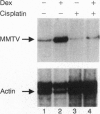
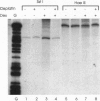
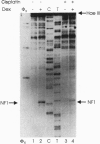
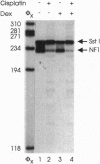
Abstract
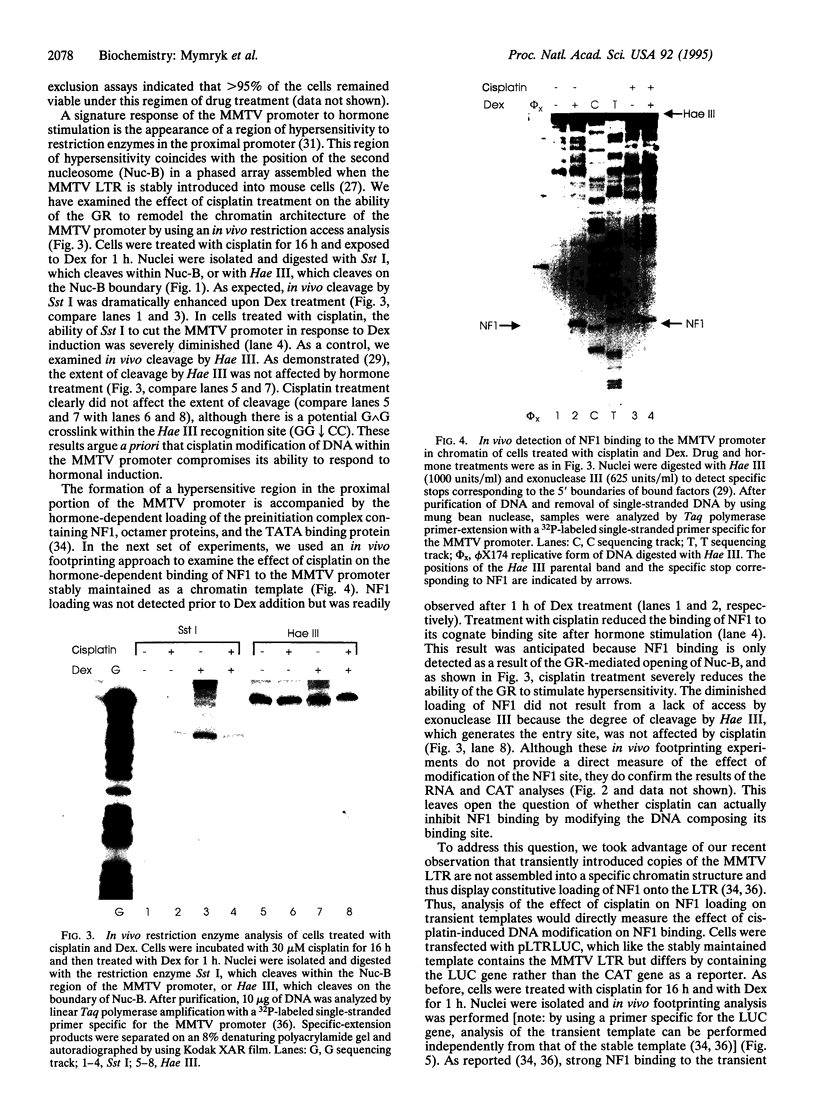
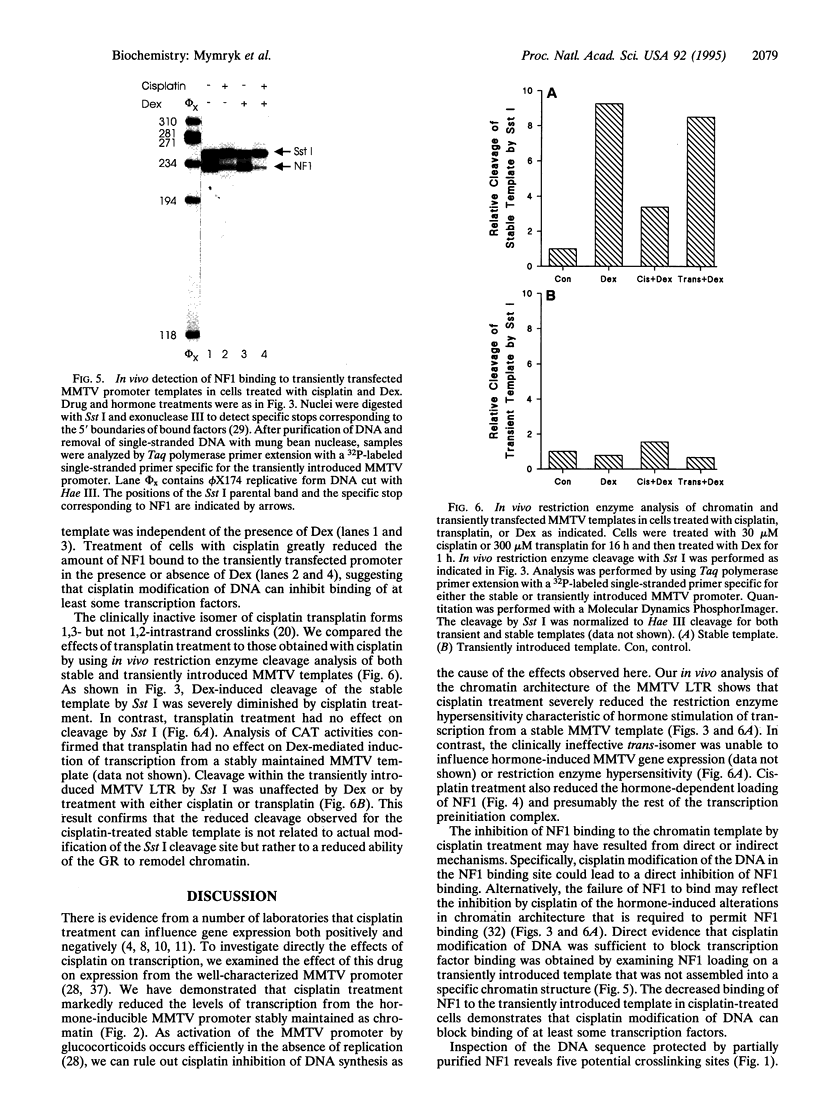
The anticancer drug cis-diamminedichloro-platinum(II) (cisplatin) covalently modifies DNA, and these lesions are thought to lead to cell death by inhibiting DNA and RNA synthesis. By using in vivo analysis techniques, we have investigated the influence of cisplatin on hormone-induced transcription from the mouse mammary tumor virus (MMTV) promoter. Cisplatin substantially reduced glucocorticoid-induced expression from the MMTV promoter stably incorporated into mouse tumor cells. The glucocorticoid-receptor-dependent chromatin remodeling and loading of transcription factors that is a signature response of this promoter in the context of chromatin were significantly reduced by cisplatin but not by the clinically ineffective trans-isomer trans-diamminedichloroplatinum(II) (transplatin). Additional in vivo studies on transiently introduced nonchromatin MMTV templates demonstrated that cisplatin modification of DNA blocked binding of the transcription factor NF1. These results provide strong evidence that cisplatin influences transcription by interfering with the opening of repressive chromatin structures and by blocking transcription factor binding directly, each of which could contribute substantially to its toxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. A., Jones J. A. Characterization of binding proteins from ovarian carcinoma and kidney tubule cells that are specific for cisplatin modified DNA. Cancer Commun. 1991 Mar;3(3):93–102. doi: 10.3727/095535491820873524. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Cordingley M. G., Wolford R. G., Hager G. L. Transcription factor access is mediated by accurately positioned nucleosomes on the mouse mammary tumor virus promoter. Mol Cell Biol. 1991 Feb;11(2):688–698. doi: 10.1128/mcb.11.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer T. K., Lefebvre P., Wolford R. G., Hager G. L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992 Mar 20;255(5051):1573–1576. doi: 10.1126/science.1347958. [DOI] [PubMed] [Google Scholar]
- Archer T. K., Tam S. P., Deugau K. V., Deeley R. G. Apolipoprotein C-II mRNA levels in primate liver. Induction by estrogen in the human hepatocarcinoma cell line, HepG2. J Biol Chem. 1985 Feb 10;260(3):1676–1681. [PubMed] [Google Scholar]
- Bellon S. F., Coleman J. H., Lippard S. J. DNA unwinding produced by site-specific intrastrand cross-links of the antitumor drug cis-diamminedichloroplatinum(II). Biochemistry. 1991 Aug 13;30(32):8026–8035. doi: 10.1021/bi00246a021. [DOI] [PubMed] [Google Scholar]
- Brown S. J., Kellett P. J., Lippard S. J. Ixr1, a yeast protein that binds to platinated DNA and confers sensitivity to cisplatin. Science. 1993 Jul 30;261(5121):603–605. doi: 10.1126/science.8342024. [DOI] [PubMed] [Google Scholar]
- Bruhn S. L., Pil P. M., Essigmann J. M., Housman D. E., Lippard S. J. Isolation and characterization of human cDNA clones encoding a high mobility group box protein that recognizes structural distortions to DNA caused by binding of the anticancer agent cisplatin. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2307–2311. doi: 10.1073/pnas.89.6.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan R. L., Gralla J. D. Cisplatin resistance and mechanism in a viral test system: SV40 isolates that resist inhibition by the antitumor drug have lost regulatory DNA. Biochemistry. 1990 Apr 10;29(14):3436–3442. doi: 10.1021/bi00466a003. [DOI] [PubMed] [Google Scholar]
- Chao C. C., Huang S. L., Lee L. Y., Lin-Chao S. Identification of inducible damage-recognition proteins that are overexpressed in HeLa cells resistant to cis-diamminedichloroplatinum (II). Biochem J. 1991 Aug 1;277(Pt 3):875–878. doi: 10.1042/bj2770875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda Y., Anin M. F., Leng M., Job D. RNA polymerases react differently at d(ApG) and d(GpG) adducts in DNA modified by cis-diamminedichloroplatinum(II). Biochemistry. 1992 Feb 25;31(7):1904–1908. doi: 10.1021/bi00122a002. [DOI] [PubMed] [Google Scholar]
- Cordingley M. G., Riegel A. T., Hager G. L. Steroid-dependent interaction of transcription factors with the inducible promoter of mouse mammary tumor virus in vivo. Cell. 1987 Jan 30;48(2):261–270. doi: 10.1016/0092-8674(87)90429-6. [DOI] [PubMed] [Google Scholar]
- Donahue B. A., Augot M., Bellon S. F., Treiber D. K., Toney J. H., Lippard S. J., Essigmann J. M. Characterization of a DNA damage-recognition protein from mammalian cells that binds specifically to intrastrand d(GpG) and d(ApG) DNA adducts of the anticancer drug cisplatin. Biochemistry. 1990 Jun 19;29(24):5872–5880. doi: 10.1021/bi00476a032. [DOI] [PubMed] [Google Scholar]
- Evans G. L., Gralla J. D. Cisplatin-induced imbalances in the pattern of chimeric marker gene expression in HeLa cells. Biochem Biophys Res Commun. 1992 Apr 15;184(1):1–8. doi: 10.1016/0006-291x(92)91149-k. [DOI] [PubMed] [Google Scholar]
- Evans G. L., Gralla J. D. Differential effects of cisplatin on the expression of chimeric marker genes in CV-1 cells. Biochem Pharmacol. 1992 Jul 7;44(1):107–119. doi: 10.1016/0006-2952(92)90044-j. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin as an essential part of the transcriptional mechanism. Nature. 1992 Jan 16;355(6357):219–224. doi: 10.1038/355219a0. [DOI] [PubMed] [Google Scholar]
- Ferrari S., Harley V. R., Pontiggia A., Goodfellow P. N., Lovell-Badge R., Bianchi M. E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992 Dec;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K., Amsterdam A., Grosschedl R. DNA-binding properties of the HMG domain of the lymphoid-specific transcriptional regulator LEF-1. Genes Dev. 1991 Dec;5(12B):2567–2578. doi: 10.1101/gad.5.12b.2567. [DOI] [PubMed] [Google Scholar]
- Gralla J. D., Sasse-Dwight S., Poljak L. G. Formation of blocking lesions at identical DNA sequences by the nitrosourea and platinum classes of anticancer drugs. Cancer Res. 1987 Oct 1;47(19):5092–5096. [PubMed] [Google Scholar]
- Hager G. L., Archer T. K., Fragoso G., Bresnick E. H., Tsukagoshi Y., John S., Smith C. L. Influence of chromatin structure on the binding of transcription factors to DNA. Cold Spring Harb Symp Quant Biol. 1993;58:63–71. doi: 10.1101/sqb.1993.058.01.010. [DOI] [PubMed] [Google Scholar]
- Hughes E. N., Engelsberg B. N., Billings P. C. Purification of nuclear proteins that bind to cisplatin-damaged DNA. Identity with high mobility group proteins 1 and 2. J Biol Chem. 1992 Jul 5;267(19):13520–13527. [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Kashani-Sabet M., Wang W., Scanlon K. J. Cyclosporin A suppresses cisplatin-induced c-fos gene expression in ovarian carcinoma cells. J Biol Chem. 1990 Jul 5;265(19):11285–11288. [PubMed] [Google Scholar]
- Landsman D., Bustin M. A signature for the HMG-1 box DNA-binding proteins. Bioessays. 1993 Aug;15(8):539–546. doi: 10.1002/bies.950150807. [DOI] [PubMed] [Google Scholar]
- Lee H. L., Archer T. K. Nucleosome-mediated disruption of transcription factor-chromatin initiation complexes at the mouse mammary tumor virus long terminal repeat in vivo. Mol Cell Biol. 1994 Jan;14(1):32–41. doi: 10.1128/mcb.14.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P., Berard D. S., Cordingley M. G., Hager G. L. Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol Cell Biol. 1991 May;11(5):2529–2537. doi: 10.1128/mcb.11.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez E., Dusserre Y., Wahli W., Mermod N. Synergistic transcriptional activation by CTF/NF-I and the estrogen receptor involves stabilized interactions with a limiting target factor. Mol Cell Biol. 1991 Jun;11(6):2937–2945. doi: 10.1128/mcb.11.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pil P. M., Lippard S. J. Specific binding of chromosomal protein HMG1 to DNA damaged by the anticancer drug cisplatin. Science. 1992 Apr 10;256(5054):234–237. doi: 10.1126/science.1566071. [DOI] [PubMed] [Google Scholar]
- Richard-Foy H., Hager G. L. Sequence-specific positioning of nucleosomes over the steroid-inducible MMTV promoter. EMBO J. 1987 Aug;6(8):2321–2328. doi: 10.1002/j.1460-2075.1987.tb02507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Rubin E., Kharbanda S., Gunji H., Weichselbaum R., Kufe D. cis-Diamminedichloroplatinum(II) induces c-jun expression in human myeloid leukemia cells: potential involvement of a protein kinase C-dependent signaling pathway. Cancer Res. 1992 Feb 15;52(4):878–882. [PubMed] [Google Scholar]
- Simpson R. T., Bustin M. Histone compostion of chromatin subunits studied by immunosedimentation. Biochemistry. 1976 Sep 21;15(19):4305–4312. doi: 10.1021/bi00664a026. [DOI] [PubMed] [Google Scholar]
- Treiber D. K., Zhai X., Jantzen H. M., Essigmann J. M. Cisplatin-DNA adducts are molecular decoys for the ribosomal RNA transcription factor hUBF (human upstream binding factor). Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5672–5676. doi: 10.1073/pnas.91.12.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe A. P. New approaches to chromatin function. New Biol. 1990 Mar;2(3):211–218. [PubMed] [Google Scholar]
- Zaret K. S., Yamamoto K. R. Reversible and persistent changes in chromatin structure accompany activation of a glucocorticoid-dependent enhancer element. Cell. 1984 Aug;38(1):29–38. doi: 10.1016/0092-8674(84)90523-3. [DOI] [PubMed] [Google Scholar]
- Zoumpourlis V., Kerr D. J., Spandidos D. A. Carboplatin as opposed to cisplatin does not stimulate the expression of the human immunodeficiency virus long terminal repeat sequences. Biochem Pharmacol. 1992 Feb 4;43(3):650–654. doi: 10.1016/0006-2952(92)90592-7. [DOI] [PubMed] [Google Scholar]