Abstract
Introduction
Many individual Intensive Care Unit (ICU) characteristics have been associated with patient outcomes, including staffing, expertise, continuity and team structure. Separately, many aspects of clinical care in ICUs have been operationalized through the development of complex treatment protocols. The United State Critical Illness and Injury Trials Group-Critical Illness Outcomes Study (USCIITG-CIOS) was designed to determine whether the extent of protocol availability and use in ICUs is associated with hospital survival in a large cohort of United States ICUs. Here, we describe the study protocol and analysis plan approved by the USCIITG-CIOS Steering Committee.
Methods
USCIITG-CIOS is a prospective, observational, ecological multi-centered “cohort” study of mixed ICUs in the U.S. The data collected include organizational information for the ICU (e.g., protocol availability and utilization, multi-disciplinary staffing assessment) and patient level information (e.g. demographics, acute and chronic medical conditions). The primary outcome is all-cause hospital mortality, with the objective being to determine whether there is an association between protocol number and hospital mortality for ICU patients. USCIITG-CIOS is powered to detect a 3% difference in crude hospital mortality between high and low protocol use ICUs, dichotomized according to protocol number at the median. The analysis will utilize regression modeling to adjust for outcome clustering by ICU, with secondary linear analysis of protocol number and mortality and a variety of a priori planned ancillary studies. There are presently 60 ICUs participating in USCIITG-CIOS to enroll approximately 6,000 study subjects.
Conclusions
USCIITG-CIOS is a large multicentric study examining the effect of ICU protocol use on patient outcomes. The primary results of this study will inform our understanding of the relationship between protocol availability, use, and patient outcomes in the ICU. Moreover, given the shortage of intensivists worldwide, the results of USCIITG-CIOS can be used to promote more effective ICU and care team design and will impact the delivery of intensive care services beyond individual practitioners.
Trial Registration
ClinicalTrials.gov Identifier NCT01109719
Keywords: ICU, Critical Care, Outcomes, Protocols, ICU organization
Introduction
Critically ill patients are managed in intensive care units (ICU) that contain limited, focused clinical resources and high intensity nurse and physician staffing. While ICUs were initially developed to care for all patients with high acuity illness on a single hospital ward, they have evolved over the past 50 years to include units for patients with general respiratory failure or those with more narrowly defined illnesses or injuries in subspecialty ICU’s.[1] Anecdotal and published reports indicate that there is wide variation in organization and structure of these units. For example, variation has been documented in physician staffing, hours of staffing, the presence and role of medical directors and the presence of multidisciplinary rounding teams.[2-5] Heterogeneous care provided to ICU patients may be related in part to patient differences or a lack of medical consensus. However, some of this variation in care may be related to the general characteristics of the ICU itself and the practitioners who work there. ICU factors previously shown to be associated with practice variation include the ICU type (general or specialty), its location, or the training of the lead physician.[6,7] Whether this heterogeneity leads directly to worse outcomes (cause and effect) remains to be proven, however, variation in patients admitted to specialty ICUs that do not match their particular diagnosis appear to fare worse, suggesting familiarity and possible standardization of care may influence patient outcomes.[7-9]
One method to reduce unwanted variance in clinical practice is the use of treatment protocols to drive care for patients meeting specific criteria.[10] There is little information on whether differences in processes of care (previously attributed to ICU organization and structure) might be related to the use of protocol based care. For instance, high intensity ICU organization has been shown to be associated with higher likelihood of evidence-based delivery of low tidal volume ventilation to acute lung injury patients.[6] However, the presence of a protocol has also been linked to more frequent use of a desired process of care[11] and therefore is likely to be associated with improved patient outcomes. As a result, it is logical to expect that a greater number of protocols should be associated with a reduction in unwanted practice variation and thereby improved outcomes independent of ICU organization. The United States Critical Illness and Injury Trials Group (USCIIT Group) designed the Critical Illness Outcomes Study (USCIITG-CIOS) to test the hypothesis that the number of protocols influences hospital mortality. Additionally, this study will prevalence of protocols in sixty ICU’s in the United States.
Methods
Study design
To test the hypothesis that the number of protocols used in an ICU is associated with hospital survival, the USCIIT Group organized a large prospective observational cohort study of ICU patients. Initial ICUs were selected from a range of institutions participating in USCIIT Group and other collaborative groups. After recruitment, physicians familiar with each ICU were asked to fill out a structured assessment of protocol use and general unit characteristics, including physician and nursing staffing, the presence of electronic medical records and computerized order entry. This survey determined both the presence of a priori defined care protocols and how they are utilized. In addition, respondents had the opportunity to report self-determined protocols. Clinical characteristics and outcomes of a series of patients in these same ICUs then were recorded (Figure 1).
Figure 1. Study scheme and sequence of data submission.
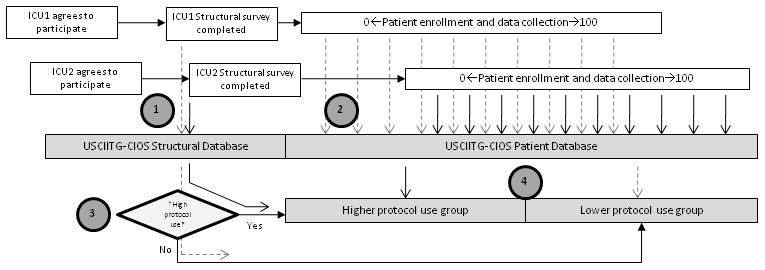
(1) Individual sites entered the study independently and submission of structural survey information was required prior to patient enrollment. Individual ICUs were then assigned to a high protocol group or low protocol group according to their specific number of protocols relative to the median for the study. (2) Patient data is entered by individual sites without the knowledge of whether their ICU is defined as high protocol or low protocol use (3) as the group assignments are made centrally at the conclusion of the study. This same procedure was followed for all 60 centers. In this example ICU1 was defined as a “lower” protocol ICU and ICU2 as a “higher” protocol site. Numbers indicate sequence of activities. *Assigned at the conclusion of data entry ICU, Intensive care unit; USCIITG-CIOS, Critical Illness Outcomes Study
At the time of initial planning, several important sub-studies were integrated into the overall study design: ICU staffing patterns, the presence of electronic medical records, and continuity of care measurements. Adherence to specific care protocols and their relationship to disease-specific outcome will also be determined (Table e1).
Site recruitment and communications
Because healthcare delivery systems and intensive care units vary between countries, we sought to identify at least sixty ICU’s in the United States only. Sites were initially petitioned by review of publications from active critical care investigators in the United States, word-of-mouth at international critical care meetings, and direct communication to previous collaborators. Specific communication was sent to representatives of the USCIIT Group and Surviving Sepsis Campaign institutions. Both academic and non-academic ICU’s were approached, regardless of specialty (medical, surgical, cardiac, neurologic, trauma, etc) and case-mix (e.g. cancer). Follow-up calls provided a more detailed discussion between a USCIITG-CIOS principal investigator (PI) and the site’s local contact. During this semi-structured interview, local ICU structure referral patterns, prior clinical research experience, the USCIITG-CIOS study protocol anticipated workflow, workload, expectations and site PI rights were discussed. Interested sites were provided with basic IRB application materials and ultimately access to the USCIITG-CIOS web interface.
Communication between the PI’s and individual site investigators was logistically complex. Formal communication from the USCIITG-CIOS PI’s to site investigators began at the time of first subject recruitment and took a variety of forms. These included face-to-face meetings, conference calls or electronic LISTSERV communications (Figure 2). Regularly scheduled emails provided updates on recruitment status, deadlines, and overall study progress. Inter-site communication was encouraged. Site-specific queries were handled by the study PI’s via electronic communication. Final participating sites are listed in Appendix A.
Figure 2. Study history timeline.
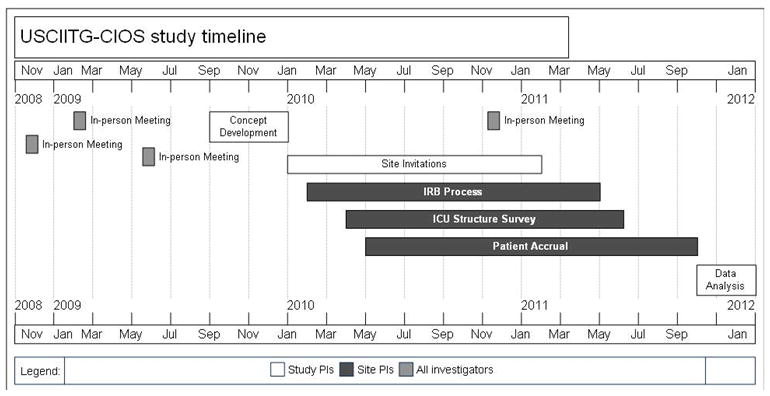
Because so many sites were recruited, the process of site engagement, approval and initiation occurred in a “rolling” fashion. In-person meetings and electronic communication was used to attempt to compress the time window during which surveys were completed and patients enrolled.
Study subjects and human subjects’ protections
Each site was asked to screen census registers weekly to enroll new ICU patients. Sites were permitted discretion in choosing survey days and encouraged to rotate days to ensure variation in sampling. Study subjects were those adult patients occupying a bed in the study ICU at 8 am on each survey day. Subjects were excluded if they were < 18 years of age or had been previously enrolled into USCIITG-CIOS. Enrollment days were not continuous in order to facilitate subject enrollment without duplication. A minimum of 100 subjects was targeted for enrollment from each study ICU (Figure 3).
Figure 3. Patient accrual scheme.
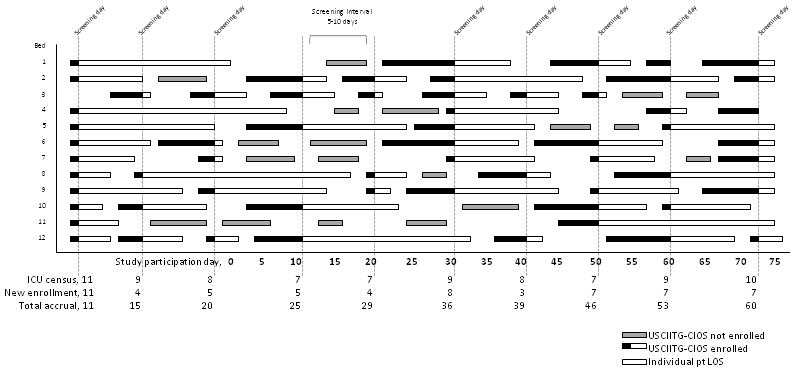
This illustrates the hypothetical patient accrual into the USCIITG-CIOS study from a single participating ICU with twelve patient care beds. ICU census registers were screened at intervals that varied between 5 and 10 days. All patients were eligible for data entry unless they were previously entered or met other exclusion criteria. The black portion of a patient stay bar indicates their enrollment in the USCIITG-CIOS patient database and the fact that patients are only entered once into the database. All enrolled subjects were followed for outcome data until the time of hospital discharge. USCIITG-CIOS, Critical Illness Outcomes Study; LOS, length of stay in intensive care; pt, patient
Since the exposure of interest (indigenous clinical care protocols) was by definition the baseline practice of any individual unit and these practices were not altered by study participation, any variation across study sites was considered local “usual care”. In addition, this study was designed to collect all patient information devoid of personally identifiable indicators. As a result, local institutional review boards were petitioned for a waiver of consent.
Sample size
The primary objective of this study is to determine if a difference in hospital mortality exists between highly protocolized ICUs and those with fewer or no protocols. Since no threshold number of protocols could be proposed from prior work, we chose to divide ICUs into two groups defined by the median number of protocols observed in our sample of ICUs (median to be determined). We estimated that the minimal significant difference in unadjusted mortality outcomes would be 3% between ICU with a “higher” and “lower” number of protocols. Based upon average mortality from surveys of individual ICU’s, we assumed that the group of ICUs with a lower number of protocols would have a baseline hospital mortality of 15%. Ignoring clustering by ICU, we would require 2791 participants per study group to detect a 3% absolute difference in hospital mortality with 90% power and a significance level of 0.05. We plan to engage at least 60 centers to accrue 6000 evaluable patients. We anticipate that one year will be required to enroll the requisite number of subjects.
Study outcomes and data collection
The primary outcome for the USCIITG-CIOS study is hospital mortality. Secondary outcomes are ICU mortality, discharge location; hospital ICU length of stay, and duration of mechanical ventilation. Observations were recorded using two separate questionnaire case report forms (CRF); one for ICU structural data and one for patient-level data. Each participating ICU completed a structural questionnaire prior to enrolling subjects. The structural questionnaire included 79 questions in seven sections: general information, bed count, utilization, staffing, rounding practices, team work tools, and ICU protocols (Table 1). A “protocol” was defined using the Medline MeSH subject heading as “precise and detailed plans for a regimen of therapy”. Investigators were left to determine whether local practice was guided by a protocol, but additional questions were asked to determine how the protocol was activated and how its recommendations were implemented. Guidance for all responses was provided in the distributed Operations Manual. After the structural questionnaire was deemed complete, the site was allowed to enroll subjects.
Table 1.
Major planned sub studies under USCIITG-CIOS (Partial list)
| Title | Description | Investigators |
|---|---|---|
| Systematic review of protocols | Systematic review of protocol efficacy for improving patient outcomes | Patil, Eberlein, Winters, Morris, Brown, Hirschburg, Prasad |
| ICU structure | Descriptive phenotype of USCIITGCIOS centers | Checkley, Sevransky, Gajic, Howell, Shahul |
| Race/ethnicity, processes and outcomes in the ICU | Analysis of the association between race or ethnicity and receipt of healthcare and associated outcomes, adjusting for confounders | Martin, Pietropaoli, Howell, Shahul, Talmor, Hunziker |
| Transfusion goals | Do we comply with transfusion protocols? | Murphy, Shahul |
| Epidemiology of FFP transfusion | Description of frequency and triggers for FFP transfusion | Netzer, Shahul |
| EMR and ICU | Is there a patient-level benefit to health information technology in the ICU? | Pickering, Howell, Talmor, Han,Shahul, Pickering, Hunziker |
| Cancer | Prevalence and outcomes | Eberlein, Pastores |
| Consultation | Epidemiology of consultative services in critically ill patients in the United States | Howell, Talmor, Goodspeed |
| Continuity of care | Does a change in resident or attending influence care delivery? | Siner, Gutteridge |
| Nutrition | Prevalence of adequate nutrition in ICU’s | Rice, Shahul |
| ALI/MODS simulation model | Validation of a multiscale simulation model of acute lung injury and multiorgan failure in the multicenter cohort of critically ill patients | Gajic, Pickering |
Enrollment days were chosen randomly, with 5-10 days between enrollments to allow for patient turnover. A case report form (CRF) was completed for all patients receiving care in an ICU on that day who met the enrollment criteria. The CRF included 136 items organized into nine sections: demographics, illness severity, organ failure data, mechanical ventilation, medications, treatment issues, diagnoses, infections, and outcomes (Table 2). Records were reviewed on the day of enrollment and periodically thereafter until the time of discharge to obtain appropriate treatment and follow-up data. Admission data on mechanical ventilation and medications was recorded from 8 am on the day of enrollment. All other data were based on the 24-hour period prior to enrollment (Table 2).
Table 2.
Major components of the Structural Survey
| Domain | Number of questions | Structure of responses |
|---|---|---|
| Hospital and ICU overview | 16 | categorical and numerical |
| Staffing | 10 | categorical and numerical |
| Organization | 9 | categorical |
| Services | 2 | categorical |
| Rounding practices | 10 | categorical |
| ICU protocols | 26 | categorical |
| Teamwork tools | 2 | categorical |
Patient charts were reviewed for documented chronic organ insufficiency and the circumstances leading to ICU admission. These categorizations and their definitions were recorded according to APACHE II definitions.[12] Researchers were not permitted to interpret records; diagnoses recorded in the CRF were all based upon physicians or other practitioner’s notes recorded in the patient’s chart. The presence of chart documentation and consult notes were recorded in order to quantify the number of disciplines involved in each patient’s care. These notes were defined as identifying a specific service by the title of the chart entry. Data on infections were completed based on progress notes from the day of enrollment and the most recent laboratory data. Outcome data were completed after the patient was discharged from the hospital. Discharge location included detail about hospital type if the patient was discharged to another inpatient facility. The presence of mechanical ventilation, renal replacement therapy or limitation-of-care orders at hospital discharge was also documented.
Primary analysis and other statistical considerations
The primary objective of this study is to determine whether an association exists between the number of ICU protocols and hospital mortality for ICU patients. The primary outcome for this study is hospital mortality. As described above, we plan to divide ICUs into two groups based upon the median number of protocols observed in the entire sample of ICUs (n=30 as having a higher number of protocol and n=30 as having a lower number of protocols). We will first compare the simple means of the hospital mortality rates between the two pre-defined groups of ICUs using standard techniques.[13] Secondly, we will perform an adjusted analysis controlling for physiologic severity of illness and other potentially confounding variables. Specifically, we will use logistic regression techniques with generalized estimating equations (GEE), a compound symmetry matrix,[14] and a robust variance estimate to adjust for clustering of hospital mortality by ICU. In a secondary analysis, we will characterize the relationship between the number of protocols as a continuous variable and hospital mortality using regression splines[15] within the context of multivariable logistic regression with GEE.
Data Quality and Management
Prior to data collection the PI’s developed an Operations Manual to ensure inter-site reliability. The operations manual included selected definitions for questionnaires as well as guidelines for data collection. The operations manual was maintained and revised as needed by the PI’s, and distributed to sites as revisions were made. Frequent communications were sent electronically that included clarifications of the operations manual, discussion of IRB approval process, and suggestions regarding data collection. The primary investigators reviewed each site’s structural questionnaire prior to allowing subject enrollment.
All patient data were recorded on paper CRFs and then entered into an online database maintained by Johns Hopkins University. The database was designed to prevent entry of duplicate patients in the following ways. First, the database created a unique patient identifier for each new patient based on the demographic information provided. This demographic information then was compared to enrollees. Subjects with an exact match on five independent variables (gender, age, race, height and weight) were blocked from entry. During data entry, computer decision support identified missing, inconsistent, or out-of-range data. A data error query was produced and either resolved within the data center by individual, manual review or returned to the local investigator for resolution. Finally, the database ensured that data is entered in structured formats to facilitate calculation of the APACHE II and SOFA scores.
Discussion
We have developed a large collaborative United States study to investigate the effect of the number of protocols used in an intensive care unit on hospital mortality. There are many factors that influence outcomes in individual ICUs. These factors can include local referral patterns[16-18], patient-specialty ICU type match[7], case-volume[8], staffing characteristics and expertise.[2,3] Presumably these superficial characteristics belie some variation in the ability to identify or treat important medical issues. Protocols have been used to reduce unwanted practice variation. Protocol driven interventions in the ICU have improved targeted process measures and disease specific outcomes[19], but the association between the number of protocols and general outcome across diverse patient groups has not been studied. In this multicentric prospective study, we test the hypothesis that there is an association between the amount of protocolization in a given ICU and outcome.
There is a significant evidence base that should influence ICU practice. Noteworthy practices include transfusion management[20,21], sedation and analgesia[22] and ventilator weaning.[23,24] Additionally, condition specific practices like the use of low tidal volume ventilation in acute lung injury patients[25] or aggressive treatment of septic shock have improved mortality in these diseases.[26] Given that these are common ICU conditions and treatments, adopting these practices effectively could improve outcomes across larger populations of general critically ill patients independent of their admitting problem. Despite knowledge of these interventions and their beneficial consequences, evidence suggests these practices are still only variably applied.[27-32]
Non adherence to recommended protocols can be related to knowledge deficits, distractions[33] or a lack of access to evidence at the point of care.[34] Knowledge of these barriers has led to the development of simple checklists for ICU teams to use to identify treatment goals, but their ability to improve adherence is dependent on knowledge of a mechanism to effect these goals.[35,36] Protocols can provide operational definitions for the delivery of evidence based therapy and accordingly increase a practitioner’s ability to understand how to apply a specific practice. When clear and readily available[37,38] protocols should improve outcomes in a variety of ways, particularly if baseline adherence to desired practice is low. In some settings, these protocols may improve the efficiency of decision making or allow the responsibility for the decision to be shifted to appropriate team members.
The organizational structure of an intensive care unit has been linked to adherence to evidence based ventilator management for acute lung injury patients.[6,39] Our study will attempt to describe whether one potential mechanism for this effect is the use of protocols. The systematic adoption of protocols, using presence of a higher number of protocols as a proxy, should be associated with improved outcomes because it marks an underlying multidisciplinary culture that defines unwanted practice variation. Alternately, if a specific ICU cares for a high proportion of patients where these practices apply (like sepsis or acute lung injury) then protocols could directly improve practice and outcome.[38] Finally a higher number of protocols could simply increase the likelihood of having the “right” protocols in place. In any case, the observation that the number of protocols is associated with outcome could have important implications for the appropriate design and structure of ICU care teams particularly when the human resources needed to develop multidisciplinary teams are limited.[40-42]
The novelty of our study is that it addresses a unique topic in critical care medicine that could not be easily studied in a prospective randomized design. It is clear that multidisciplinary teams improve outcomes in ICU’s,[5,43] but the mechanism by which this occurs is not known. By studying protocol use in general, we are analyzing an approach to care standardization and not the practices themselves. Strengths of our study design include a cohort with a broad array of ICUs of mixed type and the explicit description of their coverage model and care practices. In addition, data collection was designed to not only quantify protocols but also record the area of practice they are designed to impact. We record data about how the particular protocol is activated (standing order, individual activation, etc) to understand where in the process of clinical decision making these support tools exist. Finally, by collecting important clinical data we can generate inferences about what proportion of patients in an individual ICU would have been a candidate for protocol-driven care and appropriately risk-adjust all outcomes.
There are several potential limitations to this study. By including a broad array of ICU types, we may lose the ability to determine the importance of individual care protocols that impact a small proportion of our cohort (e.g. ventriculostomy management). Second, because we primarily measure outcome as hospital mortality, we may lose the ability to detect benefit from care protocols that have a greater impact on morbidity than mortality. Examples of these types of treatments include sedation practice and mobility protocols. It is possible that the way in which a protocol is activated is important to its ability to affect outcomes. Because we are only collecting survey data about how protocols are utilized, we lack the ability to comment on whether individual units operationalize their stated practice. Additionally, we do not review the content of each protocol so we cannot exclude the possibility that the protocols are incorrect or not explicit enough. Ideally, this question could be tested by a cluster randomized trial of protocolized general care, but the resources to perform this study and the ethics behind it, could be prohibitive barriers. Lastly, it is possible that we may fail to observe improved outcomes in general ICU patients simply because the “right” protocols have not been developed yet. We believe this is unlikely given the temporal improvements in ICU outcomes that have been observed with time and the magnitude of effect seen in homogenous patient groups[25,44]; however, this is still a possibility.
Our analysis also may be confounded by not clustering by ICU in our original sample size calculations. Controlling for this necessitates accounting for the coefficient of variation of the actual mortality between study ICUs, an additional unknown. If we were to now take this into account, we would likely trade more rigorous analysis for less power to detect the expected 3% difference in hospital mortality (assuming the coefficient of variation for hospital mortality is >0.1, Figure 4). While our intent is to enroll 6000 participants across 60 ICUs, we plan to monitor longitudinally the observed coefficient of variation for hospital mortality to obtain a better understanding of the magnitude of this potential effect.
Figure 4. Impact of variation in hospital mortality across study sites given ICU (cluster) recruitment size.
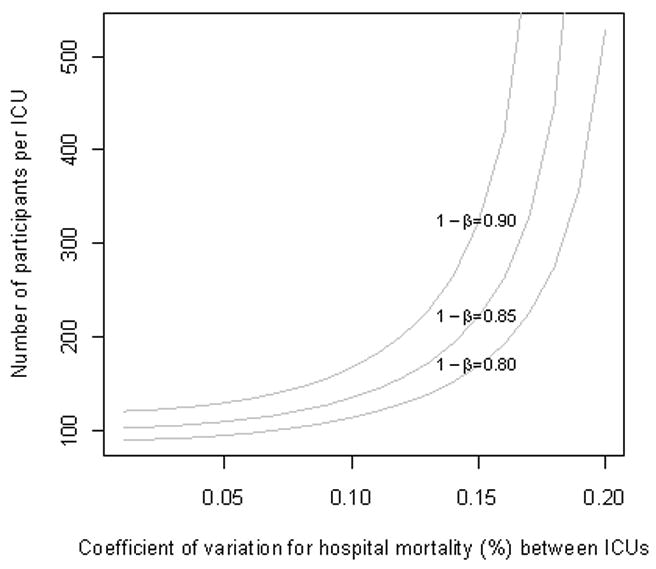
In closing, this study will examine the association between number of care protocols and patient outcome. Further, it will provide a number of important derivatives, include an ability to begin to identify what protocol characteristics determine improved practice and a relative weighting of the impact of specific protocols by ICU specialty. This new knowledge in turn can inform design of information systems to automate when possible those practices that have the greatest positive impact on outcomes and costs. This study will generate important information that can be used in the near term by unit and hospital medical directors to inform ICU organization, and in the longer term to determine bridge strategies to improve ICU outcomes in a resource limited environment.
Table 3.
Patient-level data collection
| Domain | Number of questions | Structure of responses | Time frame |
|---|---|---|---|
| Demographics | 13 | categorical and numerical | at hospital admission |
| Severity of illness | 29 | categorical and numerical | 24 hours prior to enrollment |
| Organ failure data | 6 | numerical | 24 hours prior to enrollment |
| Mechanical Ventilation | 11 | categorical and numerical | 8 am on day of enrollment |
| Medications | 6 | categorical | 24 hours prior to enrollment |
| Treatment factors | 22 | categorical | 24 hours prior to enrollment |
| Diagnoses | 6 | categorical and numerical | 24 hours prior to enrollment |
| Infections | 6 | categorical | 24 hours prior to enrollment |
| Outcomes | 9 | categorical | values recorded after discharge |
Instruments and definitions used within the survey include the following: Severity of illness, APACHE2; Organ failure, SOFA; Coagulopathy, INR > 1.5, PTT > 2x control or platelets < 50k; Hypotensive, MAP <65 mm Hg, SBP < 90 mm Hg, or drop in SBP of > 40 mm Hg despite 3 2 L fluid given; Delirium, CAM-ICU, ICDSC, Nu-DESC; Stress dose steroids, hydrocortisone ≥ 200 mg/day or equivalent dose of other corticosteroids.
Acknowledgments
We acknowledge the leadership of the USCIITG in facilitating the execution of this study and providing invaluable advice on protocol development and execution.
Appendix A
Participating centers [Listed in alphabetical order by state, then medical center. The principal investigator’s (PI) name appears first then all others are listed in alphabetical order per site.]
ARIZONA: University of Arizona Medical Center, Tucson, AZ, Terence O’Keeffe (PI), Coy Collins; CALIFORNIA: LA County-University of South California Hospital, Los Angeles, CA, Janice Liebler (PI), Ali Ahoui, Anahita Nersiseyan, Usman Shah, Hidenobu Shigemitsu, Nanditha Thaiyananthan, ; Stanford University Medical Center, Stanford, CA, Joe HSU (PI), Lawrence Ho; CONNECTICUT Bridgeport Hospital, Bridgeport, CT; Yale University Hospital, New Haven, CT, Jonathan M. Siner (PI), Mark D. Siegel; GEORGIA: Emory University Hospital, Atlanta, GA, Greg S. Martin (PI), Craig Coopersmith, Sushma, Cribbs, Annette Esper, Micah Fisher, David Gutteridge, Akrm Abdelrahman, Mona Brown, Sang Lee, Apryl Smith; Grady Memorial Hospital, Atlanta, GA, Greg S. Martin (PI), Craig Coopersmith, Sushma Cribbs, Annette Esper, Micah Fisher, David Gutteridge; ILLINOIS: Northwest Community Hospital, Arlington Heights, IL, Melanie Atkinson (PI), Aimee Draftz, Jackie Durgin, Yelena Rikhman, Jessica Scheckel, Mary Walthers; Saint Francis Hospital, Evanston, IL, Gerald Luger (PI), Carol Downer; University of Illinois Medical Center, Chicago, IL, Ruxana T. Sadikot (PI), Kamran Javaid, Daniel Rodgers, Vibhu Sharma; MARYLAND: John Hopkins Bayview Medical Center, Baltimore, MD, Jon Sevranski (PI), Will Checkley, Romer Geocadin, David Murphy, Dale Needham, Adam Sapirstein, Steven Schwartz, Glenn Whitman, Brad Winters, Addisu Workneh, Sammy Zakaria; John Hopkins Hospital, Baltimore, MD, Jon Sevranski (PI), Will Checkley, Romer Geocadin, David Murphy, Dale Needham, Adam Sapirstein, Steven Schwartz, Glenn Whitman, Brad Winters, Addisu Workneh, Sammy Zakaria; St. Agnes Hospital, Baltimore, MD, Anthony Martinez (PI), Fran Keith; University of Maryland Medical Center, Baltimore, MD, Steven Johnson (PI), Dan Herr, Carl Shanholtz, Arabela Sampaio, Jennifer Titus; NIH Hospital, Bethesda, MD; Suburban Hospital Bethesda, Bethesda, MD, Leo Rotello (PI), Jennifer Anderson; MASSACHUSETTS: Beth Israel Deaconess Medical Center, Boston, MA, Sajid Shahul (PI), Valerie Banner-Goodspeed, Michael Howell, Sabina Hunziker, Victoria Nielsen, Jennifer Stevens, Daniel Talmor; Brigham and Women’s Hospital, Boston, MA, Namrata Patil (PI), Lisa Chin, Michael Myers, Stanthia Ryan; MAINE: York Hospital, York, Maine; MICHIGAN: University of Michigan Medical Center, Ann Arbor, MI, Pauline Park (PI), Vivek Arora, James Blum, Kristin Brierley, Jessica DeVito, Elizabeth Jewell, Scott McCardle, Julie McClelland, Deborah Rohner; MINNESOTA: Mayo Clinic Rochester, Rochester, MN, Brian W. Pickering (PI), Jyothsna Gi, Rahul Kashyap, Naman Trivedi; MISSOURI: University of Missouri-Columbia Hospital, Columbia, Missouri; Kansas City VA Hospital, Kansas City, MO, Timothy Dwyer (PI), Kyle Brownback; NEW JERSEY: University of Medicine and Dentistry of New Jersey, Newark, NJ, Steven Chang (PI), Zaza Cohen, Frank Italiano, Zeeshan Kahn, Amee Patrawalla; NEW MEXICO: Presbyterian Healthcare Services, Albequerque, NM, Denise Gonzales (PI), Paul Campbell; NEW YORK: Columbia University Medical Center, New York, NY, David Chong (PI), Matthew Baldwin, Luke Benvenuto, Natalie Yip; Memorial Sloan Kettering Cancer Center, New York, NY; University of Rochester Medical Center, Rochester, NY, Anthony Pietropaoli (PI), Kathleen Faulkner, Timothy Bouck, Ann Marie Mattingly; NORTH CAROLINA: Wake Forest University Health Science, Durham, NC, Peter E. Morris (PI), Lori S. Flores; ECU Hospital, Greenville, NC, Abid Butt, Mark Mazer, Kelly Jernigan; Moses Cone Health, Greensboro, NC, Patrick Wright (PI), Sarah Groce, Jeanette McLean, Arshena Overton; OHIO: Cleveland Clinic, Cleveland, OH, Jorge A. Guzman (PI), Mohammed Abou El Fadl, Tonya Frederick, Gustavo-Cumbo-Nacheli, John Komara; The Ohio State University Medical Center, Columbus, OH, James M. O’Brien (PI), Naeem Ali, Matthew Exline; PENNSYLVANIA: Eastern Regional Medical Center, Cancer Treatment Centers of America, Philadelphia, PA, Jeffrey Hoag (PI), Daniela Albu, Pat McLaughlin; Hahnemann University Hospital, Philadelphia, PA; Hospital of the University of Pennsylvania, Philadelphia, PA, Meeta Prasad (PI), Scott Zuick; TENNESSEE: Meharry Medical College Hospital, Nashville, TN, Richard D. Fremont (PI), Chinenye O. Emuwe, Victor C. Nwazue, Olufemi S. Owolabi; Vanderbilt University Medical Center, Nashville, TN, Bryan Cotton (PI), George Hart, Judy Jenkins; Vanderbilt University Medical Center, Nashville, TN, Todd W. Rice (PI), Tim Girard, Margaret Hays, Susan Morgan; TEXAS: University of Texas-Houston Medical Center, Houston, TX; UTAH: Intermountain Medical Center, Murray, Utah, Samuel Brown (PI), Colin Grissom, Russ Miller III, Anita Austin, Heather Gallo, Naresh Kumar, David Murphy; VIRGINIA: Inova Health Systems, Falls Church, VA, Maryann Putman (PI), Joanne Ondrush.
Footnotes
Author’s contributions
NA, participated in the study design and primarily drafted the manuscript. WC participated in the study design, analysis plan and revised sample size estimates by clustering as well as drafted the statistical manuscript sections. DG developed the data collection and integrity plan and drafted the data collection section of the manuscript. GM participated in the study design and site recruitment process and further drafted sections of the manuscript. JS principally conceived of the study and led initial study protocol efforts and drafted portions of this manuscript. SS participated in site communication planning and drafted portions of the manuscript. All authors provided critical revisions to the manuscript and approved of the final manuscript content.
Competing Interests
None of the authors report they have a significant financial or other competing interest related to the content of this manuscript.
Reference List
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, Rubenfeld GD. Critical care and the global burden of critical illness in adults. Lancet. 2010;376:1339–1346. doi: 10.1016/S0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review. JAMA. 2002;288:2151–2162. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Rapoport J, Lemeshow S, Chalfin DB, Phillips G, Danis M. Association between critical care physician management and patient mortality in the intensive care unit. Ann Intern Med. 2008;148:801–809. doi: 10.7326/0003-4819-148-11-200806030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gajic O, Afessa B, Hanson AC, Krpata T, Yilmaz M, Mohamed SF, Rabatin JT, Evenson LK, Aksamit TR, Peters SG, et al. Effect of 24-hour mandatory versus on-demand critical care specialist presence on quality of care and family and provider satisfaction in the intensive care unit of a teaching hospital. Crit Care Med. 2008;36:36–44. doi: 10.1097/01.CCM.0000297887.84347.85. [DOI] [PubMed] [Google Scholar]
- 5.Kim MM, Barnato AE, Angus DC, Fleisher LF, Kahn JM. The effect of multidisciplinary care teams on intensive care unit mortality. Arch Intern Med. 2010;170:369–376. doi: 10.1001/archinternmed.2009.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke CR, Watkins TR, Kahn JM, Treggiari MM, Caldwell E, Hudson LD, Rubenfeld GD. The effect of an intensive care unit staffing model on tidal volume in patients with acute lung injury. Crit Care. 2008;12:R134. doi: 10.1186/cc7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lott JP, Iwashyna TJ, Christie JD, Asch DA, Kramer AA, Kahn JM. Critical illness outcomes in specialty versus general intensive care units. Am J Respir Crit Care Med. 2009;179:676–683. doi: 10.1164/rccm.200808-1281OC. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JM, Goss CH, Heagerty PJ, Kramer AA, O’Brien CR, Rubenfeld GD. Hospital volume and the outcomes of mechanical ventilation. N Engl J Med. 2006;355:41–50. doi: 10.1056/NEJMsa053993. [DOI] [PubMed] [Google Scholar]
- 9.Kahn JM, Ten Have TR, Iwashyna TJ. The relationship between hospital volume and mortality in mechanical ventilation: an instrumental variable analysis. Health Serv Res. 2009;44:862–879. doi: 10.1111/j.1475-6773.2009.00959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prasad M, Christie JD, Bellamy SL, Rubenfeld GD, Kahn JM. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25:610–619. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Umoh NJ, Fan E, Mendez-Tellez PA, Sevransky JE, Dennison CR, Shanholtz C, Pronovost PJ, Needham DM. Patient and intensive care unit organizational factors associated with low tidal volume ventilation in acute lung injury. Crit Care Med. 2008;36:1463–1468. doi: 10.1097/CCM.0b013e31816fc3d0. [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 13.Hayes RJ, Moulton LH. Cluster randomised trials. Boca Raton, FL: Chapman and Hall Publishers; 2009. [Google Scholar]
- 14.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health. 1993;14:43–68. doi: 10.1146/annurev.pu.14.050193.000355. [DOI] [PubMed] [Google Scholar]
- 15.Hastie T, Tibshirani R. Generalized additive models. Boca Raton, FL: Chapman and Hall Publishers; 1990. [Google Scholar]
- 16.Escobar GJ, Greene JD, Gardner MN, Marelich GP, Quick B, Kipnis P. Intra-hospital transfers to a higher level of care: contribution to total hospital and intensive care unit (ICU) mortality and length of stay (LOS) J Hosp Med. 2011;6:74–80. doi: 10.1002/jhm.817. [DOI] [PubMed] [Google Scholar]
- 17.Golestanian E, Scruggs JE, Gangnon RE, Mak RP, Wood KE. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007;35:1470–1476. doi: 10.1097/01.CCM.0000265741.16192.D9. [DOI] [PubMed] [Google Scholar]
- 18.Kahn JM, Kramer AA, Rubenfeld GD. Transferring critically ill patients out of hospital improves the standardized mortality ratio: a simulation study. Chest. 2007;131:68–75. doi: 10.1378/chest.06-0741. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JE, Mosher BD, Morrison CA, Schneider PD, Stevens P, Kepros JP. A disciplined approach to implementation of evidence-based practices decreases ICU and hospital length of stay in traumatically injured patients. J Surg Res. 2010;163:327–330. doi: 10.1016/j.jss.2010.03.074. [DOI] [PubMed] [Google Scholar]
- 20.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 21.Corwin HL, Gettinger A, Rodriguez RM, Pearl RG, Gubler KD, Enny C, Colton T, Corwin MJ. Efficacy of recombinant human erythropoietin in the critically ill patient: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 1999;27:2346–2350. doi: 10.1097/00003246-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 23.Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–574. doi: 10.1097/00003246-199704000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Girard TD, Kress JP, Fuchs BD, Thomason JW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 25.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 26.Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, Reinhart K, Angus DC, Brun-Buisson C, Beale R, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36:296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 27.Gillis RC, Weireter LJ, Jr, Britt RC, Cole FJ, Jr, Collins JN, Britt LD. Lung protective ventilation strategies: have we applied them in trauma patients at risk for acute lung injury and acute respiratory distress syndrome? Am Surg. 2007;73:347–350. [PubMed] [Google Scholar]
- 28.Dennison CR, Mendez-Tellez PA, Wang W, Pronovost PJ, Needham DM. Barriers to low tidal volume ventilation in acute respiratory distress syndrome: survey development, validation, and results. Crit Care Med. 2007;35:2747–2754. doi: 10.1097/01.CCM.0000287591.09487.70. [DOI] [PubMed] [Google Scholar]
- 29.Chaiwat O, Vavilala MS, Philip S, Malakouti A, Neff MJ, Deem S, Treggiari MM, Wang J, Lang JD. Intraoperative adherence to a low tidal volume ventilation strategy in critically ill patients with preexisting acute lung injury. J Crit Care. 2010 doi: 10.1016/j.jcrc.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Tanios MA, de Wit M, Epstein SK, Devlin JW. Perceived barriers to the use of sedation protocols and daily sedation interruption: a multidisciplinary survey. J Crit Care. 2009;24:66–73. doi: 10.1016/j.jcrc.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor M, Bucknall T, Manias E. Sedation management in Australian and New Zealand intensive care units: doctors’ and nurses’ practices and opinions. Am J Crit Care. 2010;19:285–295. doi: 10.4037/ajcc2009541. [DOI] [PubMed] [Google Scholar]
- 32.Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 33.Cabana MD, Rand CS, Powe NR, Wu AW, Wilson MH, Abboud PA, Rubin HR. Why don’t physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 34.The McDonell Norms Group. Enhancing the use of clinical guidelines: a social norms perspective. J Am Coll Surg. 2006;202:826–836. doi: 10.1016/j.jamcollsurg.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 35.Berenholtz SM, Pronovost PJ, Lipsett PA, Hobson D, Earsing K, Farley JE, Milanovich S, Garrett-Mayer E, Winters BD, Rubin HR, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–2020. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 36.Kass N, Pronovost PJ, Sugarman J, Goeschel CA, Lubomski LH, Faden R. Controversy and quality improvement: lingering questions about ethics, oversight, and patient safetyresearch. Jt Comm J Qual Patient Saf. 2008;34:349–353. doi: 10.1016/s1553-7250(08)34044-6. [DOI] [PubMed] [Google Scholar]
- 37.Morris AH, Orme J, Jr, Truwit JD, Steingrub J, Grissom C, Lee KH, Li GL, Thompson BT, Brower R, Tidswell M, et al. A replicable method for blood glucose control in critically Ill patients. Crit Care Med. 2008;36:1787–1795. doi: 10.1097/CCM.0b013e3181743a5a. [DOI] [PubMed] [Google Scholar]
- 38.Morris AH, Hirshberg E, Sward KA. Computer protocols: how to implement. Best Pract Res Clin Anaesthesiol. 2009;23:51–67. doi: 10.1016/j.bpa.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Treggiari MM, Martin DP, Yanez ND, Caldwell E, Hudson LD, Rubenfeld GD. Effect of intensive care unit organizational model and structure on outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007;176:685–690. doi: 10.1164/rccm.200701-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Angus DC, Kelley MA, Schmitz RJ, White A, Popovich J., Jr Caring for the critically ill patient. Current and projected workforce requirements for care of the critically ill and patients with pulmonary disease: can we meet the requirements of an aging population? JAMA. 2000;284:2762–2770. doi: 10.1001/jama.284.21.2762. [DOI] [PubMed] [Google Scholar]
- 41.Nguyen YL, Kahn JM, Angus DC. Reorganizing adult critical care delivery: the role of regionalization, telemedicine, and community outreach. Am J Respir Crit Care Med. 2010;181:1164–1169. doi: 10.1164/rccm.200909-1441CP. [DOI] [PubMed] [Google Scholar]
- 42.Barnato AE, Kahn JM, Rubenfeld GD, McCauley K, Fontaine D, Frassica JJ, Hubmayr R, Jacobi J, Brower RG, Chalfin D, et al. Prioritizing the organization and management of intensive care services in the United States: the PrOMIS Conference. Crit Care Med. 2007;35:1003–1011. doi: 10.1097/01.CCM.0000259535.06205.B4. [DOI] [PubMed] [Google Scholar]
- 43.Cobb JP. Engineering health in the intensive care unit. Arch Intern Med. 2010;170:319–320. doi: 10.1001/archinternmed.2009.528. [DOI] [PubMed] [Google Scholar]
- 44.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]


