Abstract
Cannabinoids affect positive and negative affective experience and emotional perception, possibly by modulating limbic brain reactivity. In this double-blind crossover, placebo-controlled functional magnetic resonance imaging study in humans, an acute oral dose of Δ9-tetrahydrocannabinol (THC) attenuated subgenual anterior cingulate cortex (sgACC) reactivity during the induction of negative affect. This observation extends prior findings implicating a cortico-limbic, emotion-related central mechanism underlying cannabinoid function.
Keywords: Cannabinoid, Amygdala, Subgenual anterior cingulate, Emotion, fMRI
Introduction
The endogenous cannabinoid system has been identified as an important neuromodulator of emotional states, stimulating interest in this system as a potential novel target to treat affective disorders (Hill and Gorzalka 2009; Gaetani et al. 2009). Cannabis sativa, and its primary active constituent Δ9-tetrahydrocannabinol (THC) produce a complex subjective experience that involves elevating positive mood, attenuating negative mood, and enhancing relaxation (Gobbi et al. 2005; Hollister 1986; Hall and Solowij 1998; Wachtel et al. 2002; Kirk and de Wit 1999).
Cannabinoid Type 1 (CB1) receptors are densely localized in the amygdala and associated limbic frontal cortex including the anterior cingulate cortex (ACC) (Westlake et al. 1994; Tsou et al. 1998; Mailleux et al. 1992; Moldrich and Wenger 2000; Eggan and Lewis 2007), regions strongly involved in emotion and negative affect (Kober et al. 2008; Phan et al. 2002) and the pathophysiology of depression (Drevets 1998; Pezawas et al. 2005; Mayberg et al. 1999; Pizzagalli 2010). Interestingly, chronic use of marijuana is associated with selective attenuation of ACC and amygdala reactivity to affective stimuli (Gruber et al. 2009) and thus, agonism of CB1 receptors could account for the striking mood altering effects of THC.
In the present analysis, we examined the effect of THC on brain activity during a validated emotional picture processing task (the Emotional Pictures Task, EPT). The negatively valenced images typically induce negative affect while engaging limbic-paralimbic brain regions, particularly the amygdala and ACC (Phan et al. 2002). In a double-blind, randomized within-subjects crossover design, we examined the effects of a moderate dose of oral THC (7.5 mg) or placebo (PBO) in healthy volunteers (n = 16) on subjective and brain indices of stimulus-induced negative affect. We hypothesized that relative to PBO, THC would attenuate reactivity in cortico-limbic regions (amygdala and ACC) during the experience of negative emotion. The EPT also employs positively and neutral valence images, allowing for examination of the valence specificity of THC’s effects.
Materials and methods
Subjects
Sixteen healthy, right-handed volunteers (eight males; aged 18–28 years) participated in this study, previously described (Phan et al. 2008). All participants gave written informed consent after explanation of the experimental protocol, as approved by the University of Chicago Institutional Review Board.
Experimental protocol and task
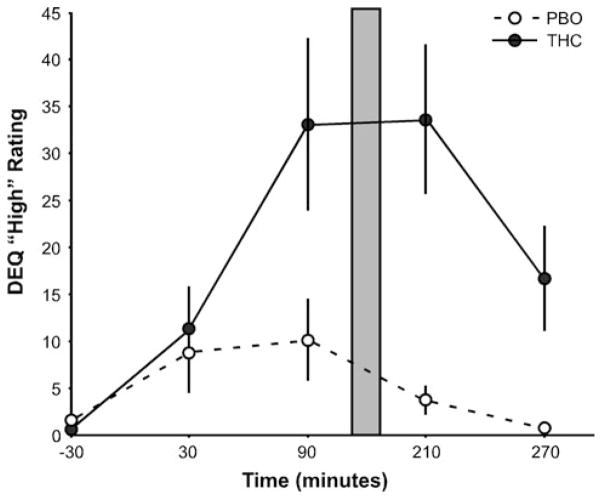
The study used a two-session, double-blind, placebo-controlled, within-subject design as previously described (Phan et al. 2008). Approximately 120 min prior to scanning participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (Marinol; 7.5 mg; Solvay Pharmaceuticals, Marietta, GA) or dextrose alone (placebo; PBO) in a random order. Subjective mood/drug effects were assessed via the Drug Effects Questionnaire (DEQ) (Johanson and Uhlenhuth 1981) throughout the experiment and under both THC and PBO, as previously reported (Phan et al. 2008). We conducted the fMRI experiment between the 90 and 210 min interval post-drug ingestion (PBO condition, 129.10 ± 3.95 min; THC condition, 130.60 ± 4.35 min) during peak levels of subjective drug “high” (Fig. 1), as measured by the DEQ. We also collected subject responses to the Addiction Research Center Inventory (ARCI) (Chait et al. 1985; Martin et al. 1971), a true–false questionnaire with empirically derived subscales sensitive to the effects of marijuana (ARCI-M), including drug-induced euphoria, stimulant effects, intellectual efficiency, sedation, dysphoria and somatic effects (Chait et al. 1985) 30 min before ingestion of the THC or PBO capsule and at 30, 90, 210, and 270 min (T1–T5) after ingestion of the capsule.
Fig. 1.
Mean DEQ “drug high” (±SEM) ratings over time before and after oral administration of THC (closed circles; solid line) or PBO (open circles; dashed line). Grey bar denotes the timeframe in which participants were engaged in the IAPS task during fMRI scanning, which also coincided with expected peak THC effects and plasma levels. Difference of rating scores between drug groups is significant at 90, 210, and 270 min after drug administration (ps < 0.05, two-tailed)
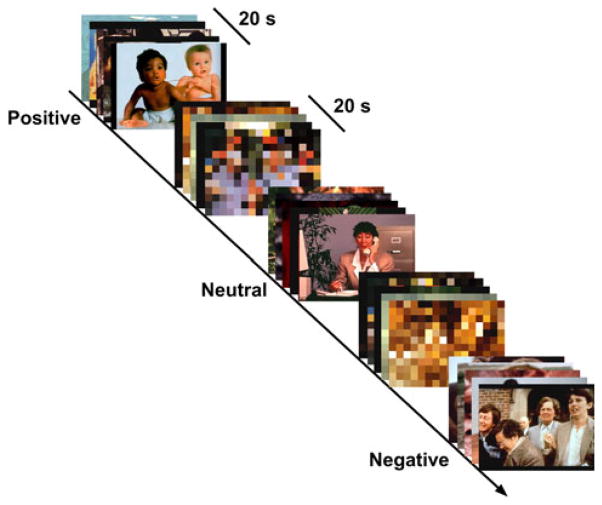
The stimuli used in the EPT consist of 180 unique images acquired from the International Affective Pictures System (IAPS) (Lang et al. 2008) classified into three valence categories (negative, n = 60; neutral, n = 60; and positive, n = 60; matched on arousal), based on normative ratings (negative = mean valence = 2.29 ± 0.50; mean arousal 5.80 ± 0.81; neutral = mean valence = 5.04 ± 0.40; mean arousal = 3.49 ± 0.97; positive = mean valence = 7.52 ± 0.44; mean arousal = 5.54 ± 0.78). All picture conditions (negative, positive, and neutral) were matched in color composition, image complexity, and content. The stimuli were divided equally across the two fMRI sessions and no picture was repeated within or between each session to avoid habituation, sensitization, and learning effects on cortico-limbic reactivity, which could bias our fMRI outcome variables. In addition, each of the 180 pictures had matched scrambled pictures generated from a randomized 16 × 16 pixilation. These scrambled images were not recognized as containing any identifiable content and served as a low-level control (baseline condition) to allow the BOLD signal evoked by the emotional images to return to baseline.
This task involved a block design in which participants viewed two 20 s blocks of each stimulus type (negative, neutral, or positive) interspersed with 20 s blocks of the scrambled (i.e., pixilated) and unrecognizable images during each functional run, over 3 runs (12 blocks per run). Each block consisted of five trials of one stimulus type or five trials of matched scrambled pictures, presented consecutively for 4 s each; block order was pseudorandomized within and across subjects (Fig. 2). During the presentation of each picture stimulus, participants were asked to rate stimulus valence (“How does the picture make you feel?”: 1 = unpleasant; 2 = neutral; 3 = pleasant) during the 4 s display duration via button press. Following the scanning session (between the 210 and 270 min interval post-drug ingestion) all participants viewed each of the 180 unique picture stimuli previously seen during the scanning session and were asked to subjectively rate the valence and arousal evoked by each image on a 9-point scale (Valence: 1, most unpleasant; 5, neither unpleasant or pleasant; 9, most pleasant; Arousal: 1, least arousing; 5, moderately arousing; 9, extremely arousing); their responses were self-paced.
Fig. 2.
Example of the EPT. Twenty-second blocks of each stimulus type (negative, neutral, or positive) interspersed with 20 s blocks of the scrambled images. Each block consisted of five trials of one stimulus type or five trials of matched scrambled pictures, presented consecutively for 4 s each. During the presentation of each picture stimulus, participants were asked to provide a relatively crude subjective measure of stimulus valence (“How does the picture make you feel?”: 1 = unpleasant; 2 = neutral; 3 = pleasant) during the 4 s display duration via button press
Functional imaging: acquisition and analysis
fMRI scanning was performed on a 3T GE Signa magnetic resonance scanner. Whole-brain functional images (i.e., blood oxygenated level-dependent [BOLD]) were collected using a T2*-sensitive gradient echo reverse spiral acquisition sequence, previously described (Phan et al. 2008).
Data from 15 subjects met criteria for high quality and scan stability with minimum motion corrections (<3 mm displacement in any one direction; 1 subject was excluded for poor data quality due to excessive head movement) and were subsequently included in fMRI analyses. The first four volumes from each run were discarded to allow for T1 equilibration effects. Functional data were processed and analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Trust Centre for Neuroimaging, London; http://www.fil.ion.ucl.ac.uk/spm) (Friston et al. 1995), previously described (Phan et al. 2008). Images were spatially realigned to correct for head movement, normalized to an EPI template in Montreal Neurological Institute (MNI) space, resampled to 2 mm3 voxels, and smoothed with an 8 mm3 Gaussian kernel to minimize noise and effects due to residual differences in functional and gyral anatomy during inter-subject averaging. The general linear model was applied to the time series, convolved with the canonical hemodynamic response function and with a 128 s high-pass filter. Condition effects were modeled with box-car regressors representing the occurrence of each block type, and effects were estimated at each voxel and for each subject. In addition, the six movement parameters obtained during realignment were included in the model as regressors to account for motion-related effects in BOLD. Contrast images (linear contrasts of negative > neutral, positive > neutral) were calculated for each participant in both PBO and THC session separately to compare brain activity during affective (negative, positive) and neutral conditions. Individual contrast maps (statistical parametric maps; SPMs) were then analyzed at the second level in a random-effects statistical model.
We had a priori hypotheses that negative affect induction would engage limbic circuitry (e.g., amygdala, ACC), and that THC would have valence-specific and localized effects on these regions. We conducted a whole-brain voxel-wise repeated-measures analysis of variance (ANOVA) with drug (THC, PBO) and valence [negative, positive (vs. neutral)] as within-subject factors and we searched for significant activations that fell within an anatomically defined amygdala and ACC (Walter et al. 2003), consistent with prior approaches (Phan et al. 2008; Zink et al. 2010). Based on our a priori hypothesis we were particularly interested in testing the main effect of drug and drug × emotion interactions.
Behavioral data analysis
Subjective mood/drug effect ratings from the DEQ and ARCI, on-line valence rating and response time, and post-scan valence and arousal ratings were analyzed using an ANOVA with the following factors: drug (THC, PBO) and time (T1–T5). Post hoc comparisons using paired t tests were performed after a significant overall F ratio was obtained. We used a significance threshold of p < 0.05 (two-tailed), corrected for multiple comparisons using Bonferroni correction.
Results
Behavioral results
Subjective data was missing from one participant on the DEQ (n = 14). THC significantly increased ratings of feeling THC effects and feeling “high,” (DEQ “feel”: main effect of drug F(1,13) = 21.21, p < 0.001; main effect of time F(4,52) = 15.58, p < 0.001; and drug-by-time interaction F(4,52) = 7.60, p < 0.001; DEQ “high”: main effect of drug F(1,13) = 12.37, p = 0.004; main effect of time F(4,52) = 7.09, p < 0.001; and drug-by-time interaction F(4,52) = 5.11, p = 0.002; ARCI-M subscale: main effect of drug F(1,14) = 8.32, p = 0.012; main effect of time F(4,56) = 13.80, p < 0.001; and drug-by-time interaction F(4,56) = 3.03, p = 0.025). Post hoc t tests showed mean scores for these measures were significantly higher for the THC session relative to the PBO session at 90, 210, and 270 min following ingestion of the capsule (ps < 0.05), and peaked at 90 and 210 min when participants were performing the fMRI EPT (Fig. 1). However, THC did not significantly affect ratings on the DEQ “like drug” and “want more drug,” or on the ARCI subscales (all ps > 0.05).
Data from on-line (during fMRI) valence ratings of the IAPS images and response times were missing from two subjects (n = 13) and from post-scanning valence and arousal ratings were missing from one subject (n = 14) due to equipment malfunction. Behavioral results from the EPT in the scanner showed that there was a main effect of emotion condition for on-line valence (F(2,24) = 74.75, p < 0.001) with negative images rated as most unpleasant (negative > neutral > positive; ps < 0.05 for all comparisons); however, the drug did not affect these ratings (drug and drug-by-emotion; ps > 0.05). For post-scanning valence ratings, there was a main effect of affect condition on valence rating (F(2,26) = 193.23, p < 0.001); similar to on-line ratings, post-scanning valence ratings of negative images were rated as significantly more unpleasant than positive and neutral images (ps < 0.05 for all comparisons). However, the drug did not affect either post-scan valence or arousal (drug, drug-by-emotion ps > 0.05).
Neuroimaging results
Within our a priori regions THC significantly reduced activity in the subgenual ACC (sgACC; BA25) (peak MNI coordinate [2, 16, −12]; volume = 280 mm3; Z = 3.82, p = 0.005, small volume corrected) (Table 1; Fig. 3a). However, this effect was not specific to valence of the pictures, and the drug did not affect activity in the amygdala. Follow-up analyses on extracted BOLD signals (parameter estimates, β weights in arbitrary units [a.u.] of activation) from functional clusters that fell within the anatomically defined sgACC from the main effect of drug revealed increased sgACC activation to both positive and negative images in PBO, which was significantly diminished during the THC session, but only during negative images (Fig. 3b; t(14) = −3.89, p = 0.002, corrected; mean β ± SEM: PBO, 0.20 ± 0.06 vs. THC, −0.10 ± 0.06); THC had no significant effect on sgACC activation during positive images (Fig. 3b; t(14) = −1.53, p = 0.15; mean β ± SEM: PBO, 0.18 ± 0.06 vs. THC, 0.03 ± 0.07).
Table 1.
Whole Brain Voxel-Wise ANOVA for the main effect of drug and drug × valence interaction
| Session | Brain Region | Laterality | Volume (mm3) | Z score | MNI coordinates
|
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Drug | |||||||
| Middle occipital gyrus | L | 5,056 | 4.58 | −40 | −70 | 22 | |
| sgACC | R | 280 | 3.82 | 2 | 16 | −12 | |
| Postcentral gyrus | R | 2,928 | 3.37 | 36 | −40 | 68 | |
| R | 1,400 | 3.03 | 52 | −22 | 36 | ||
| L | 360 | 2.99 | −60 | 2 | 14 | ||
| L | 312 | 2.83 | −34 | −30 | 42 | ||
| L | 176 | 2.82 | −50 | −28 | 58 | ||
| R | 176 | 2.79 | 34 | −30 | 40 | ||
| Superior medial frontal gyrus | L | 304 | 3.27 | −10 | 28 | 62 | |
| Superior parietal gyrus | L | 568 | 3.23 | −14 | −48 | 76 | |
| L | 512 | 3.17 | −18 | −78 | 56 | ||
| Superior frontal gyrus | R | 4,064 | 3.20 | 32 | −8 | 58 | |
| Cerebellum | R | 208 | 3.10 | 4 | −38 | −16 | |
| R | 176 | 2.83 | 36 | −56 | −26 | ||
| Calcarine fissure | R | 584 | 2.77 | 28 | −58 | 4 | |
| Drug × Valence | |||||||
| No significant activations | |||||||
A priori ROIs shown in bold and significant at p <0.05, small volume corrected for multiple comparisons within an anatomically defined mask (clusters with >20 contiguous voxels). All other activations shown at p <0.005, uncorrected (clusters with >20 contiguous voxels)
MNI Montreal Neurological Institute, sgACC, subgenual anterior cingulate cortex
Fig. 3.
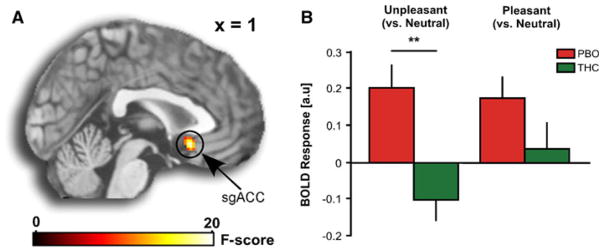
Effects of THC on brain activity. a Whole-brain voxel-wise statistical F maps overlaid on a canonical brain rendering (MNI sagittal, x-plane = 0) showing the ANOVA main effect of drug. Activations are displayed at whole-brain voxel-wise p < 0.005, uncorrected; color bar represents statistical F scores. b Mean BOLD response (β weights ± SEM) from sgACC showing activation to negative and positive (>neutral) images in the PBO session and attenuation of sgACC activity to negative (>neutral) images, but no effect on sgACC activation to positive (>neutral images) during the THC session; double asterisk significant at p < 0.05, corrected for multiple comparisons
In addition, to obviate bias and for subsequent generation of additional hypothesis, we report all activation results in brain regions other than our a priori areas in Table 1. We followed up the voxel-wise main effect of drug by looking at the within-session and between-session contrasts during negative images (>neutral) and confirmed sgACC activation during the PBO session and the absence of this activation during the THC session (Fig. 4). To explore if a relationship exists between THC-induced change in sgACC activation (PBO > THC) and THC-induced change in subjective effects (THC > PBO), we conducted both linear and non-linear regression analyses, but did not observe any significant correlations between change in sgACC activation and changes in reported subjective drug effects (DEQ “high” and “feel,” VAS “high,” and “ARCI-M”) related to drug at the 90, 210, and 270 min time points during which time THC produced significant increases in those subjective ratings (ps > 0.05 for all comparisons).
Fig. 4.
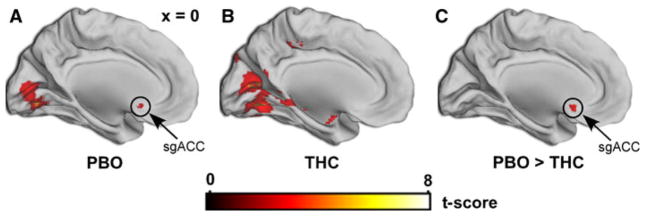
THC effects on sgACC activation to negative affect. Within-session whole-brain voxel-wise statistical t maps overlaid on a canonical brain rendering (MNI sagittal, x-plane = 0) showing sgACC activation to negative (>neutral) images is present during the PBO session [(0, 18, −10); volume = 192 mm3; Z = 3.01, p = 0.05, corrected] (a), but is absent during the THC session (b). c Between-session statistical t map showing less sgACC reactivity in the THC session [PBO > THC; (0, 16, −10); volume = 320 mm3; Z = 3.37; p = 0.02, corrected]. Activations are displayed at whole-brain voxel-wise p < 0.005, uncorrected; color bar represents statistical t scores
Discussion
Using a placebo-controlled THC administration (a CB1 receptor agonist) and a validated task to evoke negative emotions and probe cortico-limbic function, we observed that THC significantly reduced sgACC activation to pictures depicting negatively valenced content in a group of non-addicted, recreational marijuana users. This result fits with the notion that THC and other exogenous cannabinoids may have centrally mediated effects on negative mood processes, and supports the idea that endogenous cannabinoids play a role in modulating negative affect.
The sgACC is involved in emotion processing and awareness, especially the experience of negative affect (e.g. sadness) (Bush et al. 2000; Mayberg et al. 1999; George et al. 1995; Pizzagalli 2011). Patients with depressed mood have been shown to exhibit exaggerated reactivity to negative affect challenges and sustained hypermetabolic activity at rest in sgACC (Drevets et al. 2008; Drevets et al. 1997; Pizzagalli 2011) and successful treatment of depression is correlated with normalization of sgACC activation (Mayberg 2003; Mayberg et al. 2005). Evidence exists to suggest that CB1 receptor agonism and higher endocannabinoid tone produce behavior in animal models of depression that are indicative of an “anti-depressant” effect (Dubreucq et al. 2010; Hayase 2007; Gobbi et al. 2005). The findings here extend prior evidence of a central mechanism by which THC and other cannabinoid agonists could have mood altering effects in humans (Bhattacharyya et al. 2010; Fusar-Poli et al. 2010; Fusar-Poli et al. 2009; Cornelius et al. 2010; Phan et al. 2008).
This study should be considered preliminary, and several key related questions remain unanswered. Our small and selected sample may have limited our ability to detect THC’s effects on activity in our other a priori ROIs (e.g., amygdala) during negative and positive affect induction. We did observe a decrease in sgACC reactivity to positive images during the THC session, albeit not significant, and it is possible that a larger sample size would provide adequate power to detect an effect of THC to positive affect. We chose to administer a low dose of THC in this initial study, and thus, future studies are needed to determine dose-dependent effects and lend greater support to the inference for a direct effect of THC on sgACC reactivity in relation to dose (Paulus et al. 2005). The lack of THC-induced subjective and behavioral effects in the present study is consistent with prior studies on THC and other mood-modulating agents (Paulus et al. 2005; Harmer et al. 2006; Phan et al. 2008) but limits our ability to make inferences about the possible mood elevating effects of THC and whether THC-mediated effects on brain responding are related to changes in negative mood (i.e., attenuation).
In summary, our data demonstrate a significant and selective impact of the cannabinoid agonist THC on sgACC reactivity during negative affect. The current results provide a brain “target” for THC’s mood elevating effects and extend prior findings implicating a cortico-limbic, emotion-related central mechanism underlying cannabinoid function.
Acknowledgments
This research was supported in part by the Brain Research Imaging Center at University of Chicago and National Institutes of Health (NIH) Grants MH076198 (K.L.P.), DA024197 (K.L.P.), DA002812, and DA009133 (H.d.W.). The authors would like to acknowledge Jamie Golden for her assistance in participant recruitment, screening, and data collection.
Contributor Information
Christine A. Rabinak, Department of Psychiatry, University of Michigan, Rachel Upjohn Building, 4250 Plymouth Road, Box 5765, Ann Arbor, MI 48109-2700, USA. Mental Health Service, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, MI 48105, USA
Chandra Sekhar Sripada, Department of Psychiatry, University of Michigan, Rachel Upjohn Building, 4250 Plymouth Road, Box 5765, Ann Arbor, MI 48109-2700, USA.
Mike Angstadt, Department of Psychiatry, University of Michigan, Rachel Upjohn Building, 4250 Plymouth Road, Box 5765, Ann Arbor, MI 48109-2700, USA.
Harriet de Wit, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL 60637, USA.
K. Luan Phan, Email: luan@umich.edu, Department of Psychiatry, University of Michigan, Rachel Upjohn Building, 4250 Plymouth Road, Box 5765, Ann Arbor, MI 48109-2700, USA. Mental Health Service, Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, MI 48105, USA. Neuroscience Program, University of Michigan, Ann Arbor, MI 48109, USA.
References
- Bhattacharyya S, Morrison PD, Fusar-Poli P, Martin-Santos R, Borgwardt S, Winton-Brown T, Nosarti C, OCCM, Seal M, Allen P, Mehta MA, Stone JM, Tunstall N, Giampietro V, Kapur S, Murray RM, Zuardi AW, Crippa JA, Atakan Z, McGuire PK. Opposite effects of delta-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology. 2010;35(3):764–774. doi: 10.1038/npp.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. pii:S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chait LD, Fischman MW, Schuster CR. ‘Hangover’ effects the morning after marijuana smoking. Drug Alcohol Dep. 1985;15(3):229–238. doi: 10.1016/0376-8716(85)90002-x. [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Aizenstein HJ, Hariri AR. Amygdala reactivity is inversely related to level of cannabis use in individuals with comorbid cannabis dependence and major depression. Addict Behav. 2010;35(6):644–646. doi: 10.1016/j.addbeh.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med. 1998;49:341–361. doi: 10.1146/annurev.med.49.1.341. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency decreases wheel-running activity: consequences on emotional behaviours and hippocampal neurogenesis. Exp Neurol. 2010;224(1):106–113. doi: 10.1016/j.expneurol.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Eggan SM, Lewis DA. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: a regional and laminar analysis. Cereb Cortex. 2007;17(1):175–191. doi: 10.1093/cercor/bhj136. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuro Image. 1995;2(2):166–172. doi: 10.1006/nimg.1995.1019. pii:S1053811985710191. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Crippa JA, Bhattacharyya S, Borgwardt SJ, Allen P, Martin-Santos R, Seal M, Surguladze SA, O’Carrol C, Atakan Z, Zuardi AW, McGuire PK. Distinct effects of {delta}9-tetrahydrocannabinol and cannabidiol on neural activation during emotional processing. Arch Gen Psychiatry. 2009;66(1):95–105. doi: 10.1001/archgenpsychiatry.2008.519. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Allen P, Bhattacharyya S, Crippa JA, Mechelli A, Borgwardt S, Martin-Santos R, Seal ML, O’Carrol C, Atakan Z, Zuardi AW, McGuire P. Modulation of effective connectivity during emotional processing by Delta 9-tetrahydrocannabinol and cannabidiol. Int J Neuropsychopharmacol. 2010;13(4):421–432. doi: 10.1017/S1461145709990617. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Dipasquale P, Romano A, Righetti L, Cassano T, Piomelli D, Cuomo V. The endocannabinoid system as a target for novel anxiolytic and antidepressant drugs. Int Rev Neurobiol. 2009;85:57–72. doi: 10.1016/S0074-7742(09)85005-8. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, Cassano T, Morgese MG, Debonnel G, Duranti A, Tontini A, Tarzia G, Mor M, Trezza V, Goldberg SR, Cuomo V, Piomelli D. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102(51):18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Rogowska J, Yurgelun-Todd DA. Altered affective response in marijuana smokers: an FMRI study. Drug Alcohol Depend. 2009;105(1–2):139–153. doi: 10.1016/j.drugalcdep.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall W, Solowij N. Adverse effects of cannabis. Lancet. 1998;352(9140):1611–1616. doi: 10.1016/S0140-6736(98)05021-1. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, Mackay CE, Reid CB, Cowen PJ, Goodwin GM. Antidepressant drug treatment modifies the neural processing of nonconscious threat cues. Biol Psychiatry. 2006;59(9):816–820. doi: 10.1016/j.biopsych.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Hayase T. Chronologically overlapping occurrences of nicotine-induced anxiety- and depression-related behavioral symptoms: effects of anxiolytic and cannabinoid drugs. BMC Neurosci. 2007;8:76. doi: 10.1186/1471-2202-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. The endocannabinoid system and the treatment of mood and anxiety disorders. CNS Neurol Disord Drug Targ. 2009;8(6):451–458. doi: 10.2174/187152709789824624. pii:HT-2(Puffenbarger) [DOI] [PubMed] [Google Scholar]
- Hollister LE. Health aspects of cannabis. Pharmacol Rev. 1986;38(1):1–20. [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preference and mood in humans: repeated assessment of d-amphetamine. Pharmacol Biochem Behav. 1981;14(2):159–163. doi: 10.1016/0091-3057(81)90237-9. [DOI] [PubMed] [Google Scholar]
- Kirk JM, de Wit H. Responses to oral delta9-tetrahydrocannabinol in frequent and infrequent marijuana users. Pharmacol Biochem Behav. 1999;63(1):137–142. doi: 10.1016/s0091-3057(98)00264-0. [DOI] [PubMed] [Google Scholar]
- Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuro Image. 2008;42(2):998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Buthbert BN. Technical Report A-8. University of Florida; Gainsville: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Mailleux P, Parmentier M, Vanderhaeghen JJ. Distribution of cannabinoid receptor messenger RNA in the human brain: an in situ hybridization histochemistry with oligonucleotides. Neurosci Lett. 1992;143(1–2):200–204. doi: 10.1016/0304-3940(92)90265-9. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12(2):245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21(11):1735–1742. doi: 10.1016/s0196-9781(00)00324-7. pii:S0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Castillo G, Simmons AN, Stein MB. Dose-dependent decrease of activation in bilateral amygdala and insula by lorazepam during emotion processing. Arch Gen Psychiatry. 2005;62(3):282–288. doi: 10.1001/archpsyc.62.3.282. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. NeuroImage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28(10):2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2010;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183–206. doi: 10.1038/npp.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. pii:S0306452297004363. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H. Comparison of the subjective effects of Delta (9)-tetrahydro-cannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161(4):331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, MARINA Vaitl D. An easy tool for the creation for MAsks for Region of Interest Analyses. 9th international conference on functional mapping of the human brain, NeuroImage; June 2003; New York. 2003. pp. 19–22. [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiography and in situ hybridization histochemistry study of normal aged and Alzheimer’s brains. Neuroscience. 1994;63(3):637–652. doi: 10.1016/0306-4522(94)90511-8. pii:0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Zink CF, Stein JL, Kempf L, Hakimi S, Meyer-Lindenberg A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J Neurosci. 2010;30(20):7017–7022. doi: 10.1523/JNEUROSCI.4899-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]