Abstract
Objective
Nonalcoholic fatty liver disease (NAFLD) affects 9.6% of children and may put these children at elevated risk of high blood pressure and subsequent cardiovascular morbidity and mortality. Therefore, we sought to determine the prevalence of and risk factors for high blood pressure in children with NAFLD.
Methods
Cohort study performed by the NIDDK NASH Clinical Research Network. There were 484 children with NAFLD ages 2 to 17 at enrollment; 382 children were assessed both at enrollment and 48 weeks afterwards. The main outcomes were high blood pressure at baseline and persistent high blood pressure at both baseline and 48 weeks.
Results
Prevalence of high blood pressure at baseline was 35.8% and prevalence of persistent high blood pressure was 21.4%. Children with high blood pressure were significantly more likely to have worse steatosis than children without high blood pressure (mild 19.8% vs. 34.2%, moderate 35.0% vs. 30.7%, severe 45.2% vs. 35.1%; P = 0.003). Higher body mass index, low-density lipoprotein, and uric acid were independent risk factors for high blood pressure (Odds Ratios: 1.10 per kg/m2, 1.09 per 10 mg/dL, 1.25 per mg/dL, respectively). Compared to boys, girls with NAFLD were significantly more likely to have persistent high blood pressure (28.4% vs.18.9%; P = 0.05).
Conclusions
In conclusion, NAFLD is a common clinical problem that places children at substantial risk for high blood pressure, which may often go undiagnosed. Thus blood pressure evaluation, control, and monitoring should be an integral component of the clinical management of children with NAFLD.
Introduction
High blood pressure and nonalcoholic fatty liver disease (NAFLD) are two emerging clinical problems in children closely related to the epidemic of childhood obesity. NAFLD is now the most common cause of chronic liver disease in children in the United States with an estimated prevalence of 9.6%. [1] The prevalence of high blood pressure is estimated to be between 2 and 5% among children in the United States. [2], [3] High blood pressure in childhood is likely to persist into adulthood [4], [5] and is a risk factor in adulthood for atherosclerosis and coronary heart disease. [6]
NAFLD itself has been linked to cardiovascular disease in both children and adults. [7]–[9] In adults with NAFLD, cardiovascular disease is the leading cause of death. [10], [11] In children with NAFLD, studies have reported high blood pressure as part of the metabolic syndrome; however, blood pressure has not been evaluated as the focal point of any of these studies. [9], [12], [13] Thus, many questions remain about the prevalence of high blood pressure and its associated risk factors in children with NAFLD. Moreover, there have been no longitudinal studies of blood pressure among children with NAFLD. Therefore we performed a multi-center, longitudinal cohort study with the following study aims: 1) to determine the prevalence of high blood pressure in children with NAFLD in relation to demographic and key clinical risk factors, and 2) to determine the rate and risk factors of persistent (over 48 weeks) high blood pressure in children with NAFLD.
Methods
Study population
The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) NASH Clinical Research Network (NASH CRN) enrolled children with NAFLD in longitudinal cohort studies (Database and Database 2; NCT01061684) and a randomized controlled trial (TONIC; NCT00063635). These studies have been described [14], [15] and were performed at 13 pediatric clinical centers across the United States (see appendix for list). NAFLD Database began enrollment in September 2004, TONIC in August 2005 and Database 2 in October 2009. Children completed annual visits with comprehensive anthropometric and laboratory measurements described below. For this analysis, we included children who were <18 years of age at baseline with NAFLD. A diagnosis of NAFLD was based on liver histology with ≥5% of hepatocytes containing macrovesicular fat and exclusion of other causes of chronic liver disease by clinical history, laboratory studies, and histology. [16] For the baseline analysis, we excluded children with underlying renal disease, those without a histologic diagnosis of NAFLD, or missing blood pressure at baseline. For the longitudinal analysis, we excluded those without follow-up data at 48 weeks.
Ethics Statement
All studies were approved by the Institutional Review Boards at each participating center. Written informed consent was obtained from parents/guardians and written assent was obtained from children.
Liver Histology
All liver biopsy specimens were stained with hematoxylin-eosin and Masson's trichrome stains, and reviewed and scored centrally by the Pathology Committee according to the published NASH Clinical Research Network scoring system. [17] The Pathology Committee was blinded to any clinical or demographic information. Steatosis was graded according to the percentage of hepatocytes that contained fat droplets as follows: grade 0, none: <5%; grade 1, mild: 5 to 33%; grade 2, moderate: 34 to 66%; and grade 3, severe:> 66%. Fibrosis was staged as follows: a) stage 1a – mild zone 3 perisinusoidal fibrosis requiring trichrome stain; b) stage 1b – moderate zone 3 perisinusoidal fibrosis not requiring trichrome stain; c) stage 1c – portal/periportal fibrosis only; c) stage 2 –zone 3 perisinusoidal fibrosis and periportal fibrosis; d) stage 3 – bridging fibrosis; and e) stage 4 – cirrhosis. Liver biopsies were diagnosed as steatohepatitis, borderline steatohepatitis or NAFLD but not steatohepatitis, based on the aggregate presence and degree of the individual histologic features of fatty liver disease. Although no single histologic feature is considered diagnostic of NASH, a typical set of minimum criteria would include macrovesicular steatosis (more than 5%), lobular inflammation and hepatocyte injury as manifest by ballooning degeneration. Borderline cases demonstrated a lesser degree of one or more findings. Cases determined to be NAFLD but not steatohepatitis NASH show steatosis with no or minimal lobular inflammation. The assignment of a diagnosis of steatohepatitis, borderline steatohepatitis or NAFLD but not steatohepatitis was made as a consensus agreement of the NASH CRN pathology group at the time of central review of cases.
Measures
Physical measurements included height, weight, waist circumference, systolic blood pressure, and diastolic blood pressure. Blood pressure was measured and percentiles were computed as instructed in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. [18] After 5 minutes of seated rest, blood pressure was measured twice from the right arm of the seated child with an automated sphygmomanometer with 1 minute of rest between measurements. The average of the 2 measures was recorded. Cuff sizes were selected so that the cuff bladder encircled at least 80% of the mid-upper arm per standard protocol. Participants fasted overnight for 12 hours before phlebotomy. Fasting laboratory assays included glucose, insulin, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, alanine aminotransferase (ALT), aspartate aminotransferase (AST), gamma glutamyltransferase (GGT), and uric acid. Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared.
Case definitions
The definitions for blood pressure in children are based upon the normative distribution of blood pressure in healthy children. [18] High blood pressure is defined as average systolic or diastolic blood pressure that is ≥95th percentile for age, sex, and height. Hypertension is defined as high blood pressure on at least three separate occasions. For this analysis we defined High blood pressure as systolic or diastolic blood pressure ≥95th percentile for age, sex and height, or the use of antihypertensive medication for a clinical diagnosis of hypertension. Persistent high blood pressure was defined as having high blood pressure at both baseline and at 48 weeks. Clinical hypertension was determined by parental or patient report that a child had a clinical diagnosis of hypertension assigned by their treating physician.
Data Analysis
Descriptive statistics (mean, standard deviation, frequency, and percentages) were used to compare patients with and without elevated blood pressure; P values were determined either from Chi-square tests for categorical variables or from non-parametric two-sample Wilcoxon rank sum tests for continuous variables. Risk factors for high blood pressure were identified using multiple logistic regression models with the presence of high blood pressure as the binary outcome and a candidate set of risk factors: gender, age, race/ethnicity (non-Hispanic white, Hispanic, and non-Hispanic non-white), BMI, GGT, LDL, glucose, insulin, and uric acid. Goodness of fit of the logistic model was assessed using a Hosmer-Lemeshow chi-square test with P>0.05 indicating adequate fit. Parallel analyses were done for risk factors for persistently high blood pressure. Characteristics of those children with and without 48 week follow-up were compared using a logistic regression model of the odds of missing-ness in relation to the risk factors; a Wald test was performed to determine whether set of characteristics differed in those children who were not evaluated at a 48 week follow-up assessment. All analyses assumed nominal, two-sided P values as statistically significant if P≤0.05. Analyses were performed using SAS version 9.3 (SAS Institute) and Stata version 13.1 (StataCorp). Sensitivity analysis of variation in risk factors by gender showed no evidence of effect modification (interaction P range from 0.12 to 0.81). Additionally, the set of risk factors was not related to the odds of missing the 48 week follow-up visit (P = 0.41).
Results
Study population
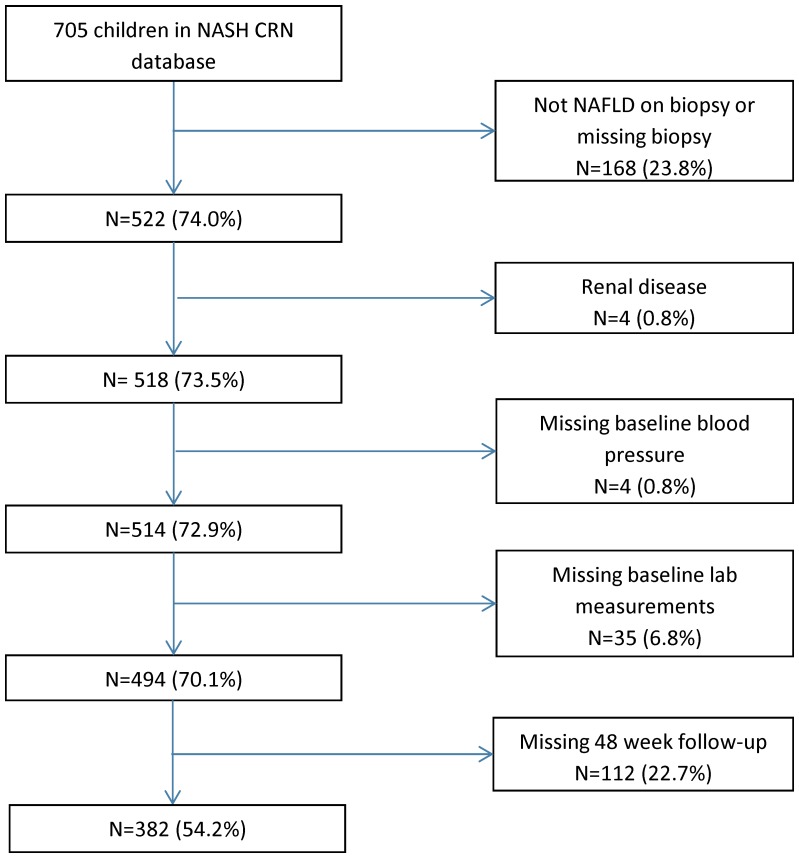
There were 494 children enrolled in the NASH CRN that met all criteria and were included in the baseline analysis. A study flow chart is shown in Figure 1 . The demographic and clinical parameters are shown in Table 1 . The mean age of the participants at baseline was 13.1 years. The mean BMI of participants at baseline was 32.7 kg/m2. The distribution of disease severity was: NAFLD but not NASH 27.5% (136/494), borderline NASH 44.7% (221/494) and definite NASH 27.7% (137/494). The majority of participants (358/494) were boys. There was no significant difference between boys and girls with respect to age or race/ethnicity. Boys had a significantly higher BMI Z-score than girls (2.4±0.4 vs 2.2±0.4; P<0.001) but no difference in BMI (32.9±6.3 vs 32.6±7.0 kg/m2; P = 0.33).
Figure 1. Flowchart shows the application of study inclusion and exclusion criteria.
Table 1. High Blood Pressure in Children with NAFLD—Baseline Characteristics.
| High Blood Pressurea | ||||
| Characteristics | No | Yes | Total | P Valueb |
| N (%) or mean ±SD | N = 317 | N = 177 | N = 494 | |
| Blood pressure | ||||
| Systolic blood pressure percentile | 64.1±25.0 | 95.8±24.0 | 75.5±26.0 | <0.0001 |
| Diastolic blood pressure percentile | 53.9±23.6 | 73.8±21.9 | 61.0±24.9 | <0.0001 |
| Demographics | ||||
| Male | 234 (73.8%) | 124 (70.1%) | 358 (72.5%) | 0.37 |
| Age (years)c | 13.1±2.7 | 12.9±2.8 | 13.1±2.7 | 0.35 |
| <8 years | 11 (3.5%) | 6 (3.4%) | 17 (3.4%) | 0.33 |
| 8–12 years | 141 (44.5%) | 91 (51.4%) | 232 (47.0%) | |
| 13–17 years | 165 (52.1%) | 80 (45.2%) | 245 (49.6%) | |
| Race/ethnicity | 0.05 | |||
| Non-Hispanic white | 85 (26.8%) | 63 (35.6%) | 148 (30.0%) | |
| Hispanic | 216 (68.4%) | 101 (57.1%) | 317 (64.2%) | |
| Other | 16 (5.1%) | 13 (7.3%) | 29 (5.9%) | |
| Anthropomorphic | ||||
| BMI z-score | 2.2±0.4 | 2.4±0.4 | 2.3±0.4 | <0.0001 |
| BMI (kg/m2) | 31.6±6.2 | 34.6±6.5 | 32.7±6.5 | <0.0001 |
| Liver enzymes | ||||
| ALT (U/L) | 105.9±84.3 | 107.1±88.8 | 106.3±85.8 | 0.83 |
| AST (U/L) | 63.2±48.5 | 64.6±45.3 | 63.7±47.3 | 0.41 |
| GGT (U/L) | 42.4±29.9 | 48.6±34.5 | 44.6±31.7 | 0.004 |
| Serum chemistries | ||||
| HDL (mg/dL) | 38.5±8.5 | 38.2±10.1 | 38.4±9.1 | 0.17 |
| LDL (mg/dL) | 100.2±29.9 | 106.4±28.7 | 102.4±29.6 | 0.04 |
| Serum glucose (mg/dL) | 87.7±18.1 | 89.3±15.0 | 88.3±17.1 | 0.05 |
| Serum insulin (µU/mL) | 31.7±38.5 | 37.4±28.5 | 33.7±35.3 | 0.001 |
| HOMA-IRd | 7.0±9.3 | 8.3±6.7 | 7.5±8.5 | 0.001 |
| Uric acid (mg/dL) | 5.9±1.4 | 6.3±1.4 | 6.0±1.4 | 0.03 |
| Liver Histology | ||||
| Steatosis grade | 0.003 | |||
| <33% | 108 (34.2%) | 35 (19.8%) | 143 (29.0%) | |
| 34–66% | 97 (30.7%) | 62 (35.0%) | 159 (32.3%) | |
| >66% | 111 (35.1%) | 80 (45.2%) | 191 (38.7%) | |
| Lobular inflammation | 0.94 | |||
| <2 under 20x | 173 (54.8%) | 95 (53.7%) | 268 (54.4%) | |
| 2–4 under 20x | 122 (38.6%) | 71 (40.1%) | 193 (39.2%) | |
| >4 under 20x | 21 (6.7%) | 11 (6.2%) | 32 (6.5%) | |
| Ballooning | 0.02 | |||
| None | 174 (55.1%) | 96 (54.2%) | 270 (54.8%) | |
| Few | 81 (25.6%) | 61 (34.5%) | 142 (28.8%) | |
| Many | 61 (19.3%) | 20 (11.3%) | 81 (16.4%) | |
| Fibrosis stage | 0.47 | |||
| None | 102 (32.4%) | 44 (25.0%) | 146 (29.7%) | |
| Zone 3, perisinusoidal or portral/periportal only | 121 (38.4%) | 73 (41.5%) | 194 (39.5%) | |
| Zone 3, periportal | 49 (15.6%) | 30 (17.1%) | 79 (16.1%) | |
| Bridging | 38 (12.1%) | 27 (15.3%) | 65 (13.2%) | |
| Cirrhosis | 5 (1.6%) | 2 (1.1%) | 7 (1.4%) | |
| Diagnosis | 0.42 | |||
| NAFLD, not NASH | 93 (29.3%) | 43 (24.3%) | 136 (27.5%) | |
| Borderline NASH | 136 (42.9%) | 85 (48.0%) | 221 (44.7%) | |
| Definite NASH | 88 (27.8%) | 49 (27.7%) | 137 (27.7%) | |
Abbreviations: BMI = body mass index, ALT = alanine aminotransferase, AST = aspartate aminotransferase, GGT = gamma-glutamyl transpeptidase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, HOMA-IR = homeostasis model of assessment - insulin resistance
We defined high blood pressure as systolic or diastolic blood pressure ≧95th percentile for age, sex and height or the use of antihypertensive medication. Blood pressure percentiles were computed as instructed in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.
P values determined from chi square tests for categorical variables and from two-sample Wilcoxon rank sum tests for continuous variables due to the presence of non-normality.
Age range from 2–17 years.
HOMA-IR units are (mg/dL×IU/mL/405).
High Blood Pressure at Baseline
The estimated prevalence of high blood pressure at baseline was 35.8% (95% CI 31.7–40.2). As shown in Table 1 , children with and without high blood pressure did not differ significantly by age, sex or race. Children with high blood pressure had a significantly higher mean BMI than children without high blood pressure (34.6 vs. 31.6 kg/m2; P<0.0001). Children with high blood pressure also had significantly higher GGT, LDL-cholesterol, serum fasting insulin, and uric acid values. In addition, children with high blood pressure had significantly more severe steatosis (mild: 19.8%, moderate: 35.0%, severe: 45.2%) than children without high blood pressure (mild: 34.2%, moderate: 30.7%, severe: 35.1%, P = 0.003). As shown in Table 2 , each one unit increase in BMI was associated with 10% greater odds of having high blood pressure (95%CI: 6%–14%). There was a significant linear relationship between LDL-cholesterol and odds of high blood pressure (OR [95%CI]: 1.09 per 10 mg/dL [1.02, 1.17]). In addition, for every 1 mg/dL of uric acid there was a 25% increase in the odds of having high blood pressure (95%CI: 6%–48%).
Table 2. Clinical, Demographic, and Biochemical Risk Factors for High Blood Pressure at Baseline.
| Odds Ratios (OR) for High Blood Pressurea | ||||
| Single variable logistic | Adjusted, Multivariable Logistic | |||
| Risk Factors | OR (95%CI) | P Valueb | OR (95% CI) | P Valueb |
| Demographics | ||||
| Male vs Female | 0.83 (0.55, 1.25) | 0.37 | 0.71 (0.45, 1.12) | 0.14 |
| Age/year | 0.98 (0.91, 1.04) | 0.47 | 0.82 (0.75, 0.90) | <0.001 |
| Race/ethnicity | 0.05 | 0.36 | ||
| Non-Hispanic white | 1.0 (Reference) | 1.0 (Reference) | ||
| Hispanic | 0.63 (0.42, 0.94) | 0.03 | 0.84 (0.53, 1.33) | 0.46 |
| Other | 1.10 (0.49, 2.44) | 0.82 | 1.51 (0.62, 3.63) | 0.36 |
| Anthropomorphic | ||||
| BMI/(kg/m2) | 1.08 (1.04, 1.11) | <0.001 | 1.10 (1.06, 1.14) | <0.001 |
| Liver enzymes | ||||
| GGT/(10 U/L) | 1.06 (1.00, 1.12) | 0.04 | 1.03 (0.96, 1.09) | 0.44 |
| Serum chemistries | ||||
| LDL/(10 mg/dL) | 1.07 (1.01, 1.14) | 0.03 | 1.10 (1.03, 1.18) | 0.006 |
| Serum glucose/(10 mg/dL) | 1.06 (0.95, 1.18) | 0.32 | 1.07 (0.96, 1.19) | 0.25 |
| Serum insulin/(10 µU/mL) | 1.05 (0.99, 1.11) | 0.11 | 1.01 (0.96, 1.07) | 0.70 |
| Uric acid/(mg/dL) | 1.18 (1.03, 1.35) | 0.02 | 1.25 (1.05, 1.49) | 0.01 |
| Liver histology | ||||
| Steatosis grade>33% | 2.11 (1.36, 3.26) | 0.001 | 2.26 (1.39, 3.66) | 0.001 |
| Intercept | 0.01 (0.001, 0.08) | <0.001 | ||
| Hosmer-Lemeshow χ2 for model fit | 0.94 | |||
Abbreviations: OR = odds ratio, CI = confidence interval, BMI = body mass index, GGT = gamma-glutamyl transpeptidase, LDL = low-density lipoprotein.
High blood pressure was defined as systolic or diastolic blood pressure ≧95th percentile for age, sex and height or the use of antihypertensive medication. Blood pressure percentiles were computed as instructed in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.
P values and 95% CI were obtained from Wald statistics.
Persistent High Blood Pressure
The estimated prevalence of persistent high blood pressure was 21.4% (95%CI 17.6–25.9). Girls with NAFLD were significantly more likely to have persistent high blood pressure than boys with NAFLD (28.4% [20.5%–38.0%] vs.18.9% [14.7%–24.0%]). As shown in Table 3 , children with persistent high blood pressure were more likely to be non-Hispanic white (36.6% vs. 27.7%). Similar to the differences seen at baseline, children with persistent high blood pressure had significantly higher values of GGT, LDL-cholesterol, insulin, and uric acid than children without persistent high blood pressure. There was no significant difference in the severity of any histologic feature between children with and without persistent high blood pressure. In multivariate analysis ( Table 4 ), boys with NAFLD had 45% lower odds of having persistent high blood pressure than girls with NAFLD (95%CI: 3–69%). BMI, LDL-cholesterol, and uric acid were all significantly positively associated with the odds of having persistent high blood pressure (OR[95%CI]: 1.10[1.05, 1.15], 1.12[1.03, 1.23], 1.32[1.06, 1.64], respectively). Sensitivity analysis of variation in risk factors by gender showed no evidence of effect modification (interaction P range from 0.12 to 0.81). Additionally, the set of risk factors was not related to the odds of missing the 48 week follow-up visit (P = 0.41).
Table 3. Persistently High Blood Pressure in Children with NAFLD—Baseline Characteristics.
| Persistently High Blood Pressurea | |||
| Characteristics | No | Yes | P Valueb |
| N (%) or mean±SD | N = 300 | N = 82 | |
| Blood pressure | |||
| Systolic blood pressure percentile | 71.0±25.9 | 95.6±12.0 | <0.0001 |
| Diastolic blood pressure percentile | 56.6±24.2 | 73.8±24.7 | <0.0001 |
| Demographics | |||
| Male | 227 (75.7%) | 53 (64.6%) | 0.05 |
| Age (years)c | 13.0±2.7 | 13.0±2.6 | 0.85 |
| <8 years | 7 (2.3%) | 2 (2.5%) | 0.96 |
| 8–12 years | 145 (48.3%) | 41 (50.0%) | |
| 13–17 years | 148 (49.3%) | 39 (47.6%) | |
| Race/ethnicity | 0.007 | ||
| Non-Hispanic white | 83 (27.7%) | 30 (36.6%) | |
| Hispanic | 205 (68.3%) | 43 (52.4%) | |
| Other | 12 (4.0%) | 9 (11.0%) | |
| Anthropomorphic | |||
| BMI z-score | 2.3±0.5 | 2.5±0.3 | <0.0001 |
| BMI (kg/m2) | 32.1±6.4 | 35.3±6.1 | <0.0001 |
| Liver enzymes | |||
| ALT (U/L) | 106.4±84.9 | 110.2±82.4 | 0.77 |
| AST (U/L) | 62.8±44.2 | 69.6±53.1 | 0.50 |
| GGT (U/L) | 43.9±31.8 | 50.7±36.7 | 0.04 |
| Serum chemistries | |||
| HDL (mg/dL) | 38.4±8.3 | 37.8±11.3 | 0.08 |
| LDL (mg/dL) | 100.3±29.6 | 108.8±29.8 | 0.03 |
| Serum glucose (mg/dL) | 88.6±18.2 | 90.7±17.2 | 0.05 |
| Serum insulin (µU/mL) | 33.5±39.3 | 39.1±32.4 | 0.04 |
| HOMA-IRd | 7.4±9.5 | 8.9±7.8 | 0.04 |
| Uric acid (mg/dL) | 6.0±1.4 | 6.4±1.5 | 0.03 |
| Liver Histology | |||
| Steatosis grade | 0.45 | ||
| <33% | 91 (30.3%) | 19 (23.2%) | |
| 34–66% | 91 (30.3%) | 27 (32.9%) | |
| >66% | 118 (49.3%) | 36 (43.9%) | |
| Lobular inflammation | 0.19 | ||
| <2 under 20x | 151 (50.3%) | 47 (57.3%) | |
| 2–4 under 20x | 133 (44.3%) | 28 (34.2%) | |
| >4 under 20x | 16 (5.3%) | 7 (8.5%) | |
| Ballooning | 0.44 | ||
| None | 162 (54.0%) | 42 (51.2%) | |
| Few | 86 (28.7%) | 29 (35.4%) | |
| Many | 52 (17.3%) | 11 (13.4%) | |
| Fibrosis stage | 0.58 | ||
| None | 101 (33.8%) | 22 (27.2%) | |
| Zone 3, perisinusoidal or portral/periportal only | 114 (38.1%) | 30 (37.0%) | |
| Zone 3, periportal | 46 (15.4%) | 16 (19.8%) | |
| Bridging | 35 (11.7%) | 11 (13.6%) | |
| Cirrhosis | 3 (1.0%) | 2 (2.5%) | |
| Diagnosis | 0.83 | ||
| NAFLD, not NASH | 87 (29.0%) | 22 (26.8%) | |
| Borderline NASH | 135 (45.0%) | 36 (43.9%) | |
| Definite NASH | 78 (26.0%) | 24 (29.3%) | |
Abbreviations: BMI = body mass index, ALT = alanine aminotransferase, AST = aspartate aminotransferase, GGT = gamma-glutamyl transpeptidase, HDL = high-density lipoprotein, LDL = low-density lipoprotein, HOMA-IR = homeostasis model of assessment - insulin resistance.
We defined persistently high blood pressure as systolic or diastolic blood pressure ≧95th percentile for age, sex and height or the use of antihypertensive medication at both baseline and 48 week follow-up. Blood pressure percentiles were computed as instructed in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.
P values determined from chi square tests for categorical variables and from two-sample Wilcoxon rank sum tests for continuous variables due to the presence of non-normality.
Age range from 2–17 years.
HOMA-IR units are (mg/dL × IU/mL/405).
Table 4. Clinical, Demographic, and Biochemical Risk Factors for Persistently High Blood Pressure.
| Odds Ratios (OR) of Persistently High Blood Pressurea | ||||
| Unadjusted | Adjusted, Multivariable Logistic | |||
| Characteristic | OR (95%CI) | P Valueb | OR (95% CI) | P Valueb |
| Demographics | ||||
| Male vs female | 0.59 (0.35, 0.99) | 0.05 | 0.49 (0.27, 0.88) | 0.02 |
| Age/years | 1.00 (0.91, 1.09) | 0.98 | 0.82 (0.73, 0.93) | 0.001 |
| Race/ethnicity | 0.009 | 0.12 | ||
| Non-Hispanic white | 1.0 (Reference) | 1.0 (Reference) | ||
| Hispanic | 0.58 (0.34, 0.99) | 0.05 | 0.88 (0.48, 1.61) | 0.67 |
| Other | 2.08 (0.79, 5.42) | 0.14 | 2.58 (0.90, 7.37) | 0.08 |
| Anthropomorphic | ||||
| BMI/(kg/m2) | 1.07 (1.03 1.11) | <0.001 | 1.10 (1.05, 1.15) | <0.001 |
| Liver enzymes | ||||
| GGT/(10 U/L) | 1.06 (0.99, 1.13) | 0.10 | 1.02 (0.95, 1.11) | 0.55 |
| Serum chemistries | ||||
| LDL/(10 mg/dL) | 1.10 (1.01, 1.19) | 0.02 | 1.14 (1.04, 1.24) | 0.006 |
| Serum glucose/(10 mg/dL) | 1.06 (0.94, 1.19) | 0.38 | 1.07 (0.94, 1.23) | 0.31 |
| Serum insulin/(10 µU/mL) | 1.03 (0.98, 1.09) | 0.26 | 1.00 (0.94, 1.07) | 0.91 |
| Uric acid/(mg/dL) | 1.24 (1.04, 1.48) | 0.02 | 1.34 (1.07, 1.67) | 0.01 |
| Liver histology | ||||
| Steatosis grade>33% | 1.44 (0.82, 2.55) | 0.21 | 1.82 (0.96, 3.44) | 0.07 |
| Intercept | 0.003 (0.0002, 0.05) | <0.001 | ||
| Hosmer-Lemeshow χ2 for model fit | 0.59 | |||
Abbreviations: OR = odds ratio, CI = confidence interval, BMI = body mass index, GGT = gamma-glutamyl transpeptidase, LDL = low-density lipoprotein.
Persistently high blood pressure was defined as systolic or diastolic blood pressure ≧95th percentile for age, sex and height or the use of antihypertensive medication at both baseline and 48 weeks. Blood pressure percentiles were computed as instructed in The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents.
P values and 95% CI were obtained from Wald statistics.
Clinical Hypertension
At baseline, 18% (32/177) of children with high blood pressure had a clinical diagnosis of hypertension. There were 10 children taking antihypertensive medication representing 5% of those with high blood pressure and 31% of those with a clinical diagnosis of hypertension. Over the course of one year of follow-up, there were an additional 10 children diagnosed with hypertension. Antihypertensive medications were prescribed to 2 additional children who had a clinical diagnosis of hypertension at baseline and to 2 of the children subsequently diagnosed with hypertension. At week 48, 28% (23/82) of children with persistent high blood pressure had a clinical diagnosis of hypertension and 13% were taking antihypertensive medications.
Discussion
We studied the prevalence of high blood pressure in a longitudinal cohort study of children with NAFLD from pediatric centers across the United States. Children with NAFLD had a high rate of high blood pressure both at baseline and again at 48 weeks. The odds of having high blood pressure at baseline and high blood pressure that persisted at 48 weeks were associated with BMI, LDL-cholesterol and uric acid. Hepatic steatosis was associated with high blood pressure at baseline. Unexpectedly, girls with NAFLD had greater odds of persistent high blood pressure than boys with NAFLD.
The rates of high blood pressure in children with NAFLD exceeded what would be expected based upon the contribution of obesity alone. Population-based cohort studies estimate the prevalence of high blood pressure in obese children to be 11%. [2], [19]–[21] Although most children with NAFLD are overweight or obese, our finding that more than one of every three children with NAFLD had high blood pressure at baseline indicates that children with NAFLD are at particularly increased risk for high blood pressure. A previous single center study in overweight and obese children with biopsy-confirmed NAFLD demonstrated that mean systolic and diastolic blood pressure were significantly higher compared to overweight and obese controls without evidence of NAFLD. [9] Similarly, studies in children have shown that hepatic steatosis, independent of degree of obesity, is associated with cardiac dysfunction. [22], [23] Notably, in our cohort, children with NAFLD who had high blood pressure at baseline had higher degrees of hepatic steatosis. Data are extremely limited on the persistence of high blood pressure in children. In our study, the prevalence of 21.4% for persistent high blood pressure over 48 weeks in children with NAFLD was much higher than reported for other groups of children with longitudinal data available. For example, in the National Heart, Lung, and Blood Institute Growth and Health Study, the rate of persistent high blood pressure over 18 months in girls was 0.6% overall and 3% in obese girls. [21]
NAFLD and high blood pressure share pathophysiologic factors such as systemic oxidative stress and vascular and adipose tissue inflammation, which can produce vascular endothelial dysfunction. [24]–[28] NAFLD is associated with endothelial dysfunction independent of obesity and other metabolic syndrome features. [26] In the setting of hepatic steatosis, liver endothelial dysfunction can occur even prior to development of hepatic inflammation and fibrosis. [29] While it is not yet clear whether hypertension is a cause or consequence of endothelial dysfunction, exogenous infusion of endothelium-derived nitric oxide synthase inhibitors can produce hypertension in humans. [30] Our finding that elevated serum levels of LDL-cholesterol and uric acid were associated with increased odds for both baseline and persistent high blood pressure in this cohort also supports a possible role for underlying endothelial dysfunction. High levels of LDL cholesterol have been shown to alter the activity of endothelial-derived nitric oxide synthase. [31] Oxidized LDL is also associated with endothelial dysfunction and activation of the renin-angiotensin system. [32], [33] Likewise, elevated uric acid has been functionally linked to decreased endothelial nitric oxide synthase activity and nitric oxide production and in turn endothelial dysfunction. [34], [35] Elevated uric acid levels have been reported in children with NAFLD, possibly due to high dietary fructose intake. [36] Serum uric acid levels in childhood have been shown to predict high blood pressure beginning in childhood and persisting into adulthood. [37], [38] Thus, common pathophysiological processes may play a role in the development of both NAFLD and high blood pressure.
High blood pressure in childhood tracks into adulthood. [4], [5] High blood pressure in children with NAFLD is, therefore, likely to persist and place these children at risk for premature morbidity and mortality. Systolic blood pressure in childhood is a consistent predictor of arterial stiffness in adults. [39] Of the various metabolic syndrome factors, systolic blood pressure in childhood has the strongest correlation with coronary artery atherosclerosis in adulthood. [6] Additionally, adolescents with NAFLD have been shown to have left ventricular dysfunction compared to obese adolescents without NAFLD. [23] In adults with NAFLD, high blood pressure is linked to each of the three most common causes of death; cardiovascular disease, cancer, and liver disease. [40] Furthermore, in adults with NASH, the presence of hypertension is an important risk factor for the development of hepatocellular carcinoma. [41] Despite the potential for adverse outcomes, our data suggest that there is likely an underdiagnosis of high blood pressure in children with NAFLD. Future studies should assess interventions to improve the detection and control of high blood pressure in children with NAFLD.
The accurate assessment of blood pressure in children with NAFLD was strengthened by the use of the NASH CRN, the largest prospectively enrolled cohort of children with NAFLD with representation from across the U.S. Participants had an accurate diagnosis of NAFLD characterized in a rigorous standardized fashion. Moreover, the inclusion of longitudinal data is rare and particularly important for blood pressure research. The study was limited by the lack of three discrete measures required to confirm a clinical diagnosis of hypertension. However, large longitudinal cohort studies have shown that a single measurement of high blood pressure in childhood is strongly associated with development of hypertension in adulthood. [4] Furthermore, persistent single-time point measurements of high blood pressure in childhood carry an even greater risk of subsequent hypertension. [42] Future studies should consider the use of ambulatory blood pressure monitoring to better define the blood pressure phenotype in children with NAFLD. [43] Finally, long-term follow-up data are needed to assess for the development of adverse clinical outcomes associated with high blood pressure in children with NAFLD.
In conclusion, NAFLD is a common clinical problem that places children at substantial risk for high blood pressure, which may often go undiagnosed. Thus blood pressure evaluation and control should be an integral component of the clinical management of children with NAFLD.
Acknowledgments
Authors' Declarations:
Jeffrey Schwimmer, MD and James Tonascia, PhD had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Members of the Nonalcoholic Steatohepatitis Clinical Research Network Pediatric Clinical Centers
Baylor College of Medicine, Houston, TX: Stephanie H. Abrams, MD, MS; Sarah Barlow, MD; Ryan Himes, MD; Rajesh Krisnamurthy, MD; Leanel Maldonado, RN (2007–2012); Beverly Morris
Cincinnati Children's Hospital Medical Center, Cincinnati, OH: Kimberlee Bernstein, BS, CCRP; Kim Cecil, PhD; Stephanie DeVore, MSPH (2009–2011); Rohit Kohli, MD; Kathleen Lake, MSW (2009–2012); Daniel Podberesky, MD; Crystal Slaughter, BA, CCRP; Stavra Xanthakos, MD
Columbia University, New York, NY: Gerald Behr, MD; Joel E. Lavine, MD, PhD; Ali Mencin, MD; Nadia Ovchinsky, MD; Elena Reynoso, MD
Emory University, Atlanta, GA: Adina Alazraki, MD; Rebecca Cleeton, MPH; Saul Karpen, MD, PhD; Nicholas Raviele; Miriam Vos, MD, MSPH
Indiana University School of Medicine, Indianapolis, IN: Elizabeth Byam, RN; Oscar W. Cummings, MD; Cynthia Fleming, RN, MSN; Ann Klipsch, RN; Jean P. Molleston, MD; Kumar Sandrasegaran, MD; Girish Subbarao, MD
Johns Hopkins Hospital, Baltimore, MD: Kimberly Pfeifer, RN; Ann Scheimann, MD; Michael Torbenson, MD
Mount Sinai Kravis Children's Hospital, New York, NY: Ronen Arnon, MD; Mariel Boyd, CCRP
Northwestern University Feinberg School of Medicine/Ann & Robert H. Lurie Children's Hospital of Chicago: Katie Amsden, Mark H. Fishbein, MD; Elizabeth Kirwan, RN; Saeed Mohammad, MD; Ann Quinn, RD (2010–2012); Cynthia Rigsby, MD; Peter F. Whitington, MD
Saint Louis University, St Louis, MO: Jose Derdoy, MD (2007–2011); Ajay Jain MD; Debra King, RN; Pat Osmack; Joan Siegner, RN; Susan Stewart, RN; Dana Romo
University of California San Diego, San Diego, CA: Jorge Angeles; Sandra Arroyo; Hannah I. Awai, MD; Cynthia Behling, MD, PhD; Craig Bross; Jennifer Collins; Janis Durelle; Michael Middleton, MD, PhD; Kimberly Newton, MD; Melissa Paiz; Jeffrey B. Schwimmer, MD; Claude Sirlin, MD; Patricia Ugalde-Nicalo, MAS; Anne Zepeda, MAS
University of California San Francisco, San Francisco, CA: Bradley Aouizerat, PhD; Linda D. Ferrell, MD; Shannon Fleck; Ryan Gill, MD, PhD; Camille Langlois; Emily Rothbaum Perito, MD; Philip Rosenthal, MD; Patrika Tsai, MD
University of Washington Medical Center and Seattle Children's Hospital, Seattle, WA: Kara Cooper; Simon Horslen, MB ChB; Evelyn Hsu, MD; Karen Murray, MD; Randolph Otto, MD; Deana Rich; Matthew Yeh, MD, PhD; Melissa Young
Washington University, St. Louis, MO: Elizabeth M. Brunt, MD; Kathryn Fowler, MD
Resource Centers
National Cancer Institute, Bethesda, MD: David E. Kleiner, MD, PhD
National Institute of Child Health and Human Development, Bethesda, MD: Gilman D. Grave, MD
National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD: Edward C. Doo, MD; Jay H. Hoofnagle, MD; Patricia R. Robuck, PhD, MPH (2002–2011); Averell Sherker, MD
Johns Hopkins University, Bloomberg School of Public Health (Data Coordinating Center), Baltimore, MD: Patricia Belt, BS; Jeanne M. Clark, MD, MPH; Erin Hallinan, MHS; Michele Donithan, MHS; Milana Isaacson, BS; Kevin P. May, MS; Laura Miriel, BS; Alice Sternberg, ScM; James Tonascia, PhD; Aynur Ünalp-Arida, MD, PhD; Mark Van Natta, MHS; Ivana Vaughn, MPH; Laura Wilson, ScM; Katherine Yates, ScM
Data Availability
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available to qualified researchers from the NIDDK NASH CRN via the following url: https://www.niddkrepository.org/studies/nafld_pediatric/.
Funding Statement
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases (www.niddk.nih.gov) grant numbers U01DK061718, U01DK061728, U01DK061731, U01DK061732, U01DK061734, U01DK061737, U01DK061738, U01DK061730, and U01DK061713. Role of the sponsor: The National Institute of Diabetes and Digestive and Kidney Diseases provided scientific advice for the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, and approval of the manuscript. However, the content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was also supported by the National Center for Advancing Translational Sciences (www.ncats.nih.gov) grant numbers UL1TR000439, UL1TR000077, UL1TR000436, UL1TR000150, UL1TR000424, UL1TR000006, UL1TR000448, UL1TR000040, UL RR031980, UL1TR000100, UL1TR000004, UL1TR000423, UL1TR000058, UL1TR000067, and UL1TR000454. The National Center for Advancing Translational Sciences had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, et al. (2006) Prevalence of fatty liver in children and adolescents. Pediatrics 118:1388–1393. [DOI] [PubMed] [Google Scholar]
- 2. Din-Dzietham R, Liu Y, Bielo MV, Shamsa F (2007) High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation 116:1488–1496. [DOI] [PubMed] [Google Scholar]
- 3. Rosner B, Cook NR, Daniels S, Falkner B (2013) Childhood blood pressure trends and risk factors for high blood pressure: the NHANES experience 1988-2008. Hypertension 62:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bao W, Threefoot SA, Srinivasan SR, Berenson GS (1995) Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens 8:657–665. [DOI] [PubMed] [Google Scholar]
- 5. Chen X, Wang Y (2008) Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 117:3171–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartiala O, Magnussen CG, Kajander S, Knuuti J, Ukkonen H, et al. (2012) Adolescence risk factors are predictive of coronary artery calcification at middle age: the cardiovascular risk in young Finns study. J Am Coll Cardiol 60:1364–1370. [DOI] [PubMed] [Google Scholar]
- 7. Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, et al. (2006) Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 44:865–873. [DOI] [PubMed] [Google Scholar]
- 8. Pacifico L, Cantisani V, Ricci P, Osborn JF, Schiavo E, et al. (2008) Nonalcoholic fatty liver disease and carotid atherosclerosis in children. Pediatr Res 63:423–427. [DOI] [PubMed] [Google Scholar]
- 9. Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S (2008) Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation 118:277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, et al. (2005) The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology 129:113–121. [DOI] [PubMed] [Google Scholar]
- 11. Ong JP, Pitts A, Younossi ZM (2008) Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol 49:608–612. [DOI] [PubMed] [Google Scholar]
- 12. Patton HM, Yates K, Unalp-Arida A, Behling CA, Huang TT, et al. (2010) Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol 105:2093–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manco M, Marcellini M, Devito R, Comparcola D, Sartorelli MR, et al. (2008) Metabolic syndrome and liver histology in paediatric non-alcoholic steatohepatitis. Int J Obes (Lond) 32:381–387. [DOI] [PubMed] [Google Scholar]
- 14. Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, et al. (2011) Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA 305:1659–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, et al. (2008) Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology 135:1961–1971.e1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Schwimmer JB, Newton KP, Awai HI, Choi LJ, Garcia MA, et al. (2013) Paediatric gastroenterology evaluation of overweight and obese children referred from primary care for suspected non-alcoholic fatty liver disease. Aliment Pharmacol Ther 38:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, et al. (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41:1313–1321. [DOI] [PubMed] [Google Scholar]
- 18. U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576. [PubMed] [Google Scholar]
- 19. Acosta AA, Samuels JA, Portman RJ, Redwine KM (2012) Prevalence of persistent prehypertension in adolescents. J Pediatr 160:757–761. [DOI] [PubMed] [Google Scholar]
- 20.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, et al. (2007) Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr 150: 640–644, 644.e641. [DOI] [PubMed]
- 21.Obarzanek E, Wu CO, Cutler JA, Kavey RE, Pearson GD, et al. (2010) Prevalence and incidence of hypertension in adolescent girls. J Pediatr 157: 461–467, 467.e461–465. [DOI] [PubMed]
- 22. Pacifico L, Di Martino M, De Merulis A, Bezzi M, Osborn JF, et al. (2014) Left ventricular dysfunction in obese children and adolescents with nonalcoholic fatty liver disease. Hepatology 59:461–470. [DOI] [PubMed] [Google Scholar]
- 23.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, et al. (2013) Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr 162: 1160–1168, 1168.e1161. [DOI] [PMC free article] [PubMed]
- 24. Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, et al. (2008) Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol 28:1654–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stanton MC, Chen SC, Jackson JV, Rojas-Triana A, Kinsley D, et al. (2011) Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J Inflamm (Lond) 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, et al. (2005) Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 42:473–480. [DOI] [PubMed] [Google Scholar]
- 27. Dharmashankar K, Widlansky ME (2010) Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep 12:448–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, et al. (2004) Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation 109:613–619. [DOI] [PubMed] [Google Scholar]
- 29. Pasarín M, La Mura V, Gracia-Sancho J, García-Calderó H, Rodríguez-Vilarrupla A, et al. (2012) Sinusoidal endothelial dysfunction precedes inflammation and fibrosis in a model of NAFLD. PLoS One 7:e32785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sander M, Chavoshan B, Victor RG (1999) A large blood pressure-raising effect of nitric oxide synthase inhibition in humans. Hypertension 33:937–942. [DOI] [PubMed] [Google Scholar]
- 31. Balligand JL (2002) New mechanisms of LDL-cholesterol induced endothelial dysfunction; correction by statins. Bull Mem Acad R Med Belg 157:427–431 discussion 431–424. [PubMed] [Google Scholar]
- 32. Woodman RJ, Watts GF, Playford DA, Best JD, Chan DC (2005) Oxidized LDL and small LDL particle size are independently predictive of a selective defect in microcirculatory endothelial function in type 2 diabetes. Diabetes Obes Metab 7:612–617. [DOI] [PubMed] [Google Scholar]
- 33. Catar RA, Müller G, Heidler J, Schmitz G, Bornstein SR, et al. (2007) Low-density lipoproteins induce the renin-angiotensin system and their receptors in human endothelial cells. Horm Metab Res 39:801–805. [DOI] [PubMed] [Google Scholar]
- 34. Zoccali C, Maio R, Mallamaci F, Sesti G, Perticone F (2006) Uric acid and endothelial dysfunction in essential hypertension. J Am Soc Nephrol 17:1466–1471. [DOI] [PubMed] [Google Scholar]
- 35. Park JH, Jin YM, Hwang S, Cho DH, Kang DH, et al. (2013) Uric acid attenuates nitric oxide production by decreasing the interaction between endothelial nitric oxide synthase and calmodulin in human umbilical vein endothelial cells: a mechanism for uric acid-induced cardiovascular disease development. Nitric Oxide 32:36–42. [DOI] [PubMed] [Google Scholar]
- 36. Vos MB, Colvin R, Belt P, Molleston JP, Murray KF, et al. (2012) Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J Pediatr Gastroenterol Nutr 54:90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Viazzi F, Antolini L, Giussani M, Brambilla P, Galbiati S, et al. (2013) Serum uric acid and blood pressure in children at cardiovascular risk. Pediatrics 132:e93–99. [DOI] [PubMed] [Google Scholar]
- 38. Alper AB, Chen W, Yau L, Srinivasan SR, Berenson GS, et al. (2005) Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 45:34–38. [DOI] [PubMed] [Google Scholar]
- 39. McGill HC, McMahan CA, Zieske AW, Tracy RE, Malcom GT, et al. (2000) Association of Coronary Heart Disease Risk Factors with microscopic qualities of coronary atherosclerosis in youth. Circulation 102:374–379. [DOI] [PubMed] [Google Scholar]
- 40. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, et al. (2003) Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 41. Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, et al. (2006) Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 43:682–689. [DOI] [PubMed] [Google Scholar]
- 42. Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, et al. (2007) Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics 119:237–246. [DOI] [PubMed] [Google Scholar]
- 43. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, et al. (2014) Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension 63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that, for approved reasons, some access restrictions apply to the data underlying the findings. Data are available to qualified researchers from the NIDDK NASH CRN via the following url: https://www.niddkrepository.org/studies/nafld_pediatric/.