Abstract
The prevalence of stress disorders differs between men and women. An understanding of how men and women vary in acute stress responses may help to understand these sex differences. We compared responses to the TSST and a control task in healthy men (N=28) and women tested in two phases (Follicular N=29, Luteal N=23) of the menstrual cycle. Men exhibited greater cortisol responses to stress than women in either phase. Luteal women exhibited the greatest subjective and allopregnanolone responses to stress, whereas follicular women exhibited blunted noradrenaline responses. Partial correlations controlling for group differences revealed that individuals who were most sensitive to the subjective effects of stress exhibited the largest salivary cortisol, noradrenaline, and allopregnanolone responses and the smallest progesterone responses to stress. We discuss our findings in the context of sex differences in the prevalence of stress-linked disorders.
Descriptors: TSST, Stress, Sex differences, Cardiovascular, Hormones, Mood
Stress contributes to many diseases, including not only cardiovascular, infectious, autoimmune, and mood disorders (al’Absi & Wittmers, 2003; Godbout & Glaser, 2006; Harbuz, Richards, Chover-Gonzalez, Marti-Sistac, & Jessop, 2006; Krishnan & Nestler, 2008; Reagan, Grillo, & Piroli, 2008) but also substance use disorders and relapse to drug abuse (Sinha, 2008). Inter-individual variation in responses to stress are likely to contribute to risk for these stress-linked disorders. For example, there is evidence that men and women vary in responses to acute stress (Kajantie & Phillips, 2006), and men and women also differ in the prevalence of stress-linked disease (Carter-Snell & Hegadoren, 2003). Thus, investigating sex differences in responses to acute stress may advance our understanding of the pathogenesis of stress-related disease.
Acute stress initiates a complex sequence of physiological and psychological events that may differ between men and women. First, activation of the sympathetic nervous system causes release of catcholamines and subsequent increases in heart rate and blood pressure, and activation of the hypothalamic-pituitary-adrenal (HPA) axis, causes secretion of corticotrophic releasing hormone (CRH), adrenocorticotrophic hormone (ACTH), and glucocorticoids including cortisol (Chrousos & Gold, 1992; Herman et al., 2003; Lopez, Akil, & Watson, 1999; Munck, Guyre, & Holbrook, 1984). Acute stress also induces emotional reactions such as increases in anxiety and irritability, which may occur independently of sympathetic or HPA axis responses. There is some debate about whether the sympathetic, HPA axis, and subjective components of the stress response are distinct events or aspects of a coordinated integrated response (Schommer, Hellhammer, & Kirschbaum, 2003; Schlotz et al., 2008). However, most studies of responses to acute stress focus upon only one or two of these measures, making it difficult to study the relationship between measures. Thus, studies that include each of these components of the stress response are needed to investigate their interrelationships and to understand how variation in stress responses, including sex differences, influences the progression toward disease.
Studies of sex differences in stress responses have yielded mixed results (see Kajantie & Phillips, 2006 for review) in part because of differences in the procedures used to invoke stress between laboratories (Dickerson & Kemeny, 2004; Kajantie & Phillips, 2006; Krantz & Manuck, 1984; Kudielka & Kirschbaum, 2005; Lundberg, 2005; Stroud, Salovey, & Epel, 2002; Uhart, Chong, Oswald, Lin, & Wand, 2006). Recently, a single standardized procedure, the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993a), has been adopted by many laboratories and facilitates comparisons across sites. The TSST incorporates elements of social-evaluative threat (public speech) and achievement (mental arithmetic) and increases cortisol, heart rate, blood pressure, and subjective reports of anxiety amongst men and women alike (Dickerson & Kemeny, 2004; Kudielka et al., 2004a; Williams, Hagerty, & Brooks, 2004). In direct comparisons of men and women, there is some evidence that women experience more pronounced emotional responses to the TSST and similar tasks (Steptoe, Fieldman, Evans, & Perry, 1996; Kirschbaum et al., 1999; Kelly, Tyrka, Anderson, Price, & Carpenter, 2008) whereas men exhibit larger blood pressure responses (Lepore, Allen, & Evans, 1993; Steptoe et al., 1996; Matthews, Gump, & Owens, 2001). However, sex differences in heart rate and HPA axis responses to speech tasks are less consistent. Some studies reported no sex differences in heart rate responses (Kirschbaum et al., 1999; Kelly et al., 2008), whilst others report that women exhibit greater heart rate increases (Kudielka et al., 2004b). In addition, in some studies ACTH (Kudielka et al., 1998; Kirschbaum et al., 1999; Uhart et al., 2006), plasma cortisol (Kudielka et al., 1998; Uhart et al., 2006), and salivary cortisol (Kirschbaum, Wust, & Hellhammer, 1992; Kirschbaum, Klauer, Filipp, & Hellhammer, 1995a, Kirschbaum, Pirke, & Hellhammer, 1995b; Steptoe et al., 1996; Nicolson, Storms, Ponds, & Sulon, 1997; Kirschbaumet al., 1999, men vs. follicular women) responses are greater in men whereas others have found no sex differences in ACTH (Kudielka et al., 2004a; Back et al., 2008), plasma cortisol (Kirschbaum et al., 1999, men vs. luteal women; Kudielka et al., 2004a; Back et al., 2008; Kelly et al., 2008), or salivary cortisol (Girdler, Turner, Sherwood, & Light, 1990; Sgoifo et al., 2003; Kudielka et al., 2004a) responses. Together, these findings suggest that men and women may respond differently on certain stress response measures.
Comparing stress responses in men and women is complicated by variations related to menstrual cycle. Several studies have investigated responses to cognitive and stressful challenges during different phases of the menstrual cycle (Polefrone& Manuck, 1988; Stoney, Owens, Matthews, Davis, & Caggiula, 1990; Weidner& Helmig, 1990; Tersman, Collins, & Eneroth, 1991; Sita& Miller, 1996; Litschauer et al., 1998; Sato & Miyake, 2004; Carter& Lawrence, 2007; Hlavacova, Wawruch, Isonova, & Jezova, 2008), although only a few have employed the TSST or a similar speech task (Kirschbaumet al., 1995a; Kirschbaumet al., 1999; Pico-Alfonso et al., 2007). In general, these studies suggest that cardiovascular, subjective, and cortisol responses to stress do not vary across the menstrual cycle (Kirschbaumet al., 1995a; Kirschbaum et al., 1999; Pico-Alfonso et al., 2007), except for one study which found greater salivary cortisol responses to stress during the luteal phase (Kirschbaum et al., 1999). Thus, additional data are needed to assess the full profile of stress responses in women in different phases of the menstrual cycle.
The present study therefore sought to examine acute stress responses in healthy men and women, and in women in relation to menstrual cycle phase, upon a comprehensive range of physiological and subjective responses to the TSST. Based upon previous reports of responses to the TSST (see earlier), we hypothesized that men would exhibit greater cardiovascular and hormonal responses to stress whereas women, especially those tested in the luteal phase, would exhibit greater subjective discomfort.
Materials and Methods
Participants
Healthy non-smoking men (n=28) and women (n=52) aged 18–32 years were recruited from the University and surrounding community by flyers, newspaper, and university website advertisements without regard to race or ethnicity. Individuals who used regular medications (including hormonal contraceptives) and those with serious medical conditions, current or prior diagnosis of Axis I disorder (APA, 1994) including drug abuse or dependence, less than a high school education (for the purposes of completing standardized questionnaires), a body mass index outside of 19–26 kg/m2, high or low blood pressure (as defined by the U.S. Centers for Disease Control and Prevention), an abnormal electrocardiogram, or those who worked night shifts were excluded. Cigarette smokers were excluded since smoking alters responses to the TSST (al’Absi et al., 2003; Kirschbaum et al., 1993b; Childs & de Wit, 2009). Women who self-reported significant, i.e., problematic, premenstrual affective symptoms or an irregular cycle (abnormal menstruation in comparison to their normal cycle) within the last 3 months were excluded. Participants signed a consent form which stated that the aim of study was to investigate the effects of alertness upon mood and physiology. They were told that they would complete short verbal tasks during each session but were not told the specific nature of the tasks until they had completed the study.
Design
This study used a mixed within- (Stress vs. No stress) and between- subjects (Men N=28 vs. Follicular Women N=29 vs. Luteal Women N=23) design. Power analyses, based upon the inter-individual variation in stress responses seen in previous studies, indicated that group sizes of 20 would be sufficient to detect significant differences. Volunteers participated in two experimental sessions in which they underwent the TSST or a control task in randomized order. The sessions were conducted between 9 am and 1 pm at least 48 hours apart. Women were randomly assigned to menstrual phase condition; follicular women were tested between days 3 and 10 of their cycle, luteal women were tested between two and 10 days after a positive urine ovulation test. A 7-day time window was used for each phase so that women could schedule both experimental sessions within a single cycle and sessions were scheduled as near to the beginning of the time window as possible. Plasma levels of estrogen and progesterone at the start of each session were used to verify self-reported menstrual cycle phase (Griffin & Ojeda, 1996). As expected, plasma levels of estrogen were significantly higher in the luteal phase (99.6 ± 8.6 pg/ml) than the follicular phase (32.9 ± 2.5 pg/ml, t(16)=7.4 p<.001 d=1.9), as were levels of progesterone [luteal 5.3 ± 0.8 ng/ml, follicular 0.4 ± 0.2 ng/ ml, t(28)=6.1 p<.001 d=1.6].
Procedure
The University of Chicago Hospital’s Institutional Review Committee for the use of human subjects approved the protocol. Participants were tested individually in patient rooms at the University of Chicago Hospital’s General Clinic Research Center (GCRC) where they stayed overnight before each session to standardize sleeping and eating. Participants awoke at 7:30 am, ate breakfast at 8 am (including their usual serving of caffeine), catheters were inserted at 8:30 am and baseline measures obtained at 9:40 am (baseline measures). The sessions were conducted based upon standardized procedures as previously described (Childs & de Wit, 2009). Briefly, the TSST consisted of 5min public speaking and 5 min arithmetic (serial subtraction) performed in front of two examiners, who were unknown to the participant, and a video camera. Throughout the TSST, participants could see their own image portrayed on a television monitor. During the control condition (also 10 min), participants spoke to the Research Assistant about their interests and hobbies without a video camera. Inclusion of the control task allowed us to identify responses that are specific to psychosocial stress, not simply cognitive challenge or postural changes. Participants were given 10 min to prepare for each task. Participants were escorted to an adjacent room to perform the tasks which always began at 10 am. Intravenous catheters, for collection of blood samples for hormonal analysis, were placed 70 min prior to collection of baseline samples. Cardiovascular and hormonal measures were obtained 20min before and at 0, 5, 10, 20, 30, 60, 90, and 120min after the tasks. Self-reported mood was assessed 20 min before and at 0, 10, 20, 30, 60, 90, and 120 min after the tasks. The subjective questionnaires were completed within 5 min at each time point so individuals had an opportunity to rest between completions of the questionnaires. We and others have used these standardized questionnaires to assess mood repeatedly with no adverse effects. At the end of the second session, participants were fully debriefed about the specific purposes of the study and received payment ($200).
Cardiovascular Measures
Participants wore a Polar chest band and monitor that recorded heart rate continuously (Mini-Logger, Mini Mitter/Respironics, Bend, OR) and blood pressure was measured at repeated intervals using a monitor (Critikon Dinamap Plus Vital Signs Monitor, GE Healthcare Technologies, Waukesha, WI).
Hormonal Measures
Saliva samples were collected using Salivette cotton wads. The GCRC Core Laboratory at the University of Chicago determined levels of cortisol in saliva and plasma (Saliva=Salimetrics LLC [State College, PA], sensitivity=0.003 ug/dL; Plasma=Immulite/ Immulite 1000 Cortisol [Siemens Healthcare Diagnostics, Deer-field, IL], sensitivity=0.2 ug/dL). The University of Chicago Medical Endocrinology Laboratory determined levels of progesterone in plasma (Immulite 2000 Progesterone, sensitivity=0.1 ng/ mL). Dr. Richard Hauger at the University of California, San Diego, determined levels of plasma allopregnanolone according to the methods of Purdy et al., 1990. Mayo Medical Laboratories (Rochester, MN) determined levels of plasma catecholamines and estrogen [Test codes 8532 and 200255, sensitivity: noradrenaline=70 pg/mL, estradiol 15 pg/mL]. Unfortunately, plasma samples collected for ACTH assay were spoiled after a freezer malfunction, and insufficient data was obtained for meaningful statistical analysis.
Subjective Measures
Subjective mood was measured using the Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971). The 72-item POMS checklist yielded eight subscales; Tension-Anxiety, Depression-Dejection, Anger-Hostility, Vigor, Fatigue, Confusion, Friendliness, Elation.
Statistical Analysis
Demographic characteristics of the three groups were compared using one-factor analysis of variance (ANOVA, continuous variables) and Pearson’s chi-square test (categorical variables). Baseline differences between the groups were assessed using one-factor (Group) ANOVA upon the average of the pre-task values during each session (since there were no significant session differences or effects of session order).
The effects of the tasks upon cardiovascular, hormonal, and subjective data across time were assessed using three-factor (Task and Time as within-subjects factors, Group as between-subjects factor) ANOVA for repeated measures. Analyses were conducted upon mean absolute scores at each time point. For continuous heart rate data, the mean heart rate during successive 10-min periods was calculated. Since there were differences at baseline for some measures, three-factor (Task*Time*Group) repeated measures ANOVAs were also performed upon data expressed as a change from the pre-task baseline at each time point and two-factor (Task*Group) repeated measures ANOVAs were performed upon peak change scores, i.e., the maximum positive or negative change from the pretask baseline. Any differences between the analyses are detailed in the results section. Effect sizes are reported using partial eta squared (ηp2) for analyses of variance; 0.01, 0.06, and 0.14 are considered, respectively, small, medium, and large effect sizes.
All analyses were conducted using SPSS version 14 (SPSS Inc., Chicago, IL) for Windows. Missing cases (due to equipment malfunction, problem with sample collection) were deleted list wise, leading to smaller sample sizes for some analyses. Due to budgetary constraints, analysis of plasma noradrenaline, progesterone, and allopregnanolone was performed in a subset of participants, thus subject numbers for these analyses were smaller (Men N=20, Follicular women N=18, Luteal women N=14). Repeated measures ANOVAs were performed with Greenhouse Geisser correction when violations of sphericity were detected. All significant findings remained significant when thus adjusted, and therefore we report degrees of freedom from uncorrected analyses.
Results
Demographic Characteristics
Most participants were European American college students in their early twenties (Table 1). Men consumed more alcoholic drinks per week than luteal women [F(2,79)=5.5 p<.01, Bonferroni post hoc p<.01] but not follicular women (Bonferroni post hoc p>.05), and more men reported having ever used marijuana than women in either phase [Men vs. Follicular women Pearson χ2(2)=9.5 p<.01, Men vs. Luteal Women χ2(2)=21.7 p<.001]. The groups did not differ upon other demographic characteristics.
Table 1.
Demographic Characteristics of Participants
| Demographic characteristic | Male | Female Follicular | Female Luteal |
|---|---|---|---|
| N | 28 | 29 | 23 |
| Age (mean ± SEM) | 21.8 ± 0.7 | 22.1 ± 0.8 | 21.6 ± 0.7 |
| BMI (mean ± SEM) | 22.4 ± 0.4 | 22.3 ± 0.4 | 22.2 ± 0.3 |
| Education (%) | |||
| High school | 7 | 17 | 13 |
| Partial college degree | 68 | 52 | 57 |
| College degree | 21 | 28 | 26 |
| Advanced degree | 4 | 3 | 4 |
| Full time student (%) | 75 | 69 | 83 |
| Race (%) | |||
| European American | 54 | 52 | 74 |
| African American | 21 | 38 | 5 |
| Asian American | 18 | 3 | 17 |
| Other | 7 | 7 | 4 |
| Current drug use (mean ± SEM) | |||
| Alcoholic drinks/wk* | 4.4 ± 1.0 | 2.2 ± 0.6 | 1.2 ± 0.4 |
| Caffeine drinks/wk | 4.5 ± 1.5 | 5.7 ± 1.6 | 8.3 ± 2.8 |
| Lifetime drug use (% ever used) | |||
| Stimulants | 7 | 7 | 0 |
| Sedatives | 0 | 3 | 0 |
| Hallucinogens | 14 | 14 | 0 |
| Opiates | 36 | 45 | 30 |
| Marijuana* | 89 | 62 | 30 |
| Inhalants | 4 | 3 | 0 |
Notes: Asterisks indicate a significant difference between males and females tested in each phase, p<.001.
indicates a significant group difference (see Results section: Demographic Characteristics).
Baseline Differences
Before the tasks, men exhibited higher systolic blood pressure [Group F(2,77)=30.5 p<.001] than women tested in either phase (Bonferroni post hoc test p<.05 for heart rate, p<.001 for systolic blood pressure). Men also exhibited significantly higher diastolic blood pressure [Group F(2,77)=4.2 p<.05] than luteal women (Bonferroni post hoc test p<.05) but not follicular women (Bonferroni post hoc test p<.06). Luteal women demonstrated significantly higher levels of plasma progesterone [Group F(2,50)=47.9 p<.001] and allopregnanolone [Group F(2,48)=27.7 p<.001] than follicular women (Bonferroni post hoc test ps<.001) and men (Bonferroni post hoc test ps<.001). At baseline, luteal women scored higher upon the POMS Tension- Anxiety [Group F(2,76)=4.6 p<.05] and Fatigue scales [Group F(2,76)=6.4 p<.006] than follicular women (Bonferroni post hoc tests, respectively, p<.05, p<.01) but not men (Bonferroni post hoc tests, respectively, p=.09, p>.3).
Effects of Stress
Cardiovascular measures
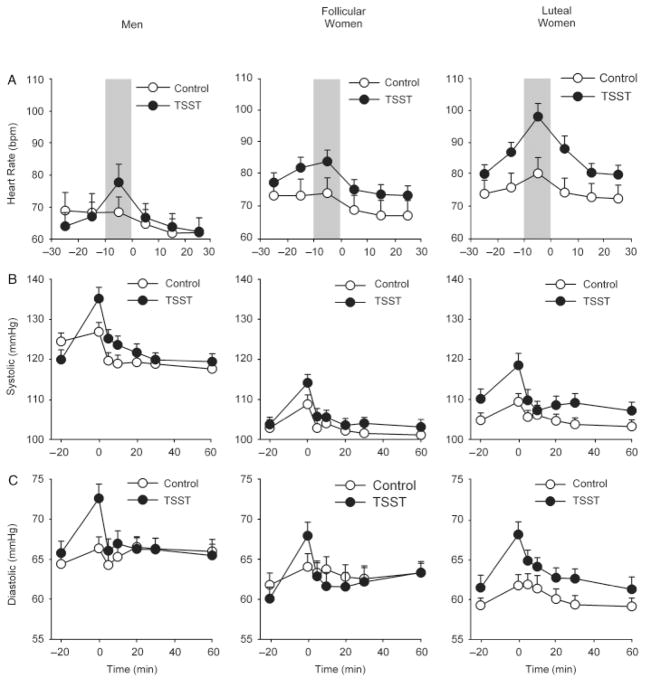
In comparison to the control task, the TSST significantly increased heart rate [Task*Time F(5,290)=7.0 p<.001 ], systolic [Task*Time F(6,462)=4.5 p<.001 ] and diastolic blood pressure [Task*Time F(6,462)=5.5 p<.001 ] similarly in all three groups [Figure 1; Task*Time*Group heart rate F(10,290)=0.9 p=.5 ; systolic blood pressure F(12,462)=1.6 p=1.6 ; diastolic blood pressure F(12,462)=0.5 p=.9 ]. Luteal women exhibited significantly higher heart rate overall in comparison to men [Group F(2,58)=3.8 p<.05 , Bonferroni post hoc test p<.05] but not follicular women (Bonferroni post hoc test p>.3). In line with differences at baseline, men exhibited significantly higher systolic blood pressure in comparison to women tested in either phase [Group F(2,77)=41.3 p<.001 ηp2 =0.52, Bonferroni post hoc test p<.001] and significantly higher diastolic blood pressure than luteal women [Group F(2,77)=4.0 p<.05 , Bonferroni post hoc test p<.05] but not follicular women (Bonferroni post hoc test p=.1). Analysis of change from baseline and peak change scores did not alter the results.
Figure 1.
Changes in heart rate (A) and systolic (B) and diastolic (C) blood pressure after the TSST (filled symbols) and control task (open symbols) for men (N=28), follicular women (N=29) and luteal women (N=23). Data represent mean ± SEM for i) heart rate averaged across successive 10-min periods, and ii) blood pressure as a function of time (Task preparation from −20 to −10 min, task performance from −10 to 0 min).
Hormonal measures
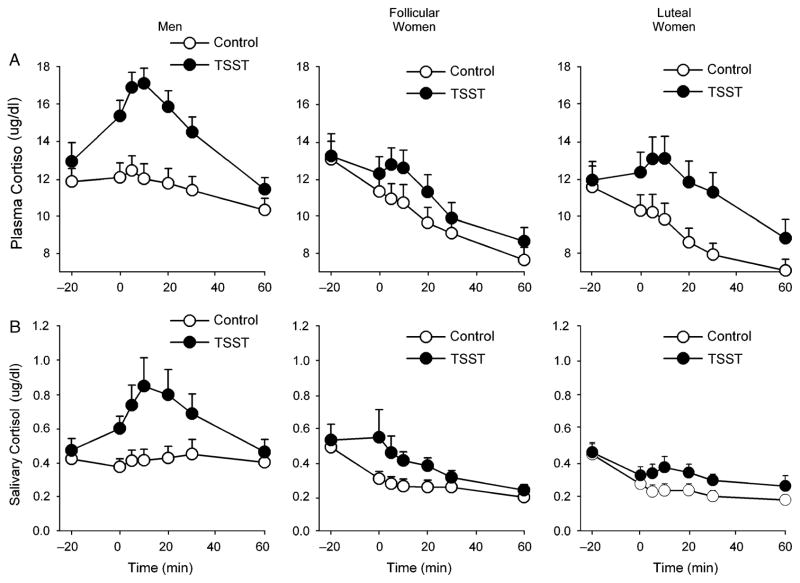
Salivary cortisol responses to the TSST differed among the groups [Task*Time*Group F(12,438)=1.8 p<.05 , Figure 2]. Simple effects analyses revealed that there was a greater, more significant difference between cortisol levels after the stressful and non-stressful tasks in men [Task F(1,24)=16.5 p<.001 ηp2 =0.41] than in women tested in either the follicular [Task F(1,28)=4.3 p<.05 ηp2 =0.13] or luteal phases [Task F(1,21)=6.2 p<.05 ηp2 =0.23]. There was a trend to a similar difference between groups in levels of plasma cortisol after the tasks, although this did not reach significance [Task*Time*Group F(12,420)=1.6 p<.09 ηp2 =0.04, Figure 2]. Analysis of change from baseline and peak change scores did not alter the results for salivary cortisol responses, however, there was a significant Task*Time*Group interaction for plasma cortisol change scores [F(10,365)=2.8 p<.01 ηp2 =0.07]. Simple effects analyses revealed that only men exhibited a significant cortisol response to the TSST [Task*Time F(5,135)=6.5 p<.001 ηp2 =0.27; effect sizes for main effect of task, respectively, 0.09 and 0.15 for follicular and luteal women, ps>.1].
Figure 2.
Changes in plasma (A) and salivary (B) cortisol after the TSST (filled symbols) and control tasks (open symbols) for men (N=28), follicular women (N=29) and luteal women (N=23). Data represent mean ± SEM as a function of time after the task (Task preparation from −20 to −10 min, task performance from −10 to 0 min).
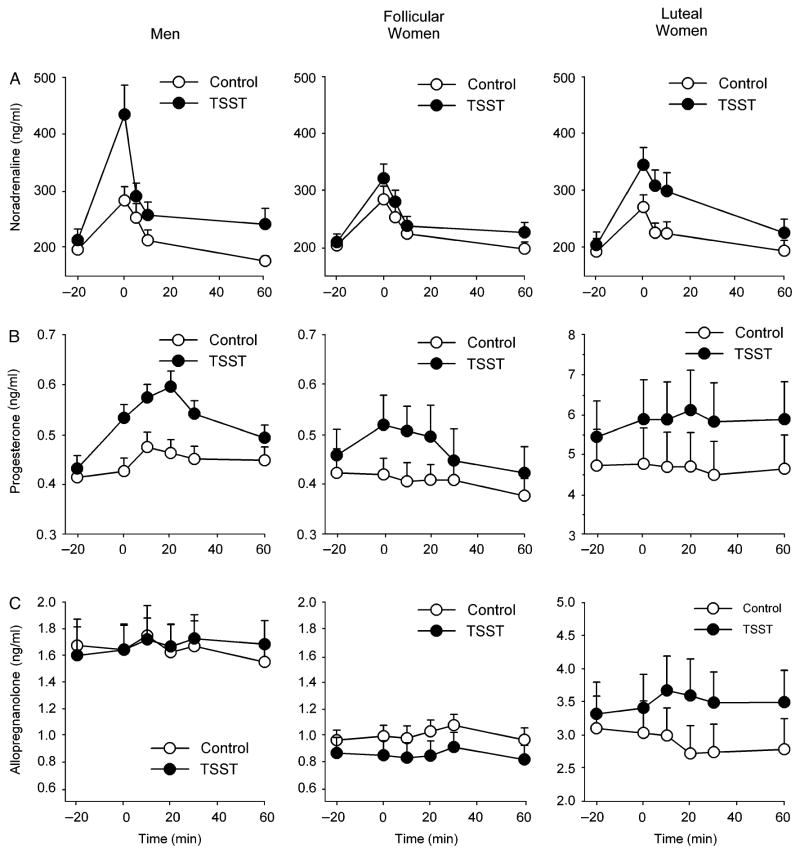
There were significant group differences in the effects of the tasks upon plasma noradrenaline [Task*Time*Group F(8,188)=2.0 p<.05 ηp2 =0.08, Figure 3]. Simple effects analyses revealed a significant main effect of task upon noradrenaline among men [Task F(1,19)=13.0 p<.01 ηp2 =41] and luteal women [Task F(1,12)=5.6 p<.05 ηp2 =0.32] but not among follicular women [Task F(1,16)=1.3 p>.2 ηp2 =0.08]. Similarly, there were group differences in the effects of the tasks upon plasma progesterone [Task*Time*Group F(10,230)=1.9 p<.05 ] and allopregnanolone [Task*Time*Group F(10,225)=2.1 p<.05 ηp2 =0.09, Figure 3]. The TSST significantly increased plasma progesterone among men [Task F(1,19)=18.6 p<.001 ηp2 =0.50] and follicular women [Task F(1,14)=5.0 p<.05 ηp2 =0.26] but not luteal women [Task F(1,13)=2.2 p>.1 ηp2 =0.14]. Stress significantly influenced plasma allopregnanolone among luteal women [Task*Time F(5,55)=2.8 p<.05 ηp2 =0.20] and there was a trend to decreased allopregnanolone among follicular women [Task*Time F(1,17)=4.2 p=.055 ηp2 =0.20] but levels among men were unchanged [Task F(1,17)=0.02 p>.8 ηp2 =0.001]. Analysis of change from baseline and peak change scores for noradrenaline, progesterone, and allopregnanolone did not alter the results.
Figure 3.
Changes in plasma noradrenaline (A), progesterone (B), and allopregnanolone (C) after the TSST (filled symbols) and control tasks (open symbols) for men (N=20), follicular women (N=18) and luteal women (N=14). Data represent mean ± SEM as a function of time after the task (Task preparation from −20 to −10 min, task performance from −10 to 0 min).
Subjective measures
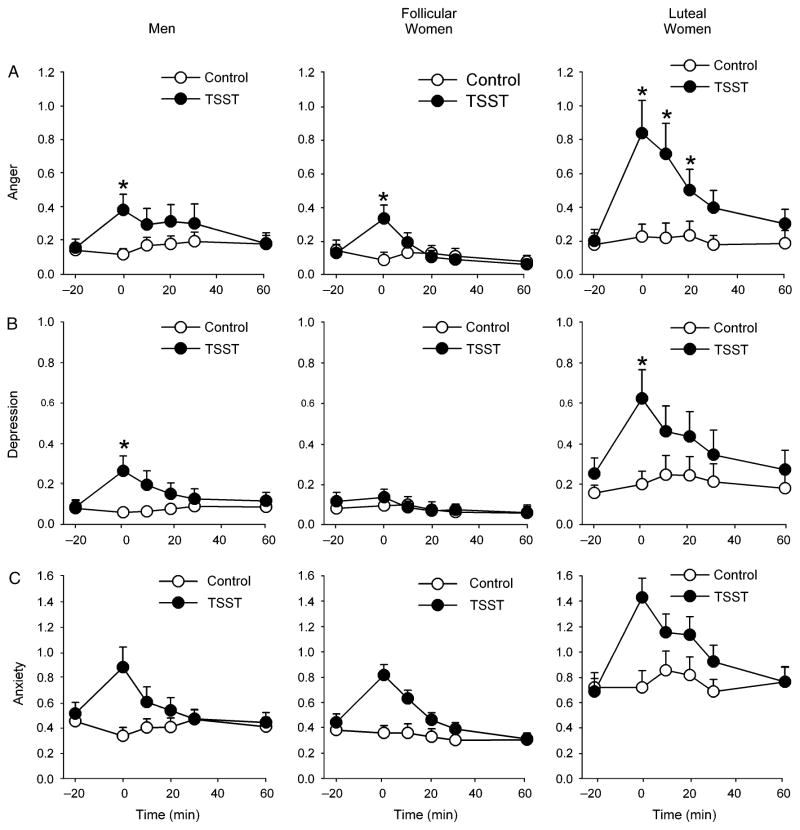
The TSST significantly increased scores upon the POMS Anger-Hostility [F(5,380)=14.2 p<.001 ηp2 =0.16], Tension-Anxiety [Task*Time F(5,380)=22.1 p<.001 ηp2 =0.23], Confusion [Task*Time F(5,380)=10.7 p<.001 ηp2 =0.12], and Depression-Dejection subscales [Task*Time F(5,380)=7.9 p<.001 ηp2 =0.09], and decreased scores upon the POMS Elation [Task*Time F(5,380)=14.7 p<.001 ηp2 =0.16], Friendliness [Task*Time F(5,380)=20.3 p<.001 ηp2 =0.21], and Vigor subscales [Task*Time F(5,380)=5.5 p<.001 ηp2 =0.07]. There were differences between groups in the effects of stress upon the Anger-Hostility [Task*Time*Group F(10,380)=1.9 p<.05 ηp2 =0.05] and Depression-Dejection [Task*Time*Group F(10,380)=2.1 p<.05 ηp2 =0.05] scales (Figure 4). Post hoc analyses revealed that women in the luteal phase exhibited more prolonged increases in ratings of anger and hostility than women in the follicular phase and men; luteal women exhibited significantly elevated Anger-Hostility scores for 20 min after the TSST whereas scores for men and follicular women were not significantly different from baseline 10min after the task (see Figure 4). In contrast, simple effects analyses revealed that women in the follicular phase did not exhibit stress-induced changes in ratings of feeling depressed [Task*Time F(5,140)=1.1 p>.3 ηp2 =0.04] whereas stress increased scores among men [Task*Time F(5,130)=6.5 p<.001 0.20] and luteal phase women [Task*Time F(5,110)=3.0 p<.05 0.12].
Figure 4.
Changes in POMS Anger (A), Depression (B), and Anxiety (C) after the TSST (filled symbols) and control tasks (open symbols) for men (N=28), follicular women (N=29) and luteal women (N=23). Data represent mean ± SEM as a function of time after the task (Task preparation from −20 to −10 min, task performance from −10 to 0min). Asterisks represent a significant difference from pre-task baseline value at −20 min (Student’s paired t-test corrected for multiple comparisons, p<.05).
In line with differences at baseline, luteal women scored higher overall upon the POMS Tension-Anxiety subscale [Group F(2,76)=11.9 p<.001 ηp2 =0.24] than the other groups (Bonferroni post hoc test ps<.01). Luteal women also scored higher upon the POMS Confusion scale [Group F(2,76)=6.1 p<.01 ηp2 =0.14] after each task than follicular women (Bonferroni post hoc test p<.01) but not men.
Analysis of peak change from baseline scores confirmed these findings; women tested in the luteal phase exhibited significantly larger increases in Anger [Group F(2,76)=4.7 p<.01 ηp2 =0.11], Depression [Group F(2,76)=6.6 p<.01 ] and Anxiety [Group F(2,76)=6.1 p<.01 ηp2 =0.14] than follicular women (Bonferroni post hoc ps<.01) and men (Bonferroni post hoc ps<.05 for Depression and Anxiety only, p>.1 for Anger).
Correlational analyses
Partial correlations between the outcome measures controlling for Group revealed some interesting relationships. As expected, salivary and plasma cortisol responses to stress were correlated (r2=0.64 p<.001), as were systolic and diastolic blood pressure responses (r2=0.35 p=.05) and systolic blood pressure responses were correlated with heart rate responses (r2=0.40 p<.05). Subjective sensitivity to stress was associated with hormonal responses; negative mood was positively correlated with salivary cortisol (Elation r2= −0.37 p<.05, Fatigue r2=0.40 p<.05, Anxiety r2=0.3 p=.08), allopregnanolone (Anxiety r2=0.36 p<.05, Anger r2=0.44 p<.05, Depression r2=0.34 p<.06) and noradrenaline (Anger r2=0.36 p<.05) responses, and negatively correlated with progesterone responses (Anger r2= −0.36 p<.05, Friendliness r2=0.38 p<.05, Depression r2= minus;0.33 p<.07). Allopregnanolone responses to stress were not significantly associated with progesterone responses (r2=0.06 p=.76), although they were positively correlated with noradrenaline responses (r2=0.37 p<.05) and marginally positively correlated with diastolic blood pressure responses (r2=0.33 p=.06).
Discussion
Sex differences in physiological or subjective responses to acute stress may contribute to sex differences in the prevalence of stress-related disorders. In this study, we found evidence of both sex and menstrual cycle-related differences in cardiovascular, hormonal, and subjective responses to stress. First, men exhibited greater increases in serum and salivary cortisol compared to women tested in either phase. Second, women tested during the luteal phase exhibited larger allopregnanolone responses to stress and expressed greater feelings of Anger and Depression than men and women tested in the follicular phase. Furthermore, women tested in the luteal phase exhibited greater heart rate responsivity and anxiety overall. Finally, whereas men and women tested in the luteal phase exhibited stress-induced increases in noradrenaline and negative mood, compared to the control task, women tested in the follicular phase did not.
It has been suggested that responses to stress differ fundamentally between men and women. Taylor et al. (2000) proposed that men typically exhibit a so-called “fight-or-flight” response to stress, characterized by sympathetic and HPA axis responses, whereas women tend to exhibit a “tend-and-befriend” response, which is more favorable for protecting offspring in the face of a threat. The marked difference in cortisol responses to stress between men and women supports this idea; greater cortisol responses in men may reflect a physical preparedness to “fight” or “flee.” In addition, men exhibited a more pronounced increase in plasma noradrenaline compared to women tested in the follicular (but not luteal) phase. Conversely, women, especially those tested during the luteal phase, were more reactive to stress on subjective self-report measures, consistent with the idea that stress affects psychosocial responses more in women than in men. Sex differences in cortisol and noradrenaline responses to stress may have had evolutionary significance and conferred sex-specific advantages for survival, allowing males to mount a physical response to threatening stimuli. However, today, stressors do not typically affect survival and may be more subtle and repetitive, and sex differences in responses to acute stress may provide clues regarding sex differences in the prevalence of stress-linked disorders. For example, chronic inflammatory disease has been linked to hypoactivity of the HPA axis (Harbuz, 2002) and primarily affects women (Whitacre, 2001; Lockshin, 2006; Fairweather, Frisancho-Kiss, & Rose, 2008). Thus, blunted adrenocortical responses to acute stress among women may contribute to a heightened susceptibility to some autoimmune diseases.
Some of our findings are in line with previous findings. That is, some investigators have reported blunted salivary or plasma cortisol responses to the TSST in women compared to men (Kirschbaum et al., 1992; Kirschbaum et al., 1995a; Kirschbaum et al., 1995b; Kirschbaum et al., 1999, follicular women vs. males; Uhart et al., 2006) and other studies have reported more pronounced emotional responses to stress in women (Steptoe et al., 1996; Kirschbaum et al., 1999; Kelly et al., 2008). Further, studies have reported greater basal levels and stress-induced increases in plasma allopregnanolone among women in the luteal, compared to the follicular phase (Girdler et al., 2001; Girdler, Beth Mechlin, Light, & Morrow, 2006). These cycle-related differences may be due to a higher availability of progesterone, a precursor of allopregnanolone, in luteal women. Finally, women in the luteal phase appear to exhibit greater heart rate responses to stressors including mental stress (Manhem, Jern, Pilhall, Shanks, & Jern, 1991), shock avoidance (Hastrup & Light, 1984), and the cold pressor test (Tersman et al., 1991) compared to women in the follicular phase, possibly because of elevated estrogen levels during the luteal phase (Pollard, Pearce, Rousham, & Schwartz, 2007).
A novel finding in this study was that the TSST increased plasma progesterone among men and women tested in the follicular phase. Other studies have reported increases in progesterone in men and women after emotion-arousing stimuli (Schultheiss, Wirth, & Stanton, 2004; Wirth & Schultheiss, 2006; Wirth, Meier, Fredrickson, & Schultheiss, 2007), yet this is among the first demonstrations of stress-induced increases in progesterone in humans. Previously, we (Childs & de Wit, 2009) reported significant increases in progesterone among men, and now we extend this finding to women tested in the follicular phase. The stress-induced increases in progesterone were particularly evident during the luteal phase, although the cycle-related difference, i.e., that luteal women appeared to exhibit larger increases, did not reach significance probably because of the large variability and high background levels of progesterone. Stress-induced increases in progesterone have also been reported in animal studies (Barbaccia et al., 1996). It is thought that progesterone is released from the adrenals upon stress-induced HPA axis activation (Genazzani et al., 1998) and that this may be a homeostatic mechanism to counteract the effects of stress (Barbaccia et al., 1996).
Partial correlations controlling for group differences revealed interesting relationships among the stress response measures. Individuals who exhibited the greatest subjective sensitivity to stress also exhibited the greatest salivary cortisol, noradrenaline, and allopregnanolone responses to stress. These results support those of a recent study, which found evidence of cross-correlations between emotional and endocrine responses to the TSST (Schlotz et al., 2008). This suggests that systems mediating hormonal release after stress also mediate psychological changes. Another interesting finding of the correlational analyses was that the increase in progesterone after stress was not correlated with increases in allopregnanolone. It has been suggested that progesterone released after stress is the source of allopregnanolone, which serves as a counter-regulatory mechanism to limit the effects of stress (Barbaccia et al., 1996). Instead, our results showing dissociation between progesterone and allopregnanolone suggest that progesterone is not the source of stress-induced allopregnanolone, but that release of allopregnanolone is more directly linked to activation of the sympathoadrenal system. Indeed, a more direct relation between activation of stress systems and allopregnanolone might be a more efficient mechanism to limit the physiological and psychological effects of stress.
The present study has some limitations. First, we were unable to analyze levels of plasma ACTH, which would have provided important information about whether pituitary sensitivity to ACTH is different inmen and women (Kirschbaum et al., 1999). In addition, our sample was mainly Caucasian, which limits application of the results to a broader population. This is of particular concern because some studies have reported ethnic differences in stress responses (Sherwood, May, Siegel, & Blumenthal, 1995; Mills et al., 1996; Ahwal et al., 1997; Harshfield et al., 2007; Chong et al., 2008). Finally, for scheduling reasons, stress sessions were conducted in the morning at a time when cortisol levels are declining. While baseline levels of cortisol did not differ in men and women, other studies have reported a higher cortisol awakening response among women (Kunz-Ebrecht et al., 2004; Maina et al., 2007,) and inspection of cortisol levels between the groups during the control condition illustrates a steeper decline across the session among women. Thus, it is possible that greater increases in cortisol might have been observed in women if testing had occurred later in the day. Nevertheless, women did exhibit a significant cortisol response relative to the non-stressful control condition, i.e., the slope of the decline was significantly less steep after stress. Indeed, it is a strength of the study that a control condition was included in the design and we are able to dissociate the effects of stress from effects of cognitive challenge or postural changes. For example, there were significant increases in noradrenaline exhibited by all groups in the control condition that likely reflect the influences of postural changes (Mlynarik et al., 2007).
In conclusion, we found evidence of sex- and menstrual cycle-related differences in acute responses to mild speaking stress. To the extent that responses to acute stress may predict stress-linked diseases, the sex differences in stress responses reported here may provide clues regarding sex differences in the long-term adverse consequences of stress. A major strength of this study was the inclusion of a range of measures across the physiological and psychological domains. This provided a complete profile of variations in responses to the stress, and notably allowed us to demonstrate correlations among the different stress measures after taking sex- and cycle-related differences into account.
Acknowledgments
We thank Ben Cunningham and Stephen Sittler, who conducted the TSST interviews, Lisa Vicini and Heather Phillips, who assisted with collection of the data, and Nicholas Van Dam, who assisted with data analysis and preparation of the manuscript. This research was supported by NIDA (DA02812) and the University of Chicago Hospital’s GCRC (USPHS MO1RR000555).
References
- Ahwal WN, Mills PJ, Kalshan DA, Nelesen RA. Effects of race and sex on blood pressure and hemodynamic stress response as a function of the menstrual cycle. Blood Pressure Monitoring. 1997;2:161–167. [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE, Erickson J, Hatsukami D, Crouse B. Attenuated adrenocortical and blood pressure responses to psychological stress in ad libitum and abstinent smokers. Pharmacology, Biochemistry, and Behaviour. 2003;74:401–410. doi: 10.1016/s0091-3057(02)01011-0. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Wittmers LE., Jr Enhanced adrenocortical responses to stress in hypertension-prone men and women. Annals of Behavioral Medicine: A Publication of the Society of Behavioral Medicine. 2003;25:25–33. doi: 10.1207/S15324796ABM2501_04. [DOI] [PubMed] [Google Scholar]
- APA. American Psychiatric Association diagnostic and statistical manual of psychiatry. 4. Washington, DC: APA; 1994. [Google Scholar]
- Back SE, Waldrop AE, Saladin ME, Yeatts SD, Simpson A, McRae AL, et al. Effects of gender and cigarette smoking on reactivity to psychological and pharmacological stress provocation. Psychoneuroendocrinology. 2008;33:560–568. doi: 10.1016/j.psyneuen.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbaccia ML, Roscetti G, Trabucchi M, Mostallino MC, Concas A, Purdy RH, Biggio G. Time-dependent changes in rat brain neuroactive steroid concentrations and GABAA receptor function after acute stress. Neuroendocrinology. 1996;63:166–172. doi: 10.1159/000126953. [DOI] [PubMed] [Google Scholar]
- Carter-Snell C, Hegadoren K. Stress disorders and gender: Implications for theory and research. The Canadian Journal of Nursing Research. 2003;35:34–55. [PubMed] [Google Scholar]
- Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses tomental stress in humans. The Journal of Physiology. 2007;585:635–641. doi: 10.1113/jphysiol.2007.141051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs E, de Wit H. Hormonal, cardiovascular, and subjective responses to acute stress in smokers. Psychopharmacology (Berl) 2009;203:1–12. doi: 10.1007/s00213-008-1359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RY, Uhart M, McCaul ME, Johnson E, Wand GS. Whites have a more robust hypothalamic-pituitary-adrenal axis response to a psychological stressor than blacks. Psychoneuroendocrinology. 2008;33:246–254. doi: 10.1016/j.psyneuen.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA: The Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: A theoretical integration and synthesis of laboratory research. Psychological Bulletin. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Fairweather D, Frisancho-Kiss S, Rose NR. Sex differences in autoimmune disease from a pathological perspective. The American Journal of Pathology. 2008;173:600–609. doi: 10.2353/ajpath.2008.071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, et al. Circulating levels of allopregnanolone in humans: Gender, age, and endocrine influences. The Journal of Clinical Endocrinology and Metabolism. 1998;83:2099–2103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Beth Mechlin M, Light KC, Morrow LA. Ethnic differences in allopregnanolone concentrations in women during rest and following mental stress. Psychophysiology. 2006;43:331–336. doi: 10.1111/j.1469-8986.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biological Psychiatry. 2001;49:788–797. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Turner JR, Sherwood A, Light KC. Gender differences in blood pressure control during a variety of behavioral stressors. Psychosomatic Medicine. 1990;52:571–591. doi: 10.1097/00006842-199009000-00009. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Glaser R. Stress-induced immune dysregulation: Implications for wound healing, infectious disease and cancer. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on NeuroImmune Pharmacology. 2006;1:421–427. doi: 10.1007/s11481-006-9036-0. [DOI] [PubMed] [Google Scholar]
- Griffin JE, Ojeda SR. Textbook of endocrine physiology. 3. New York: Oxford University Press; 1996. [Google Scholar]
- Harbuz M. Neuroendocrinology of autoimmunity. International Review of Neurobiology. 2002;52:133–161. doi: 10.1016/s0074-7742(02)52008-0. [DOI] [PubMed] [Google Scholar]
- Harbuz MS, Richards LJ, Chover-Gonzalez AJ, Marti-Sistac O, Jessop DS. Stress in autoimmune disease models. Annals of the New York Academy of Sciences. 2006;1069:51–61. doi: 10.1196/annals.1351.005. [DOI] [PubMed] [Google Scholar]
- Harshfield GA, Hanevold C, Kapuku GK, Dong Y, Castles ME, Ludwig DA. The association of race and sex to the pressure natriuresis response to stress. Ethnicity & Disease. 2007;17:498–502. [PubMed] [Google Scholar]
- Hastrup JL, Light KC. Sex differences in cardiovascular stress responses: Modulation as a function of menstrual cycle phases. Journal of Psychosomatic Research. 1984;28:475–483. doi: 10.1016/0022-3999(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: Hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in Neuroendocrinology. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hlavacova N, Wawruch M, Tisonova J, Jezova D. Neuroendocrine activation during combined mental and physical stress in women depends on trait anxiety and the phase of the menstrual cycle. Annals of the New York Academy of Sciences. 2008;1148:520–525. doi: 10.1196/annals.1410.030. [DOI] [PubMed] [Google Scholar]
- Kajantie E, Phillips DI. The effects of sex and hormonal status on the physiological response to acute psychosocial stress. Psychoneuroendocrinology. 2006;31:151–178. doi: 10.1016/j.psyneuen.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Kelly MM, Tyrka AR, Anderson GM, Price LH, Carpenter LL. Sex differences in emotional and physiological responses to the Trier Social Stress Test. Journal of Behavior Therapy and Experimental Psychiatry. 2008;39:87–98. doi: 10.1016/j.jbtep.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosomatic Medicine. 1995a;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993a;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995b;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Strasburger CJ, Langkrar J. Attenuated cortisol response to psychological stress but not to CRHor ergometry in young habitual smokers. Pharmacology, Biochemistry, and Behavior. 1993b;44:527–531. doi: 10.1016/0091-3057(93)90162-m. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Hellhammer D. Consistent sex differences in cortisol responses to psychological stress. Psychosomatic Medicine. 1992;54:648–657. doi: 10.1097/00006842-199211000-00004. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodologic critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: Impact of age and gender. Psychoneuroendocrinology. 2004a;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: The impact of age and gender. International Journal of Behavioral Medicine. 2004b;11:116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Hellhammer J, Hellhammer DH, Wolf OT, Pirke KM, Varadi E, et al. Sex differences in endocrine and psychological responses to psychosocial stress in healthy elderly subjects and the impact of a 2-week dehydroepiandrosterone treatment. The Journal of Clinical Endocrinology and Metabolism. 1998;83:1756–1761. doi: 10.1210/jcem.83.5.4758. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: A review. Biological Psychology. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;294:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Lepore SJ, Allen KA, Evans GW. Social support lowers cardiovascular reactivity to an acute stressor. Psychosomatic Medicine. 1993;55:518–524. doi: 10.1097/00006842-199311000-00007. [DOI] [PubMed] [Google Scholar]
- Litschauer B, Zauchner S, Huemer KH, Kafka-Lutzow A. Cardiovascular, endocrine, and receptor measures as related to sex and menstrual cycle phase. Psychosomatic Medicine. 1998;60:219–226. doi: 10.1097/00006842-199803000-00019. [DOI] [PubMed] [Google Scholar]
- Lockshin MD. Sex differences in autoimmune disease. Lupus. 2006;15:753–756. doi: 10.1177/0961203306069353. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Akil H, Watson SJ. Neural circuits mediating stress. Biological Psychiatry. 1999;46:1461–1471. doi: 10.1016/s0006-3223(99)00266-8. [DOI] [PubMed] [Google Scholar]
- Lundberg U. Stress hormones in health and illness: The roles of work and gender. Psychoneuroendocrinology. 2005;30:1017–1021. doi: 10.1016/j.psyneuen.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Maina G, Palmas A, Bovenzi M, Filon FL. Sex specific differences in physiologic response to stress evaluated by means of salivary cortisol. Giornale Italiano di Medicina del Lavoro ed Ergonomia. 2007;29:359–360. [PubMed] [Google Scholar]
- Manhem K, Jern C, Pilhall M, Shanks G, Jern S. Haemodynamic responses to psychosocial stress during the menstrual cycle. Clinical Science (London, England: 1979) 1991;81:17–22. doi: 10.1042/cs0810017. [DOI] [PubMed] [Google Scholar]
- Matthews KA, Gump BB, Owens JF. Chronic stress influences cardiovascular and neuroendocrine responses during acute stress and recovery, especially in men. Health Psychology: Official Journal of the Division of Health Psychology, American Psychological Association. 2001;20:403–410. [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman L. Profile of mood states. San Diego: Eduational and Industrial Testing Service; 1971. [Google Scholar]
- Mills PJ, Nelesen RA, Ziegler MG, Parry BL, Berry CC, Dillon E, Dimsdale JE. Menstrual cycle effects on catecholamine and cardiovascular responses to acute stress in black but not white normotensive women. Hypertension. 1996;27:962–967. doi: 10.1161/01.hyp.27.4.962. [DOI] [PubMed] [Google Scholar]
- Mlynarik M, Makatsori A, Dicko I, Hinghofer-Szalkay HG, Jezova D. Postural changes associated with public speech tests lead to mild and selective activation of stress hormone release. Journal of Physiology and Pharmacology: An Official Journal of the Polish Physiological Society. 2007;58:95–103. [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocrine Reviews. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nicolson N, Storms C, Ponds R, Sulon J. Salivary cortisol levels and stress reactivity in human aging. The Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 1997;52:M68–75. doi: 10.1093/gerona/52a.2.m68. [DOI] [PubMed] [Google Scholar]
- Pico-Alfonso MA, Mastorci F, Ceresini G, Ceda GP, Manghi M, Pino O, et al. Acute psychosocial challenge and cardiac autonomic response in women: The role of estrogens, corticosteroids, and behavioral coping styles. Psychoneuroendocrinology. 2007;32:451–463. doi: 10.1016/j.psyneuen.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Polefrone JM, Manuck SB. Effects of menstrual phase and parental history of hypertension on cardiovascular response to cognitive challenge. Psychosomatic Medicine. 1988;50:23–36. doi: 10.1097/00006842-198801000-00004. [DOI] [PubMed] [Google Scholar]
- Pollard TM, Pearce KL, Rousham EK, Schwartz JE. Do blood pressure and heart rate responses to perceived stress vary according to endogenous estrogen level in women? American Journal of Physical Anthropology. 2007;132:151–157. doi: 10.1002/ajpa.20468. [DOI] [PubMed] [Google Scholar]
- Purdy RH, Moore PH, Jr, Rao PN, Hagino N, Yamaguchi T, Schmidt P, et al. Radioimmunoassay of 3 alpha-hydroxy-5 alpha-pregnan-20-one in rat and human plasma. Steroids. 1990;55:290–296. doi: 10.1016/0039-128x(90)90031-6. [DOI] [PubMed] [Google Scholar]
- Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: Metabolic, morphological and behavioral consequences. European Journal of Pharmacology. 2008;585:64–75. doi: 10.1016/j.ejphar.2008.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Miyake S. Cardiovascular reactivity to mental stress: Relationship with menstrual cycle and gender. Journal of Physiological Anthropology and Applied Human Science. 2004;23:215–223. doi: 10.2114/jpa.23.215. [DOI] [PubMed] [Google Scholar]
- Schlotz W, Kumsta R, Layes I, Entringer S, Jones A, Wust S. Covariance between psychological and endocrine responses to pharmacological challenge and psychosocial stress: A question of timing. Psychosomatic Medicine. 2008;70:787–796. doi: 10.1097/PSY.0b013e3181810658. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosomatic Medicine. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Schultheiss OC, Wirth MM, Stanton SJ. Effects of affiliation and power motivation arousal on salivary progesterone and testosterone. Hormones and Behavior. 2004;46:592–599. doi: 10.1016/j.yhbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Braglia F, Costoli T, Musso E, Meerlo P, Ceresini G, Troisi A. Cardiac autonomic reactivity and salivary cortisol in men and women exposed to social stressors: Relationship with individual ethological profile. Neuroscience and Biobehavioral Reviews. 2003;27:179–188. doi: 10.1016/s0149-7634(03)00019-8. [DOI] [PubMed] [Google Scholar]
- Sherwood A, May CW, Siegel WC, Blumenthal JA. Ethnic differences in hemodynamic responses to stress in hypertensive men and women. American Journal of Hypertension. 1995;8:552–557. doi: 10.1016/0895-7061(95)00036-O. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sita A, Miller SB. Estradiol, progesterone and cardiovascular response to stress. Psychoneuroendocrinology. 1996;21:339–346. doi: 10.1016/0306-4530(95)00053-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Fieldman G, Evans O, Perry L. Cardiovascular risk and responsivity to mental stress: The influence of age, gender and risk factors. Journal of Cardiovascular Risk. 1996;3:83–93. [PubMed] [Google Scholar]
- Stoney CM, Owens JF, Matthews KA, Davis MC, Caggiula A. Influences of the normal menstrual cycle on physiologic functioning during behavioral stress. Psychophysiology. 1990;27:125–135. doi: 10.1111/j.1469-8986.1990.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: Social rejection versus achievement stress. Biological Psychiatry. 2002;52:318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychological Review. 2000;107:411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- Tersman Z, Collins A, Eneroth P. Cardiovascular responses to psychological and physiological stressors during the menstrual cycle. Psychosomatic Medicine. 1991;53:185–197. doi: 10.1097/00006842-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Weidner G, Helmig L. Cardiovascular stress reactivity and mood during the menstrual cycle. Women & Health. 1990;16:5–21. doi: 10.1300/J013v16n03_02. [DOI] [PubMed] [Google Scholar]
- Whitacre CC. Sex differences in autoimmune disease. Nature Immunology. 2001;2:777–780. doi: 10.1038/ni0901-777. [DOI] [PubMed] [Google Scholar]
- Williams RA, Hagerty BM, Brooks G. Trier Social Stress Test: A method for use in nursing research. Nursing Research. 2004;53:277–280. doi: 10.1097/00006199-200407000-00011. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Meier EA, Fredrickson BL, Schultheiss OC. Relationship between salivary cortisol and progesterone levels in humans. Biological Psychology. 2007;74:104–107. doi: 10.1016/j.biopsycho.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Schultheiss OC. Effects of affiliation arousal (hope of closeness) and affiliation stress (fear of rejection) on progesterone and cortisol. Hormones and Behavior. 2006;50:786–795. doi: 10.1016/j.yhbeh.2006.08.003. [DOI] [PubMed] [Google Scholar]