Abstract
It is generally accepted that dioecious plants occur more frequently in dry and nutrient-poor habitats, suggesting that abiotic stress factors could contribute to evolution of dioecy from hermaphrodite. Therefore, experimental investigations on the responses of subdioecious species, a special sexual system comprising male, female, and hermaphrodite plants, to abiotic stress factors could quantify the contribution of selective pressure on the evolution of dioecy. In this study, we evaluated the physiological responses of different sex morphs of Oxyria sinensis Hemsley, a perennial herb native to the East Himalayas, to drought stress. Male, female, and hermaphrodite plants of O. sinensis were subjected to low, moderate, and high drought stress conditions in a glasshouse. Generally, with increasing water stress, the values of most measured variables slightly decreased, whereas water-use efficiency slightly increased. Furthermore, there were no significant differences in most of the measured parameters among the sex morphs under each drought stress treatment, indicating that O. sinensis might be well-adapted to drought stress conditions as its typical habitat is the dry and hot habitats of xerothermic river valleys. However, nitrogen-use efficiency was significantly higher in male and female plants than in hermaphrodite plants under high drought stress conditions, suggesting that that nitrogen-use efficiency under conditions of drought stress might have contributed to the evolution of dioecy from the hermaphrodite to some degree.
Keywords: Dioecy, drought stress, Hengduan Mountains, physiological response, xerothermic river valley
Introduction
Plants are exposed to biotic and abiotic stresses with varying degrees of severity throughout their lifetime. Water deficit is one of the most important abiotic stresses, and it can have severe negative effects on plant growth, reproduction, and production (Bartels and Sunkar 2005). When plants are exposed to drought, there are several general reactions, such as stomatal closure, decreased gas exchange rates, and accumulation of osmolytes (Chaves et al. 2009). Unisexual plants often show sex-specific characters, which may result from different resource constraints on sexual functions and the ecological differentiation of male and female plants. It is generally accepted that female plants often invest more into reproduction than do male plants (e.g., Cipollini and Whigham 1994; Bochenek and Eriksen 2010; but see Zhao and Yang 2008). Regarding the different responses of dioecious plants to drought, females are considered to be more sensitive than males to drought stress (Li et al. 2004; Xu et al. 2008; Chen et al. 2010), which might result from the higher proportional investment into reproduction in females than in males (Garcia and Antor 1995; Bochenek and Eriksen 2010). A strong association was found between harsh abiotic environments and the occurrence of sexual dimorphism (Ashman 2006), suggesting that abiotic environmental stresses might have contributed to the evolution of dioecy. However, this hypothesis has rarely been tested experimentally.
Generally, for the gynodioecious species in dry or nutrient-poor habitats, hermaphrodites often produced fewer seeds than female plants (Ashman 2006), indicating that hermaphrodites in low-resources populations may be genetically more male than those in high-resources sites because female frequency would increase with resource decline and the high frequencies of females would select for the increased maleness of hermaphrodites (Charlesworth 1989). Therefore, it would be reasonable to speculate that dry habitats might contribute to the evolution of dioecy, but it is still unclear whether there is a differentiation of drought tolerance between hermaphrodite and dioecious plants because almost no research has been performed to quantify the ability of drought tolerance of hermaphroditic and dioecious plants. Subdioecy, in which there are male, female, and hermaphrodite plants of a species, is generally considered to be an intermediate phase between cosexuality and dioecy (Barrett 1992), although there is little experimental evidence supporting this idea. Therefore, by employing the subdioecious species, examinations on the different responses to drought stress of hermaphroditic and dioecious plants would specifically help us to understand the role of abiotic factors on the evolution of dioecy.
Oxyria, a small genus in the Polygonaceae, is composed of two species, Oxyria digyna (L.) Hill and Oxyria sinensis Hemsley (Sun et al. 2012), reported as hermaphroditic and dioecious species, respectively (Li et al. 2003). Nevertheless, O. sinensis, has both hermaphroditic and dioecious plants that coexist in some populations, as determined during our field investigations, but most of the populations we evaluated consisted of male and female plants only. Flowers of O. digyna are hermaphroditic, and thus, the dioecious plants of O. sinensis should be a derived phase (Barrett 1992). Collectively, considering the wide distribution and the derived the stage of dioecious plants of O. sinensis and their typical habitats in the xerothermic valleys of the Hengduan Mountains, we hypothesized that hermaphroditic plants of O. sinensis might be less tolerant than dioecious plants to drought stress. If this is the case, the differences in drought tolerance may contribute to the evolution of dioecy in O. sinensis to some degree (e.g., Hart 1985; Weller and Sakai 2005). To test our hypothesis, we subjected hermaphroditic and dioecious plants of O. sinensis to different soil water content treatments and analyzed the drought tolerance of the sex morphs. Specifically, our aims were as follows: (1) to determine whether there are differences in drought tolerance between hermaphroditic and dioecious plants; and (2) to determine whether there are differences in drought tolerance between male and female plants.
Material and Methods
Plant materials and experimental design
Oxyria sinensis is a perennial herb native to the East Himalayas (Fig. 1). It can reproduce sexually via seeds and asexually via rhizomes (Zhao and Yang 2008). This plant is characterized by tiny flowers, densely branched panicles, and winged achenes that might favor wind dispersal. In the Hengduan Mountains, O. sinensis is abundantly distributed along the roadsides and in abandoned farmlands along the riversides at altitudes below 3800 m, in xerothermic valleys. Most populations of O. sinensis consist of male and female plants only, but in some populations, especially those along the Jinshajiang River (the upper Yangtze River), there are some hermaphroditic plants. Furthermore, the hermaphroditic and dioecious plants were mixed and occupied the same habitats in these populations.
Figure 1.

Plants and the habitat of Oxyria sinensis.
The seeds of O. sinensis are easily collected in the field, but their germination rate was very low in our preliminary experiments. Therefore, we used rhizomes to cultivate experimental plants. We collected rhizomes 10 cm in length from field populations containing male, female, and hermaphroditic plants in April, 2013. The rhizomes were then brought to the experimental site at Yunnan Normal University, Kunming, Yunnan Province. The rhizomes were grown into plants in a canopied and naturally lit glasshouse. The sides of the glasshouse were always opened for aeration throughout the experiment, so that the temperature inside the glasshouse was closely linked to the ambient outside temperature. In the glasshouse, the rhizomes were planted in 90 pots containing a homogeneous mixture of equal volumes of peat and perlite. Ten “empty” pots were filled with the same amount of the soil mixture, but without seedlings, to measure evaporation rates. Each pot was 24 cm high with upper and lower diameters of 21 cm and 17 cm, respectively. All pots were filled with the same weight of a homogeneous soil mixture (1.32 kg), and the soil water content of each pot was completed to maximum field capacity (FC) by adding 2.95 kg water. All pots were periodically watered to maximum FC for 2 months after repotting to allow the seedlings to become established.
The experimental period commenced on July 8, 2013, and continued until August 23, 2013. Thirty plants of each sex morph were subjected to each of the low, moderate, and high drought stress treatments, each group comprising 10 plants. In each treatment, five plants were used for photosynthesis measurements and the other five plants were used for carbon isotope composition and leaf elemental analyses. The different levels of drought stress (low, medium, and high) were imposed by watering to 80%, 50%, and 20% of maximum FC, respectively. Soil water content was maintained at these levels by weighing the pots every 2 days, recording the amount of water lost, and then immediately rewatering to reach the desired water content.
Measurements of leaf photosynthesis, transpiration, and chlorophyll fluorescence
Maximum photosynthetic rate (Amax), stomatal conductance (g), and transpiration (E) were measured using a LI-COR 6400XT infrared gas analyzer (IRGA; LI-COR Biosciences, Lincoln, NE). Measurements were taken between 11:00 and 14:00 h on a sunny day (August 24, 2013) from five plants in each treatment for each sex morph. Light levels were maintained at 1200 μmol·m−2·s−1, which was higher than the light-saturation point of all three sex morphs (light-saturation points were derived from light response curves determined before the experiment). Light was provided by an LI-6400-02B LED lamp (LI-COR Biosciences). The external CO2 concentration was maintained at 400 μmol·mol−1 using portable tanks containing a CO2/air mixture. The output of the tanks was controlled by a LI-6400-01 CO2 injector (LI-COR Biosciences). Temperature and relative humidity were maintained as 24–26°C and 23–29%, respectively. Instantaneous water-use efficiency (WUEi) was calculated and defined as Amax/E.
Chlorophyll fluorescence parameters were measured between (0500 and 0600 h) on leaves that had been dark adapted for 30 min. These measurements were taken on the same day as the leaf gas exchange measurements. Maximum quantum yield of photosystem II (PSII) (Fv/Fm = (Fm − Fo) / Fm) was measured using a LI-6400-40 leaf chamber fluorometer (LI-COR Biosciences). Five plants in each treatment per sex morph were analyzed.
Carbon isotope composition and leaf elemental analysis
Carbon isotope composition (δ13C) has been shown to serve as a proxy for integrated water-use efficiency (Jones 1993; Zhang and Marshall 1994; Osorio et al. 1998). Thus, to evaluate the differences in the integrated water-use efficiency among the three sex morphs, we measured the carbon isotope composition of the leaves from wild plants and the treated plants under different drought stress treatments. In the laboratory, leaves with a dry weight of approximately 0.2 g were collected and then finely ground with a Tissuelyzer (Retsch, Haan, Germany). Each sample was divided into two subsets. One subset was used to analyze nitrogen and carbon contents of the needles with a CHN analyzer (Vario EL; Elementar, Hanau, Germany). The nitrogen-use efficiency (NUE), defined as the amount of organic matter produced per unit of nitrogen taken up (Vitousek 1982), was calculated as the reciprocal of the nitrogen content. The second subset was used to analyze the carbon isotope composition of leaves (δ13C). The samples were combusted in an elemental analyzer EA1108 (Carlo Erba, Milan, Italy) and analyzed using a Finnigan Delta Plus isotope mass spectrometer (Thermo Finnigan MAT GmbH, Bremen, Germany). Carbon isotope composition was calculated relative to the Pee Dee Belemnite (PDB) standard as the ratio (‰): δ13C = (Rsample/Rstandard − 1) × 1000, where Rsample and Rstandard are the ratios of 13C:12C in the sample and the standard, respectively. All measurements were performed at the Key Laboratory of Plateau Lake Ecology and Global Change, Yunnan Normal University, China.
Statistical analyses
Data for all measured variables were analyzed by the general linear model (Proc GLM) to test the effects of different species, different drought treatments, and the interactions between them. Significant differences among sex morphs in a particular treatment, or among treatments for a particular sex morph, were compared using one-way analysis of variance (ANOVA). The homogeneity of variances was tested before performing ANOVA. All statistical analyses were carried out using the SPSS statistical software package.
Results
Leaf photosynthesis and chlorophyll fluorescence
All the measured variables were significantly affected by the watering treatment, but not by sex, or by the treatment × sex interaction (Table 1). Generally, there were no significant differences among the sex morphs in each drought stress treatment for most of the measured variables, including Amax, g, E, WUEi, and Fv/Fm (Tables 2), except for Amax of different sex morphs subjected to 20% FC (Table 2).
Table 1.
Measured parameters for male, female, and hermaphrodite plants of Oxyria sinensis, with degrees of freedom in the brackets.
| Parameter | Abbrev. | Watering treatment | Sex | Treatment × Sex interaction |
|---|---|---|---|---|
| Maximal rate of photosynthesis | Amax | 5.91** (2) | 0.82 (2) | 0.95 (4) |
| Stomatal conductance | g | 8.08** (2) | 1.60 (2) | 0.45 (4) |
| Transpiration | E | 6.08** (2) | 2.17 (2) | 0.45 (4) |
| Instantaneous water-use efficiency | WUEi | 4.19** (2) | 1.18 (2) | 0.04 (4) |
| Maximum quantum yield of PS II | Fv/Fm | 4.63* (2) | 0.54 (2) | 2.09 (4) |
| Carbon content | C% | 1.70 (2) | 3.16 (2) | 0.77 (4) |
| Nitrogen-use efficiency | NUE | 1.84 (2) | 4.07* (2) | 1.20 (4) |
| Carbon isotope composition | δ13C | 18.08** (2) | 0.24 (2) | 1.73 (4) |
Significance is shown for watering treatment, sex morph, and their interactions.
P < 0.05;
P < 0.01.
Table 2.
Comparison of measured parameters among three sex morphs of Oxyria sinensis under three different drought treatments (soil water at 80% of maximal field capacity (FC), 50% FC, and 20% FC).
| Drought treatments (% of maximum FC) |
|||
|---|---|---|---|
| Low stress | Moderate stress | High stress | |
| Variable and species | 80% FC | 50% FC | 20% FC |
| Maximal rate of photosynthesis (Amax) (μmol m−2 s−1) | |||
| Male | 15.41 ± 0.94 A,X | 15.02 ± 1.56 A,X | 11.16 ± 0.83 A,Y |
| Female | 13.51 ± 0.99 A,XY | 14.31 ± 0.45 A,X | 11.79 ± 0.88 AB,Y |
| Hermaphrodite | 14.32 ± 0.85 A,X | 14.74 ± 1.38 A,X | 13.58 ± 0.26 B,X |
| Stomatal conductance (g) (mmol m−2 s−1) | |||
| Male | 0.53 ± 0.11 A,X | 0.61 ± 0.12 A,X | 0.25 ± 0.04 A,Y |
| Female | 0.36 ± 0.09 A,X | 0.46 ± 0.02 A,X | 0.28 ± 0.05 A,X |
| Hermaphrodite | 0.50 ± 0.12 A,XY | 0.62 ± 0.09 A,X | 0.34 ± 0.04 A,Y |
| Transpiration (E) (mmol m−2 s−1) | |||
| Male | 3.72 ± 0.47 A,XY | 4.40 ± 0.37 A,X | 2.80 ± 0.35 A,Y |
| Female | 3.11 ± 0.52 A,X | 3.96 ± 0.11 A,X | 2.99 ± 0.44 A,X |
| Hermaphrodite | 3.76 ± 0.54 A,X | 4.59 ± 0.38 A,X | 3.78 ± 0.31 A,X |
| Instantaneous water-use efficiency (WUEi) (Amax/E) | |||
| Male | 4.36 ± 0.49 A,X | 3.42 ± 0.28 A,X | 4.21 ± 0.50 A,X |
| Female | 4.62 ± 0.44 A,X | 3.61 ± 0.11 A,X | 4.26 ± 0.56 A,X |
| Hermaphrodite | 4.08 ± 0.49 A,X | 3.21 ± 0.11 A,X | 3.71 ± 0.36 A,X |
| Maximum quantum yield of PS II (Fv/Fm) | |||
| Male | 0.82 ± 0.007 A,X | 0.83 ± 0.002 A,X | 0.83 ± 0.002 A,X |
| Female | 0.82 ± 0.004 A,X | 0.83 ± 0.002 A,X | 0.83 ± 0.004 A,X |
| Hermaphrodite | 0.83 ± 0.005 A,X | 0.83 ± 0.003 A,X | 0.83 ± 0.005 A,X |
Values are mean ± SE.
Different letters after values indicate significant differences (P < 0.05) among different drought treatments (X, Y, Z) and different sex morphs (A, B).
The value of Amax decreased significantly under high drought stress in male and female plants, but not in hermaphrodite plants. The values of g and E decreased significantly under high drought stress only in male plants (Table 2). There were no significant differences in WUEi or Fv/Fm among the drought stress treatments for each sex morph (Table 2).
Nitrogen-use efficiency and carbon content
Overall, the NUE was significantly different between sex morphs, but not affected significantly by the drought treatment or the drought treatment × sex interaction (Table 1). Under 20% FC, the NUE was significantly lower in hermaphrodite plants than in male and female plants, but there were no significant differences among the three sex morphs under 50% FC or 80% FC (Fig. 2A). In addition, the NUE of male plants was higher under 20% FC, but there was no significant difference in the NUE of female and hermaphrodite plants among the three drought treatments (Fig. 2A). Carbon concentration (C%) was not significantly affected by drought treatment, sex morph, or the drought treatment × sex morph interaction (Table 1). Thus, there was no significant difference in C% among sex morphs in each drought treatment and among drought treatments for each sex morph (Fig. 2B).
Figure 2.

(A) Nitrogen-use efficiency (NUE) and (B) carbon content (C%) in leaves of male (black bars), female (gray bars), and hermaphrodite (open bars) plants of Oxyria sinensis under different drought conditions (soil water at 80% of maximal field capacity (FC), 50% FC, 20% FC). Data are means + SE. Different letters indicate significant differences (P < 0.05) among different drought treatments (X, Y) and different sex morphs (A, B).
Carbon isotope composition (δ13C)
For each sex morph, the amount of δ13C was significantly higher under 20% FC and 80% FC than under 50% FC. There was no significant difference in δ13C among sex morphs across all drought treatments (Fig. 3). Collectively, δ13C was affected significantly by drought treatment, but not by sex morph and or the drought treatment × sex morph interaction.
Figure 3.
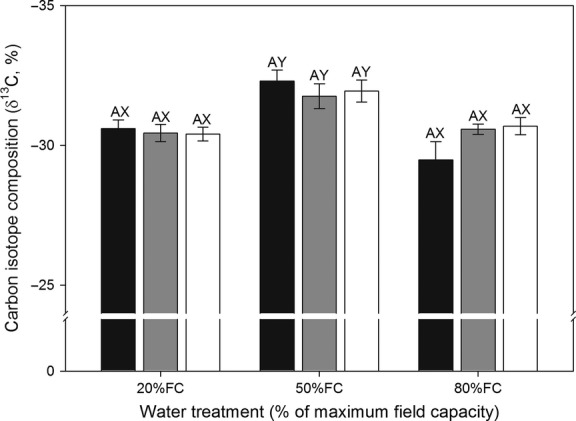
Carbon isotope compositions (δ13C) of leaves of male (black bars), female (gray bars), and hermaphrodite (open bars) plants of Oxyria sinensis under different drought conditions (soil water at 80% of maximal field capacity (FC), 50% FC, 20% FC). Data are means + SE. There were no significant differences among different drought treatments and different sex morphs.
Discussion
Ecophysiological responses of Oxyria sinensis to drought stress
Water availability is one of the most important factors constraining plant growth and distribution (Kramer and Boyer 1995). Thus, plants growing in water-limited regions have developed various mechanisms to tolerate drought. Such mechanisms include adjustments of physiological and morphological characteristics, such as changes in structure, water-use efficiency, and stomatal conductance (Kozlowski and Pallardy 2002). We observed similar patterns in O. sinensis. In the present study, high drought stress (20% FC) resulted in decreases in Amax, g, and E in each of the sex morphs, although the differences were not significant across all variables. Under drought stress, g is believed to be more sensitive than other parameters (Schulze et al. 1998); this is consistent with the results for each sex morph of O. sinensis obtained in the present study. Under moderate drought stress conditions, drought-stressed male, female, and hermaphrodite plants showed 59, 39, and 45% lower values of g, respectively, but only 26, 18, and 8% lower values of Amax, respectively, compared with their respective values under low drought stress conditions (Table 2). Changes in stomatal conductance may contribute to similar decreases in transpiration rates under increasing drought stress. The male, female, and hermaphrodite plants showed 26, 24, and 18% reductions in transpiration rates, respectively, under high drought stress relative to moderate drought stress. Greater decreases in g than in Amax under drought stress have also been observed in other plant species (e.g., Ismail and Hall 1992; Zhang and Marshall 1994; Ma et al. 2010).
We found no decreases in WUEi, δ13C, or Fv/Fm under increasing drought stress, although g showed a decreasing trend. One possible explanation for these results might be the physiological attributes of O. sinensis, which usually inhabits hot, dry xerothermic valleys. Therefore, O. sinensis may be well-adapted to drought conditions, and thus, the level of drought stress in our experiments was not strong enough to damage PS II in this species. Therefore, the decreasing trends in Amax and E might be caused by stomatal limitation (Flexas et al. 2014), even under conditions of high drought stress.
For plant species with separate sex morphs, the various morphs might be affected differently under water stress, because male and female plants differ in mass, nutrient composition, and water content (Harris and Pannell 2008). However, few studies have focused on evaluating sex-related differences in stress tolerance in plants. The limited studies that have been conducted have suggested that reduced performance of the female under stressful environments is because of its greater investment in reproduction (Li et al. 2007; Xu et al. 2008; Rozas et al. 2009; Zhang et al. 2010), although this is not a generalized pattern (Onate and Munne-Bosch 2009). For O. sinensis, no significant differences were found in Amax, g, and E between male and female plants, probably because of the habitat of this species, as mentioned above.
Implications for the evolution of dioecy
As speculated by Darwin (1887), “a very dry station apparently favours the presence of the female form”. Thus, drought stress could be one of the drivers for the evolution from the hermaphrodite to dioecy, although the exact reason remains unclear (Ashman 2006). Considering the hermaphroditic O. digyna, the only congener of O. sinensis, male and female plants could be a derived stage. Therefore, as we have predicted, if drought stress has contributed to the evolution from the hermaphrodite to dioecy in O. sinensis, then hermaphrodite plants should be more sensitive than male and female plants to drought stress. However, our results suggested that only NUE under drought conditions was affected significantly by the sex morphs (Table 1). The NUE reflects the ecological fitness of plants, because nitrogen is necessary for their growth (Aerts and Chapin 2000). In our study on O. sinensis, under increasing water stress, the NUE increased in male and female plants but decreased in hermaphrodite plants. Also, the NUE of male and female plants was significantly higher than that of hermaphrodite plants under high drought stress conditions (Fig. 2A). Considering the dry and hot habitat of O. sinensis, the high NUE of male and female plants under high drought stress might have contributed to the evolution of dioecy to a certain degree. However, this species has a strong capacity for clonal growth, resulting in a high density of ramets within one ramet, and there is also potential for wind pollination (Zhao and Yang 2008). These attributes can lead to intense inbreeding. The resulting inbreeding depression in hermaphrodite plants growing in hot, dry habitats may have played a major role in driving the evolution of dioecy (Ashman 2006), although our results indicated that high NUE contributed to the evolution of dioecy to some degree.
Acknowledgments
We are grateful to Prof. Ming Gong, Jin-Hong Cai, and Guang-Jie Chen for their great helps in the experiments. This work was financially supported by the National Natural Science Foundation of China (31160084) and the Ph.D. Programs Foundation of Ministry of Education of China (20115303120001).
Conflict of Interest
The authors declared no conflicts of interest.
References
- Aerts R. The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns. In: Fitter AH, Raffaelli DG, Chapin FS, editors. Advances in ecological research. Vol. 30. San Diego: Elsevier Academic Press Inc; 2000. pp. 1–67. [Google Scholar]
- Ashman TL. The evolution of separate sexes: a focus on the ecological context. In: Harder LD, Barrett SCH, editors. The ecology and evolution of flowers. Oxford: Oxford Univ. Press; 2006. pp. 204–222. [Google Scholar]
- Barrett SCH. Gender variation and the evolution of dioecy in Wurmbea dioica (liliaceae) J. Evol. Biol. 1992;5:423–444. [Google Scholar]
- Bartels D, Sunkar R. Drought and salt tolerance in plants. Crit. Rev. Plant Sci. 2005;24:23–58. [Google Scholar]
- Bochenek GM, Eriksen B. Annual growth of male and female individuals of the common ash (Fraxinus excelsior L.) Plant Ecol. Divers. 2010;3:47–57. [Google Scholar]
- Charlesworth D. Allocation to male and female function in hermaphrodites, in sexually polymorphic populations. J. Theor. Biol. 1989;139:327–342. [Google Scholar]
- Chaves MM, Flexas J, Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LH, Zhang S, Zhao HX, Korpelainen H, Li CY. Sex-related adaptive responses to interaction of drought and salinity in Populus yunnanensis. Plant, Cell Environ. 2010;33:1767–1778. doi: 10.1111/j.1365-3040.2010.02182.x. [DOI] [PubMed] [Google Scholar]
- Cipollini ML, Whigham DF. Sexual dimorphism and cost of reproduction in the dioecious shrub Lindera benzoin (Lauraceae) Am. J. Bot. 1994;81:65–75. [Google Scholar]
- Darwin CR. The different forms of flowers on plants of the same species. London: John Murray; 1887. [Google Scholar]
- Flexas J, Diaz-Espejo A, Gago J, Gallé A, Galmés J, Gulías J, et al. Photosynthetic limitations in mediterranean plants: a review. Environ. Exp. Bot. 2014;103:12–23. [Google Scholar]
- Garcia MB, Antor RJ. Age and size structure in populations of a long-lived dioecious geophyte: Borderea pyrenaica (Dioscoreaceae) Int. J. Plant Sci. 1995;156:236–243. [Google Scholar]
- Harris MS, Pannell JR. Roots, shoots and reproduction: sexual dimorphism in size and costs of reproductive allocation in an annual herb. Proc. R. Soc. B-Biol. Sci. 2008;275:2595–2602. doi: 10.1098/rspb.2008.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart JA. Evolution of dioecism in Lepechinia willd sect parviflorae (Lamiaceae) Syst. Bot. 1985;10:147–154. [Google Scholar]
- Ismail AM, Hall AE. Correlation between water-use efficiency and carbon isotope discrimination in diverse cowpea genotypes and isogenic lines. Crop Sci. 1992;32:7–12. [Google Scholar]
- Jones HG. Drought tolerance and water-use efficiency. In: Smith JAC, Griffiths H, editors. Water deficits: plant responses from cell to community. Oxford: BIOS Scientific Publishers; 1993. pp. 93–203. [Google Scholar]
- Kozlowski TT, Pallardy SG. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 2002;68:270–334. [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soils. San Diego: Academic Press; 1995. [Google Scholar]
- Li A, Bao B, Grabovskaya-borodina AE, Hong S, Mcneill J, Mosyakin SL, et al. Polygonaceae. In: Wu ZY, Raven PH, editors. Flora of China. Beijing and St Louis: Science Press and Missouri Botanical Garden; 2003. pp. 277–350. [Google Scholar]
- Li CY, Ren J, Luo JX, Lu RS. Sex-specific physiological and growth responses to water stress in Hippophae rhamnoides L populations. Acta Physiol. Plant. 2004;26:123–129. [Google Scholar]
- Li CY, Xu G, Zang RG, Korpelainen H, Berninger F. Sex-related differences in leaf morphological and physiological responses in Hippophae rhamnoides along an altitudinal gradient. Tree Physiol. 2007;27:399–406. doi: 10.1093/treephys/27.3.399. [DOI] [PubMed] [Google Scholar]
- Ma F, Zhao CM, Milne R, Ji MF, Chen LT, Liu JQ. Enhanced drought-tolerance in the homoploid hybrid species Pinus densata: implication for its habitat divergence from two progenitors. New Phytol. 2010;185:204–216. doi: 10.1111/j.1469-8137.2009.03037.x. [DOI] [PubMed] [Google Scholar]
- Onate M, Munne-Bosch S. Influence of plant maturity, shoot reproduction and sex on vegetative growth in the dioecious plant Urtica dioica. Ann. Bot. 2009;104:945–956. doi: 10.1093/aob/mcp176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio J, Osorio ML, Chaves MM, Pereira JS. Water deficits are more important in delaying growth than in changing patterns of carbon allocation in eucalyptus globulus. Tree Physiol. 1998;18:363–373. doi: 10.1093/treephys/18.6.363. [DOI] [PubMed] [Google Scholar]
- Rozas V, DeSoto L, Olano JM. Sex-specific, age-dependent sensitivity of tree-ring growth to climate in the dioecious tree Juniperus thurifera. New Phytol. 2009;182:687–697. doi: 10.1111/j.1469-8137.2009.02770.x. [DOI] [PubMed] [Google Scholar]
- Schulze ED, Williams RJ, Farquhar GD, Schulze W, Langridge J, Miller JM, et al. Carbon and nitrogen isotope discrimination and nitrogen nutrition of trees along a rainfall gradient in northern australia. Aust. J. Plant Physiol. 1998;25:413–425. [Google Scholar]
- Sun YS, Wang AL, Wan DS, Wang Q, Liu JQ. Rapid radiation of Rheum (Polygonaceae) and parallel evolution of morphological traits. Mol. Phylogenet. Evol. 2012;63:150–158. doi: 10.1016/j.ympev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Vitousek P. Nutrient cycling and nutrient use efficiency. Am. Nat. 1982;119:553–572. [Google Scholar]
- Weller SG, Sakai AK. Selfing and resource allocation in Schiedea salicaria (Caryophyllaceae), a gynodioecious species. J. Evol. Biol. 2005;18:301–308. doi: 10.1111/j.1420-9101.2004.00835.x. [DOI] [PubMed] [Google Scholar]
- Xu X, Peng GQ, Wu CC, Korpelainen H, Li CY. Drought inhibits photosynthetic capacity more in females than in males of Populus cathayana. Tree Physiol. 2008;28:1751–1759. doi: 10.1093/treephys/28.11.1751. [DOI] [PubMed] [Google Scholar]
- Zhang J, Marshall JD. Population differences in water-use efficiency of well-watered and water-stressed western larch seedlings. Can. J. For. Res. 1994;24:92–99. [Google Scholar]
- Zhang CY, Zhao XH, Gao LS, von Gadow K. Gender-related distributions of Fraxinus mandshurica in secondary and old-growth forests. Acta Oecol.-Int. J. Ecol. 2010;36:55–62. [Google Scholar]
- Zhao F, Yang YP. Reproductive allocation in a dioecious perennial Oxyria sinensis (Polygonaceae) along altitudinal gradients. J. Syst. Evol. 2008;46:830–835. [Google Scholar]


