Abstract
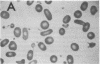
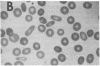
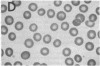
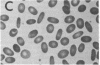
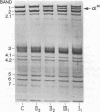
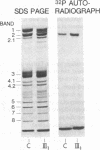
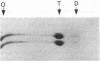
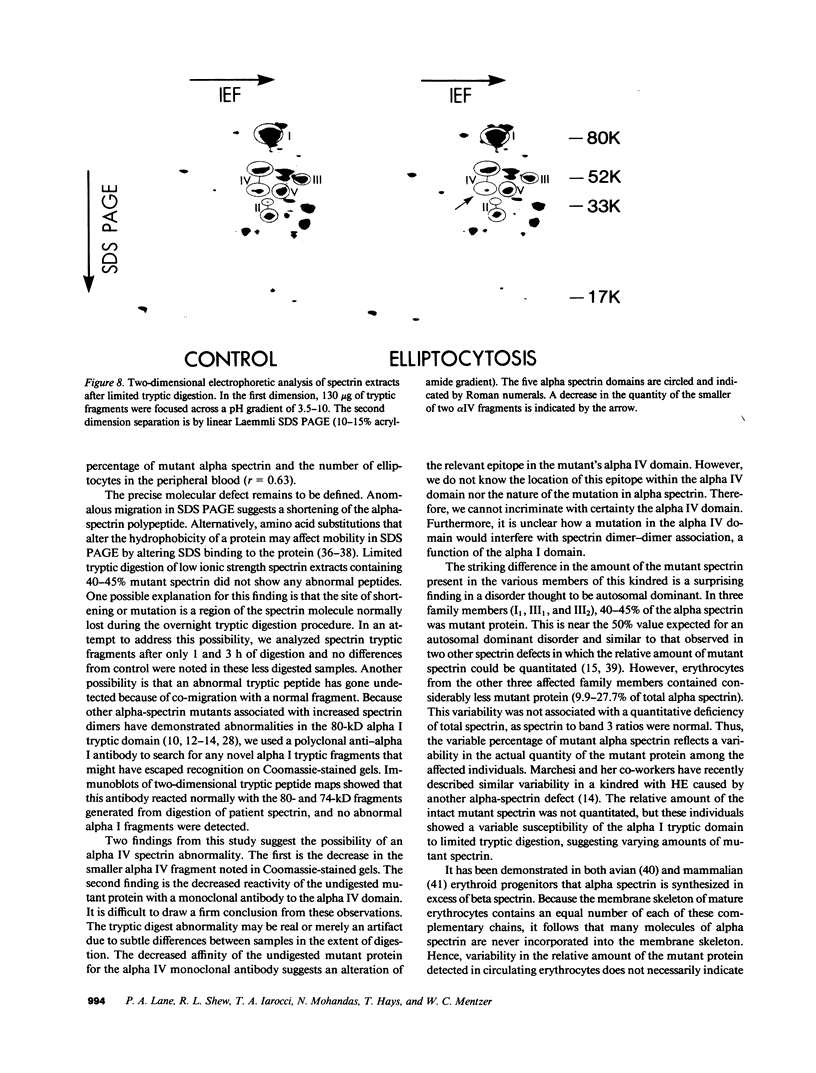
We report here a unique variant of alpha spectrin in a kindred with hereditary elliptocytosis. This novel red blood cell-membrane protein migrated to a position between the normal alpha- and beta-spectrin subunits in SDS polyacrylamide gel electrophoresis. It was identified as an alpha spectrin by its binding to anti-alpha spectrin antibodies, by the absence of a phosphorylation site, and by the normal 1:1 stoichiometry between total alpha- and beta-spectrin molecules. The quantity of the alpha-spectrin mutant, expressed as a percentage of the total alpha spectrin, varied from 9.9-45.2% among six affected individuals. Two-dimensional electrophoretic analysis of spectrin tryptic digests was qualitatively normal but showed a decreased quantity of a normal alpha IV fragment. The variable quantity of alpha-spectrin mutant among family members correlated directly with the increased percentage of spectrin dimers in cold low ionic strength spectrin extracts (r = 0.92) and inversely with red blood cell ghost mechanical stability (r = -0.98). The data suggest that this new alpha-spectrin mutant is responsible for decreased spectrin dimer-dimer association and for red cell instability in affected individuals.
Full text
PDF

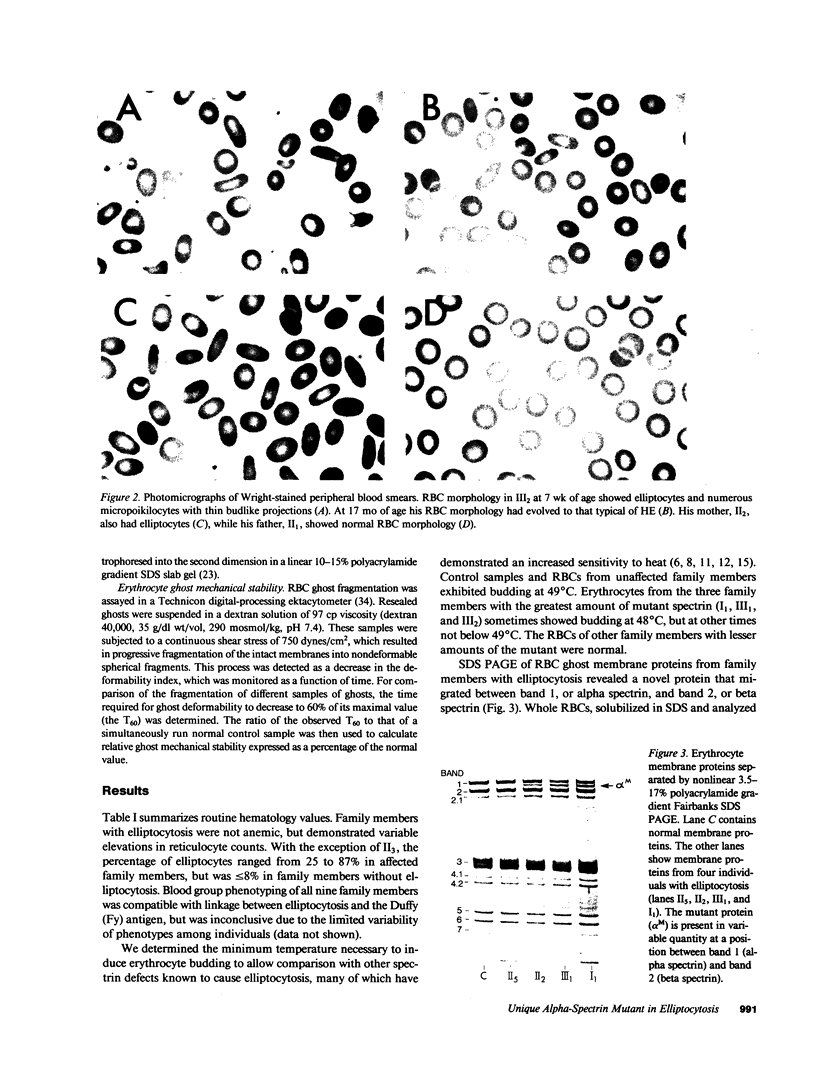




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agre P., Orringer E. P., Chui D. H., Bennett V. A molecular defect in two families with hemolytic poikilocytic anemia: reduction of high affinity membrane binding sites for ankyrin. J Clin Invest. 1981 Dec;68(6):1566–1576. doi: 10.1172/JCI110411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Blikstad I., Nelson W. J., Moon R. T., Lazarides E. Synthesis and assembly of spectrin during avian erythropoiesis: stoichiometric assembly but unequal synthesis of alpha and beta spectrin. Cell. 1983 Apr;32(4):1081–1091. doi: 10.1016/0092-8674(83)90292-1. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Clark M. R., Unger R. C., Shohet S. B. Monovalent cation composition and ATP and lipid content of irreversibly sickled cells. Blood. 1978 Jun;51(6):1169–1178. [PubMed] [Google Scholar]
- Coetzer T., Zail S. S. Tryptic digestion of spectrin in variants of hereditary elliptocytosis. J Clin Invest. 1981 May;67(5):1241–1248. doi: 10.1172/JCI110151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzer T., Zail S. Spectrin tetramer-dimer equilibrium in hereditary elliptocytosis. Blood. 1982 May;59(5):900–905. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Dhermy D., Lecomte M. C., Garbarz M., Bournier O., Galand C., Gautero H., Feo C., Alloisio N., Delaunay J., Boivin P. Spectrin beta-chain variant associated with hereditary elliptocytosis. J Clin Invest. 1982 Oct;70(4):707–715. doi: 10.1172/JCI110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein E. H., Jr, Bonifas J. M. Recessive X-linked ichthyosis: lack of immunologically detectable steroid sulfatase enzyme protein. Hum Genet. 1985;71(3):201–205. doi: 10.1007/BF00284573. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Huebner K., Palumbo A. P., Isobe M., Kozak C. A., Monaco S., Rovera G., Croce C. M., Curtis P. J. The alpha-spectrin gene is on chromosome 1 in mouse and man. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3790–3793. doi: 10.1073/pnas.82.11.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles W. J., Bologna M. L. Isolation of the chemical domains of human erythrocyte spectrin. Methods Enzymol. 1983;96:305–313. doi: 10.1016/s0076-6879(83)96028-7. [DOI] [PubMed] [Google Scholar]
- Knowles W. J., Morrow J. S., Speicher D. W., Zarkowsky H. S., Mohandas N., Mentzer W. C., Shohet S. B., Marchesi V. T. Molecular and functional changes in spectrin from patients with hereditary pyropoikilocytosis. J Clin Invest. 1983 Jun;71(6):1867–1877. doi: 10.1172/JCI110942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawler J., Coetzer T. L., Palek J., Jacob H. S., Luban N. Sp alpha I/65: a new variant of the alpha subunit of spectrin in hereditary elliptocytosis. Blood. 1985 Sep;66(3):706–709. [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. A molecular defect of spectrin in a subset of patients with hereditary elliptocytosis. Alterations in the alpha-subunit domain involved in spectrin self-association. J Clin Invest. 1984 Jun;73(6):1688–1695. doi: 10.1172/JCI111376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Liu S. C., Palek J., Prchal J. Molecular defect of spectrin in hereditary pyropoikilocytosis. Alterations in the trypsin-resistant domain involved in spectrin self-association. J Clin Invest. 1982 Nov;70(5):1019–1030. doi: 10.1172/JCI110689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecomte M. C., Dhermy D., Solis C., Ester A., Féo C., Gautero H., Bournier O., Boivin P. A new abnormal variant of spectrin in black patients with hereditary elliptocytosis. Blood. 1985 May;65(5):1208–1217. [PubMed] [Google Scholar]
- Liu S. C., Fairbanks G., Palek J. Spontaneous, reversible protein cross-linking in the human erythrocyte membrane. Temperature and pH dependence. Biochemistry. 1977 Sep 6;16(18):4066–4074. doi: 10.1021/bi00637a020. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J., Prchal J. T. Defective spectrin dimer-dimer association with hereditary elliptocytosis. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2072–2076. doi: 10.1073/pnas.79.6.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux S. E. Spectrin-actin membrane skeleton of normal and abnormal red blood cells. Semin Hematol. 1979 Jan;16(1):21–51. [PubMed] [Google Scholar]
- Marchesi S. L., Knowles W. J., Morrow J. S., Bologna M., Marchesi V. T. Abnormal spectrin in hereditary elliptocytosis. Blood. 1986 Jan;67(1):141–151. [PubMed] [Google Scholar]
- Marchesi V. T. The red cell membrane skeleton: recent progress. Blood. 1983 Jan;61(1):1–11. [PubMed] [Google Scholar]
- Mentzer W. C., Turetsky T., Mohandas N., Schrier S., Wu C. S., Koenig H. Identification of the hereditary pyropoikilocytosis carrier state. Blood. 1984 Jun;63(6):1439–1446. [PubMed] [Google Scholar]
- Mohandas N., Chasis J. A., Shohet S. B. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983 Jul;20(3):225–242. [PubMed] [Google Scholar]
- Mohandas N., Clark M. R., Health B. P., Rossi M., Wolfe L. C., Lux S. E., Shohet S. B. A technique to detect reduced mechanical stability of red cell membranes: relevance to elliptocytic disorders. Blood. 1982 Apr;59(4):768–774. [PubMed] [Google Scholar]
- Nielsen J. A., Praktitioner S. Homozygous hereditary elliptocytosis as the cause of haemolytic anemia in infancy. Scand J Haematol. 1968;5(6):486–496. doi: 10.1111/j.1600-0609.1968.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Noel D., Nikaido K., Ames G. F. A single amino acid substitution in a histidine-transport protein drastically alters its mobility in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Biochemistry. 1979 Sep 18;18(19):4159–4165. doi: 10.1021/bi00586a017. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ohanian V., Evans J. P., Gratzer W. B. A case of elliptocytosis associated with a truncated spectrin chain. Br J Haematol. 1985 Sep;61(1):31–39. doi: 10.1111/j.1365-2141.1985.tb04057.x. [DOI] [PubMed] [Google Scholar]
- Palek J. Hereditary elliptocytosis and related disorders. Clin Haematol. 1985 Feb;14(1):45–87. [PubMed] [Google Scholar]
- Speicher D. W., Morrow J. S., Knowles W. J., Marchesi V. T. A structural model of human erythrocyte spectrin. Alignment of chemical and functional domains. J Biol Chem. 1982 Aug 10;257(15):9093–9101. [PubMed] [Google Scholar]
- Tchernia G., Mohandas N., Shohet S. B. Deficiency of skeletal membrane protein band 4.1 in homozygous hereditary elliptocytosis. Implications for erythrocyte membrane stability. J Clin Invest. 1981 Aug;68(2):454–460. doi: 10.1172/JCI110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Leavitt J., Kakunaga T., Weber K. Coexpression of a mutant beta-actin and the two normal beta- and gamma-cytoplasmic actins in a stably transformed human cell line. Cell. 1980 Dec;22(3):893–899. doi: 10.1016/0092-8674(80)90566-8. [DOI] [PubMed] [Google Scholar]
- Wolfe L. C., John K. M., Falcone J. C., Byrne A. M., Lux S. E. A genetic defect in the binding of protein 4.1 to spectrin in a kindred with hereditary spherocytosis. N Engl J Med. 1982 Nov 25;307(22):1367–1374. doi: 10.1056/NEJM198211253072203. [DOI] [PubMed] [Google Scholar]
- Wyatt J. L., Greenquist A. C., Shohet S. B. Analyses of phosphorylated tryptic peptide of spectrin from human erythrocyte membrane. Biochem Biophys Res Commun. 1977 Dec 21;79(4):1279–1285. doi: 10.1016/0006-291x(77)91144-5. [DOI] [PubMed] [Google Scholar]
- Zail S. S., Coetzer T. L. Defective binding of spectrin to ankyrin in a kindred with recessively inherited hereditary elliptocytosis. J Clin Invest. 1984 Sep;74(3):753–762. doi: 10.1172/JCI111491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]