amphetamine would be increased on the second administration in a consistent environment (Paired group) but not in an inconsistent environment (Unpaired group).
METHODS
Subjects
Healthy male (n = 25) and female (n = 9) volunteers aged 18–40 were recruited from the Chicago area and local community using advertisements and flyers. They underwent an in-person screening interview that included a physical examination and electrocardiogram (ECG). Exclusion criteria included a current or prior diagnosis of a major axis I DSM-IV disorder (APA 1994), including past year drug dependence, serious medical conditions, high blood pressure, abnormal ECG, use of medications, body mass index outside of 19–26 kg/m2, less than high school education or lack of fluency in English, nightshift work and pregnancy or lactation in women. Women had to be using hormonal birth control because menstrual cycle is known to influence the subjective effects of d-amphetamine (White, Justice & de Wit 2002b). Eligible candidates signed a consent form which stated that the aim of the study was to investigate interactions between drug effects and the environment, and that they may receive a stimulant, sedative or a placebo.
Design
Subjects completed six separate sessions conducted at least 2 days but no more than 5 days apart; one 1-hour orientation session, four 4-hour conditioning sessions and one 1-hour re-exposure choice test session. During the orientation session, subjects completed an initial choice test during which they explored and rated the two testing rooms to be used for the conditioning sessions. During the four conditioning sessions, which were conducted between 9 a.m. and 1 p.m., participants received either d-amphetamine (20 mg) or placebo under double-blind conditions. One group (Paired, n = 19) received d-amphetamine (A) on two occasions in the room they preferred least at the initial choice test and placebo (P) on two occasions in the room they initially preferred the most. The order of treatments alternated across successive sessions, i.e. A, P, A, P or P, A, P, A. A second group (Unpaired, n = 15) received d-amphetamine and placebo in both rooms, and the order of treatments was randomized. Male and female subjects were randomized separately to the two groups. At the re-exposure choice test session, participants completed a second choice test during which they were allowed to explore the two testing rooms and rate their liking and preference for each room.
Experimental procedure
The University of Chicago Hospital’s Institutional Review Committee for the use of human subjects approved the protocol. Procedures were conducted at the Human Behavioral Pharmacology Laboratory at the University of Chicago Hospital. Conditioning sessions were performed in two same-sized, adjacent testing rooms that were comfortably furnished as a living room environment. Each room contained a couch, an easy chair, a desk, a computer (for administering questionnaires), a television/video player and reading materials. The two rooms were distinct in terms of accent colors, i.e. cushions, flowers and scents (citrus and clean linen), but they were designed to be equal in attractiveness. When participants were not completing study measures (questionnaires and vital signs), they were allowed to relax in the testing room and read or watch television/movies.
At the orientation session (conducted in a different room to the conditioning sessions), subjects signed the consent form which stated that the experiment aimed to study interactions between drugs and the environment. They were allowed to explore the two testing rooms to be used during conditioning sessions in an initial choice test. Their liking and preference ratings for the two rooms (see Dependent Measures section) were used to assign the drug- and placebo-paired rooms for Paired group subjects.
Upon arrival at the conditioning sessions, subjects provided breath and urine samples to test for the presence of drugs and alcohol, and for pregnancy in women. No one tested positive. Levels of carbon monoxide in expired air were obtained to control for time since last smoking; there were no differences between the groups. Subjects were then escorted to the testing room for that session, where they relaxed for 30 minutes before baseline measures of mood, heart rate and blood pressure were obtained. They then consumed a capsule containing 20 mg of d-amphetamine or placebo. Subjects completed mood and drug effects questionnaires, and vital signs were monitored every 30 minutes for 3 hours after consumption of the capsule. At the end of the session, final measures were collected and participants were allowed to leave.
At the final test session (conducted in a different room from the conditioning sessions), subjects completed a re-exposure choice test during which they explored the two testing rooms and rated their liking and preference for each room. They were then fully debriefed about the aims of the experiment, the drug they had received and received payment ($200).
Dependent measures
Subjective mood and drug effects were assessed using standardized self-report questionnaires: the Profile of Mood States (POMS; McNair & Droppleman 1971), the Addiction Research Center Inventory (ARCI; Haertzen 1966) and the Drug Effects Questionnaire (DEQ; Johanson & Uhlenhuth 1980).
Participants rated their liking and preference for the testing rooms on a Room Preference Questionnaire that consisted of a series of 100-mm visual analogue scales (VAS; Folstein & Luria 1973). First, participants rated how much they liked each room; each testing room (A and B) was associated with a 100-mm line labeled at one end with ‘Dislike’ (scored −50), in the center with ‘Neutral’ (scored 0) and at the other end with ‘Like very much’ (scored +50). Then, participants rated their relative preference for the two rooms upon a 100-mm line labeled at one end with ‘Prefer Room A’ and at the other with ‘Prefer Room B’. This questionnaire was completed twice; once at the orientation session and once at the re-exposure test session.
Drugs
d-Amphetamine sulphate (four 5-mg tablets; Mallinkrodt, Hazelwood, MO, USA) was administered in opaque gelatin capsules (size 00) with dextrose filler. Placebo capsules contained dextrose filler.
Data analysis
Demographic characteristics and substance use were compared between participants in each group using independent samples t-test (for continuous variables) or Pearson’s chi-squared analysis (for categorical variables).
Ratings of liking and preference for each of the rooms were compared between the pre- and post-conditioning choice tests in each group using Paired samples t-test (for room preference ratings) and two-factor (Room × Time) repeated measures analysis of variance (ANOVA; for room liking ratings).
Subjective and physiological responses to d-amphetamine and placebo during each session were calculated as the area under the curve relative to baseline using the trapezoid method (Pruessner et al. 2003). We explored relationships between subjective responses to d-amphetamine and changes in ratings of room liking and preference using a multiple linear regression model as previously described (Childs & de Wit 2009). Finally, we compared physiological and subjective responses to d-amphetamine on the first and second administrations (AUC2 − AUC1) between the groups using a two-factor Group × Drug repeated measured ANOVA.
All analyses were conducted using SPSS® Version 18 (SPSS Inc., Chicago, IL, USA) for Windows. Differences were considered significant at P < 0.05.
RESULTS
Demographic characteristics
Most participants were European American full-time students in their early twenties (see Table 1). The groups differed on habitual smoking (but not in CO levels on arrival at the sessions) and history of cannabis use. Participants in the Paired group smoked more cigarettes per week [t(32) = 2.4, P < 0.05], while more participants in the Unpaired group reported having ever used cannabis [χ2(1) = 3.9, P < 0.05]. These variables did not influence subjective or cardiovascular responses to d-amphetamine or changes in liking and preference ratings and so were not included in further analyses.
Table 1.
Demographic characteristics and drug use history of participants in each group.
| Paired (n = 19) | Unpaired (n = 15) | |
|---|---|---|
| Sex (male/female) | 13/6 | 12/3 |
| Age | 23.6 ± 0.9 | 23.4 ± 0.9 |
| Body mass index (kg/m2) | 22.4 ± 0.3 | 22.5 ± 0.5 |
| Full-time student (%) | 78.9 | 86.7 |
| Education level (%) | ||
| High school | 26 | 7 |
| Partial degree or in process | 68 | 80 |
| Advanced degree | 5 | 13 |
| Caffeine consumption (cups/week) | 5.0 ± 0.9 | 8.4 ± 1.8 |
| Alcohol consumption (drinks/week) | 5.8 ± 1.1 | 7.1 ± 2.0 |
| Smoking status (cigarettes/week) | 6.6 ± 2.4 | 0.2 ± 0.1* |
| Cannabis use (times/month) | 4.8 ± 1.7 | 3.7 ± 2.0 |
| Race (%) | ||
| White | 53 | 67 |
| Black | 0 | 7 |
| Asian | 16 | 13 |
| Other | 32 | 13 |
| Drug use (% ever used) | ||
| Cannabis | 26 | 60* |
| Stimulants (d-amphetamine) | 26 (26) | 20 (15) |
| Opiates | 21 | 13 |
| Tranquilizers | 0 | 7 |
| Hallucinogens | 21 | 20 |
Asterisks indicate a significant difference between groups. Independent samples t-test for continuous variables, Pearson’s Chi-squared for categorical variables,
P < 0.05.
Room preference
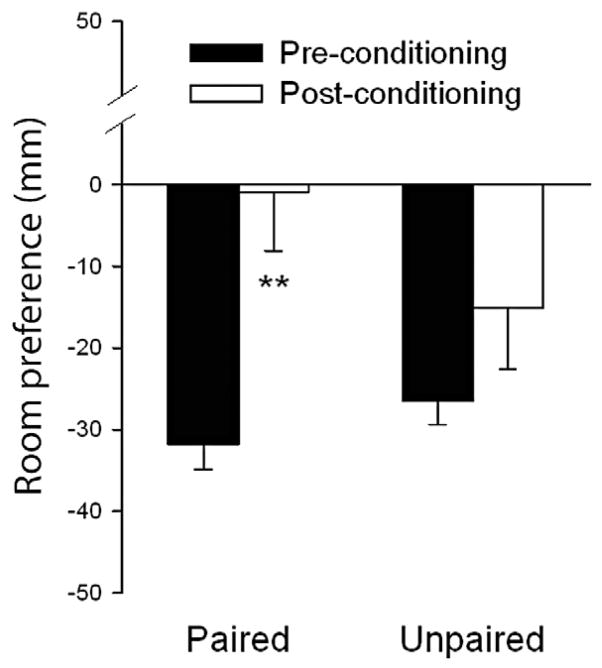
A significantly larger proportion of subjects in the Paired group switched their preference to the initially non-preferred room at the re-exposure choice test (53%) in comparison with the Unpaired group (27%, P < 0.05, binomial test). The Paired group also exhibited a significant increase in subjective preference ratings for the initially non-preferred room relative to the pre-conditioning test [t(18) = −3.7, P < 0.01, see Fig. 1]. In contrast, in the Unpaired Group, room preference ratings did not change [t(14) = −1.5, P > 0.1].
Figure 1.
Room preference scores for Paired group (n = 19, solid bars) and Unpaired group subjects (n = 15, open bars) before and after conditioning sessions with 20-mg d-amphetamine and placebo. The Paired group always received d-amphetamine in the room that they initially did not like, whereas the Unpaired group received d-amphetamine and placebo in both rooms. Bars represent mean ±SEM and asterisks indicate a significant difference between pre- and post-conditioning scores (Student’s paired t-test, P < 0.01)
Room liking and acute subjective responses to d-amphetamine
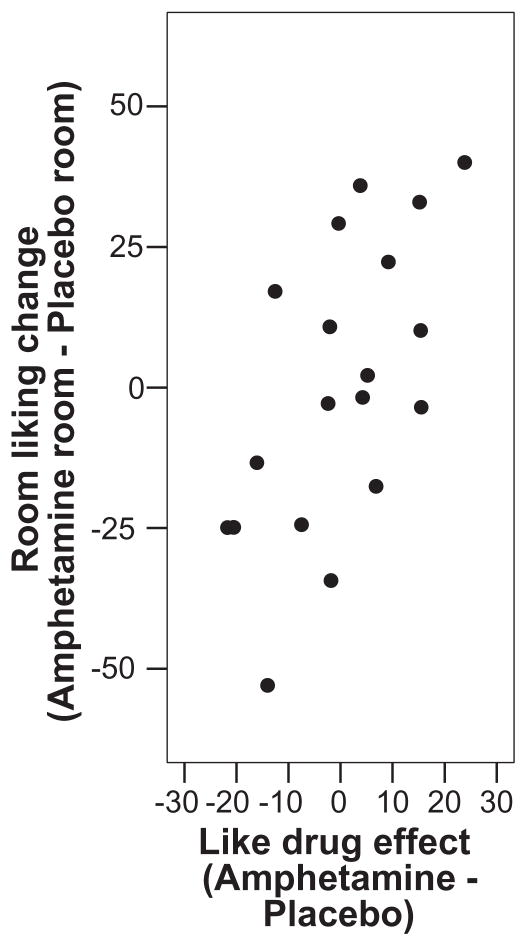
Both positive and negative acute subjective effects of d-amphetamine during conditioning sessions predicted changes in room liking ratings in the Paired group at test. Together, ratings of drug ‘liking’, positive mood (POMS), anxiety (POMS) and dysphoria (ARCI LSD) during conditioning sessions accounted for 52% of the variation in room liking ratings at test in the Paired group [adjusted R2 = 0.39, F(4,14) = 3.9, P < 0.05]. Ratings of drug ‘liking’ significantly independently predicted the change in ratings of room liking [β = 0.6, t(18) = 3.0, P = 0.01; see Fig. 2]. By contrast, the acute subjective effects of d-amphetamine did not predict changes in room liking ratings in the Unpaired group at test [adjusted R2 = −0.05, F(4,9) = 0.8, P > 0.5].
Figure 2.
Partial correlation between changes in room liking ratings (drug room minus placebo room) and peak drug liking ratings (drug minus placebo) in the Paired group (n = 19). Drug liking significantly independently predicted room liking scores
Changes in acute subjective responses to d-amphetamine
Responses to d-amphetamine changed in relation to the context in which the drug was administered. When d-amphetamine was administered in a consistent environment (Paired group), subjects’ ratings of want more drug [DEQ; Drug*Group F(1,31) = 17.8, P < 0.001] and stimulation [ARCI BG; Drug*Group F(1,31) = 5.1, P < 0.05] increased on the second administration, but this did not occur in the Unpaired group (Fig. 3). Conversely, the Unpaired group reported lower ratings of feel high [DEQ; Drug*Group F(1,31) = 8.1, P < 0.01] on the second administration, compared with the Paired group who received the drug in a consistent environment (see Fig. 3).
Figure 3.
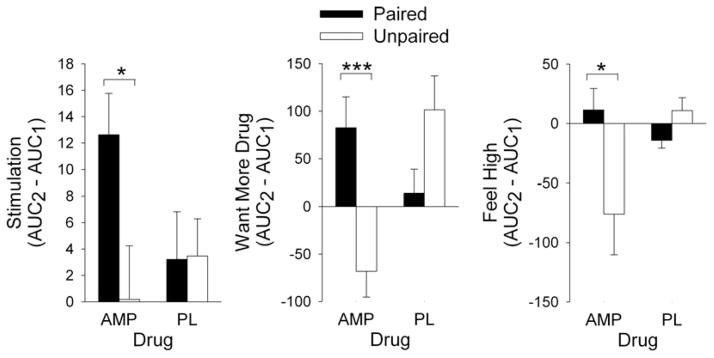
Changes in acute subjective effects of d-amphetamine and placebo from the first administration to the second administration, when the drug was experienced in a consistent environment (Paired group, n = 19, filled bars) or in a different environment (Unpaired group, n = 14, open bars). Ratings of Stimulation (ARCI BG) and Want More were significantly greater on the second administration in the Paired group compared with the Unpaired group. In contrast, ratings of Feel High after amphetamine were significantly lower on the second administration in the Unpaired group compared with the Paired group. Data represent mean ±SEM and asterisks indicate a significant difference between the groups (independent samples t-test, *P < 0.05, ***P < 0.001)
DISCUSSION
This study investigated de novo contextual conditioning with d-amphetamine in humans using methodology based upon the animal CPP paradigm. Using a biased stimulus assignment procedure (Cunningham et al. 2003), we confirmed that healthy men and women exhibit a significant increase in preference for an environment paired consistently with administration of this rewarding drug. Furthermore, in line with our previous findings (Childs & de Wit 2009), acute positive and negative subjective responses to d-amphetamine, in particular ratings of drug liking, significantly predicted changes in room liking after conditioning. Interestingly, we also found that in humans, as in animals (Hinson & Poulos 1981; Post et al. 1981; Vezina & Stewart 1984), the context of drug administration can significantly influence acute drug effects on re-administration. Our findings replicate and extend our previous findings and confirm that methodology based upon the animal place preference procedure can be used to study drug contextual conditioning processes in humans.
One difference between the procedures used here and those employed in our first study (Childs & de Wit 2009) was that in the current study room preference ratings were obtained both before and after conditioning had occurred. In this way, we were able to control for initial room preferences by using a biased procedure for stimulus assignment. In our first study, results from the Unpaired group suggested a mild pre-existing preference for one room. Pre-existing preferences might make it difficult to detect a conditioned increase in liking among the Paired subjects because of ceiling effects (Cunningham et al. 2003). In addition, in the present study room ratings were obtained during a re-exposure test in the presence of the conditioned stimuli, whereas in our first study, the rooms were rated from memory in a separate room. It could be argued that ratings obtained outside the conditioning environment (in our earlier study) did not measure Pavlovian conditioning (Stephens et al. 2010) because conditioned responses are usually considered involuntary reflexes (Everitt & Robbins 2005). We postulate that allowing participants to rate their room liking while they were in the rooms mirrors more closely the animal CPP methodology. Nevertheless, critiques of both of the studies point to the need for improved, objective behavioral measures of preference in humans in order to fully validate the model.
Interestingly, the Paired group, but not the Unpaired group, reported greater subjective stimulation and drug craving after d-amphetamine on the second administration relative to the first, whereas the Unpaired group reported less drug-induced high on the second administration relative to the first. These findings parallel those of animal studies that have reported context-dependent increases or decreases in drug effects (Le, Poulos & Cappell 1979; Tiffany, McCal & Maude-Griffin 1987; Cunningham & Noble 1992; White, Roberts & Best 2002a; Schiltz, Kelley & Landry 2005; Ostlund & Balleine 2008; Blaser, Koid & Poliner 2010). That is, with repeated administration of a drug in a consistent context, sensitization or tolerance may develop to certain physiological or behavioral effects of the drug. In non-humans, repeated administration of d-amphetamine in a consistent environment produces a context-dependent increase in its locomotor activating effects, which is only expressed in the drug-paired environment (Stewart & Vezina 1991; Stewart 1992; Anagnostaras & Robinson 1996; Robinson et al. 1998; Tirelli & Terry 1998; Anagnostaras et al. 2002; Badiani & Robinson 2004). Boileau and colleagues (Boileau et al. 2006) have demonstrated a parallel, context-specific increase in d-amphetamine-induced dopamine release in humans. In our study, we found evidence of context-dependent increases in amphetamine-induced stimulation and craving in the Paired group, and evidence for non-context-specific tolerance to feeling high, in the Unpaired group. To the extent that the psychomotor stimulant effects of stimulant drugs have been considered a homologue for rewarding subjective effects of the drug in humans (Wise & Bozarth 1987), our results in the Paired group are consistent with reports of sensitization to the psychomotor stimulant effects of amphetamine in animals and humans. Context-dependent increases in the stimulant and incentive motivational properties of d-amphetamine may enhance the drug’s rewarding properties and its abuse potential in a drug-paired context with implications for the development of drug abuse and addiction.
This study addressed some of the limitations of our previous study investigating contextual drug associations in humans, but there are still some issues to be addressed in future studies. First, and perhaps most significant to the validation of animal CPP in humans, it will be important to develop an objective behavioral measure of contextual drug conditioning in future studies. The sample size was also relatively small for a human study, and future studies of contextual conditioning should be replicated in larger groups. Another interesting question for future study is whether contextual conditioning can be established in the absence of strong subjective drug effects. For example, sex hormones are known to significantly influence the magnitude of subjective responses to amphetamine in women, thus it would be interesting to compare the development of contextual conditioning in women tested at different phases of the menstrual cycle. Finally, it will be interesting to determine whether drug contextual conditioning is related to or influences the reinforcing efficacy of a drug in that environment.
In conclusion, this study demonstrates the validity of a translational procedure to study drug reward and contextual conditioning in humans. Contextual conditioning is posited to play a central role in the development of drug addiction although very little is known about the phenomenon in humans. Thus, our model provides a method to investigate the robust, long-lasting links formed between drugs and the places they are used. Our findings also support theories of drug addiction which emphasize the importance of enhanced drug responses over time and contextual conditioning in the addiction cycle (Ludwig 1986; Robinson & Berridge 1993; O’Brien et al. 1998; Kelley 2004; Hyman et al. 2006).
Acknowledgments
We would like to thank Charlotte McGinnis, Jaclyn Zembrodt, Erin Prater and Megan Leino for technical assistance and Dr Royce Lee for medical support. These experiments complied with current US laws, and this research was supported by NIDA (DA02812).
Footnotes
Authors Contribution
EC and HDW were responsible for the study concept and design. EC contributed to the acquisition of data, analysis and interpretation of findings. EC drafted the manuscript and HDW provided critical revision of the manuscript. All authors critically reviewed content and approved the final version for publication.
References
- Anagnostaras SG, Robinson TE. Sensitization to the psychomotor stimulant effects of amphetamine: modulation by associative learning. Behav Neurosci. 1996;110:1397–1414. doi: 10.1037//0735-7044.110.6.1397. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Schallert T, Robinson TE. Memory processes governing amphetamine-induced psychomotor sensitization. Neuropsychopharmacol. 2002;26:703–715. doi: 10.1016/S0893-133X(01)00402-X. [DOI] [PubMed] [Google Scholar]
- APA. American Psychiatric Association Diagnostic and Statistical Manual of Psychiatry. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Badiani A, Robinson TE. Drug-induced neurobehavioral plasticity: the role of environmental context. Behav Pharmacol. 2004;15:327–339. doi: 10.1097/00008877-200409000-00004. [DOI] [PubMed] [Google Scholar]
- Blaser RE, Koid A, Poliner RM. Context-dependent sensitization to ethanol in zebrafish (Danio rerio) Pharmacol Biochem Behav. 2010;95:278–284. doi: 10.1016/j.pbb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Boileau I, Dagher A, Leyton M, Gunn RN, Baker GB, Diksic M, Benkelfat C. Modeling sensitization to stimulants in humans: an [11C]raclopride/positron emission tomography study in healthy men. Arch Gen Psychiatry. 2006;63:1386–1395. doi: 10.1001/archpsyc.63.12.1386. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Amphetamine-induced place preference in humans. Biol Psychiatry. 2009;65:900–904. doi: 10.1016/j.biopsych.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Ferree NK, Howard NMA. Apparatus bias and place conditioning with ethanol in mice. Psychopharmacology (Berl) 2003;170:409–422. doi: 10.1007/s00213-003-1559-y. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Conditioned activation induced by ethanol: role in sensitization and conditioned place preference. Pharmacol Biochem Behav. 1992;43:307–313. doi: 10.1016/0091-3057(92)90673-4. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Haertzen CA. Development of scales based on patterns of drug effects, using the addiction Research Center Inventory (ARCI) Psychol Rep. 1966;18:163–194. doi: 10.2466/pr0.1966.18.1.163. [DOI] [PubMed] [Google Scholar]
- Hinson RE, Poulos CX. Sensitization to the behavioral effects of cocaine: modification by Pavlovian conditioning. Pharmacol Biochem Behav. 1981;15:559–562. doi: 10.1016/0091-3057(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Janak PH, Chaudhri N. The potent effect of environmental context on relapse to alcohol-seeking after extinction. Open Addict J. 2010;3:76–87. doi: 10.2174/1874941001003010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: diazepam. Psychopharmacology (Berl) 1980;71:269–273. doi: 10.1007/BF00433061. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Memory and addiction: shared neural circuitry and molecular mechanisms. Neuron. 2004;44:161–179. doi: 10.1016/j.neuron.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Cappell H. Conditioned tolerance to the hypothermic effect of ethyl alcohol. Science. 1979;206:1109–1110. doi: 10.1126/science.493999. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kwon H, Choi J, Yang BH. Cue-exposure therapy to decrease alcohol craving in virtual environment. Cyberpsychol Behav. 2007;10:617–623. doi: 10.1089/cpb.2007.9978. [DOI] [PubMed] [Google Scholar]
- Ludwig AM. Pavlov’s ‘bells’ and alcohol craving. Addict Behav. 1986;11:87–91. doi: 10.1016/0306-4603(86)90032-8. [DOI] [PubMed] [Google Scholar]
- McNair D, Droppleman MLL, editors. Profile of Mood States. San Diego, CA: Educational and Industrial Testing Service; 1971. [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Balleine BW. On habits and addiction: an associative analysis of compulsive drug seeking. Drug Discov Today Dis Models. 2008;5:235–245. doi: 10.1016/j.ddmod.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post RM, Lockfeld A, Squillace KM, Contel NR. Drug-environment interaction: context dependency of cocaine-induced behavioral sensitization. Life Sci. 1981;28:755–760. doi: 10.1016/0024-3205(81)90157-0. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Browman KE, Crombag HS, Badiani A. Modulation of the induction or expression of psychostimulant sensitization by the circumstances surrounding drug administration. Neurosci Biobehav Rev. 1998;22:347–354. doi: 10.1016/s0149-7634(97)00020-1. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Kelley AE, Landry CF. Contextual cues associated with nicotine administration increase arc mRNA expression in corticolimbic areas of the rat brain. Eur J Neurosci. 2005;21:1703–1711. doi: 10.1111/j.1460-9568.2005.04001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC. Reward sensitivity: issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- Stewart J. Neurobiology of conditioning to drugs of abuse. Ann N Y Acad Sci. 1992;654:335–346. doi: 10.1111/j.1749-6632.1992.tb25979.x. [DOI] [PubMed] [Google Scholar]
- Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- Tiffany ST, McCal KJ, Maude-Griffin PM. The contribution of classical conditioning to tolerance to the antinociceptive effects of ethanol. Psychopharmacology (Berl) 1987;92:524–528. doi: 10.1007/BF00176489. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Terry P. Amphetamine-induced conditioned activity and sensitization: the role of habituation to the test context and the involvement of Pavlovian processes. Behav Pharmacol. 1998;9:409–419. doi: 10.1097/00008877-199809000-00004. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol. 2007;12:227–462. doi: 10.1111/j.1369-1600.2007.00070.x. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. Conditioning and place-specific sensitization of increases in activity induced by morphine in the VTA. Pharmacol Biochem Behav. 1984;20:925–934. doi: 10.1016/0091-3057(84)90018-2. [DOI] [PubMed] [Google Scholar]
- White AM, Roberts DC, Best PJ. Context-specific tolerance to the ataxic effects of alcohol. Pharmacol Biochem Behav. 2002a;72:107–110. doi: 10.1016/s0091-3057(01)00731-6. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of d-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002b;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]