Abstract
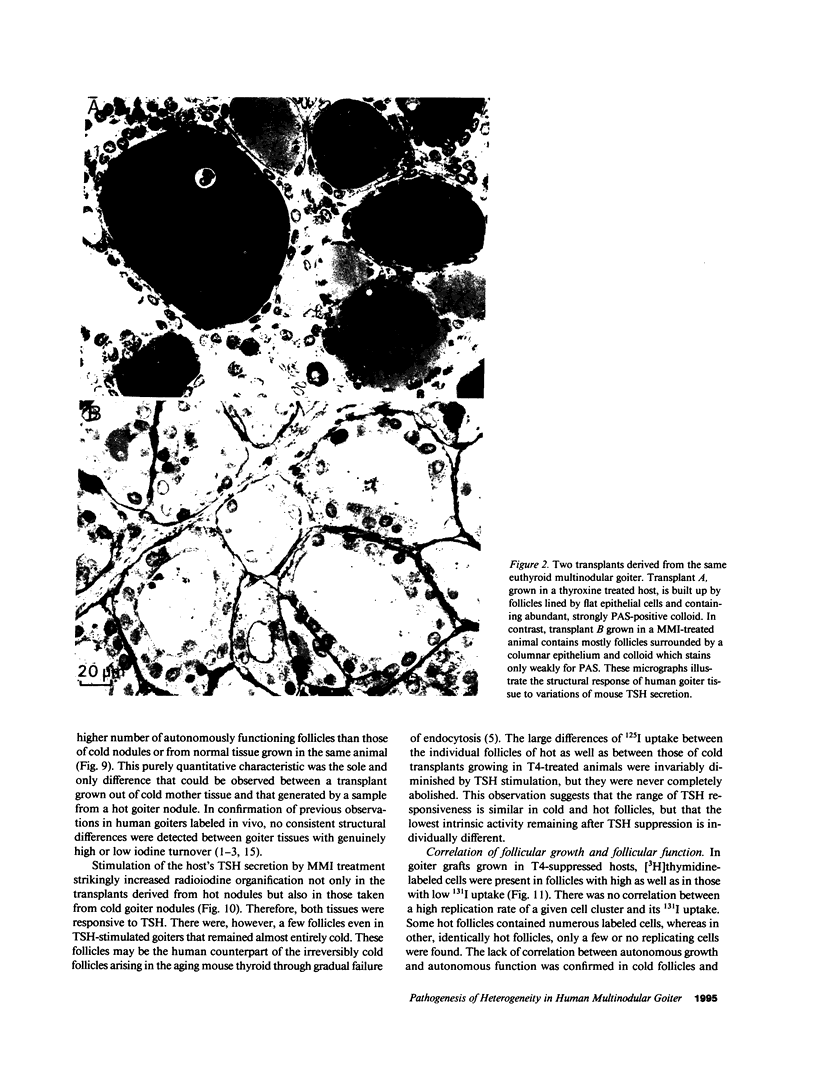
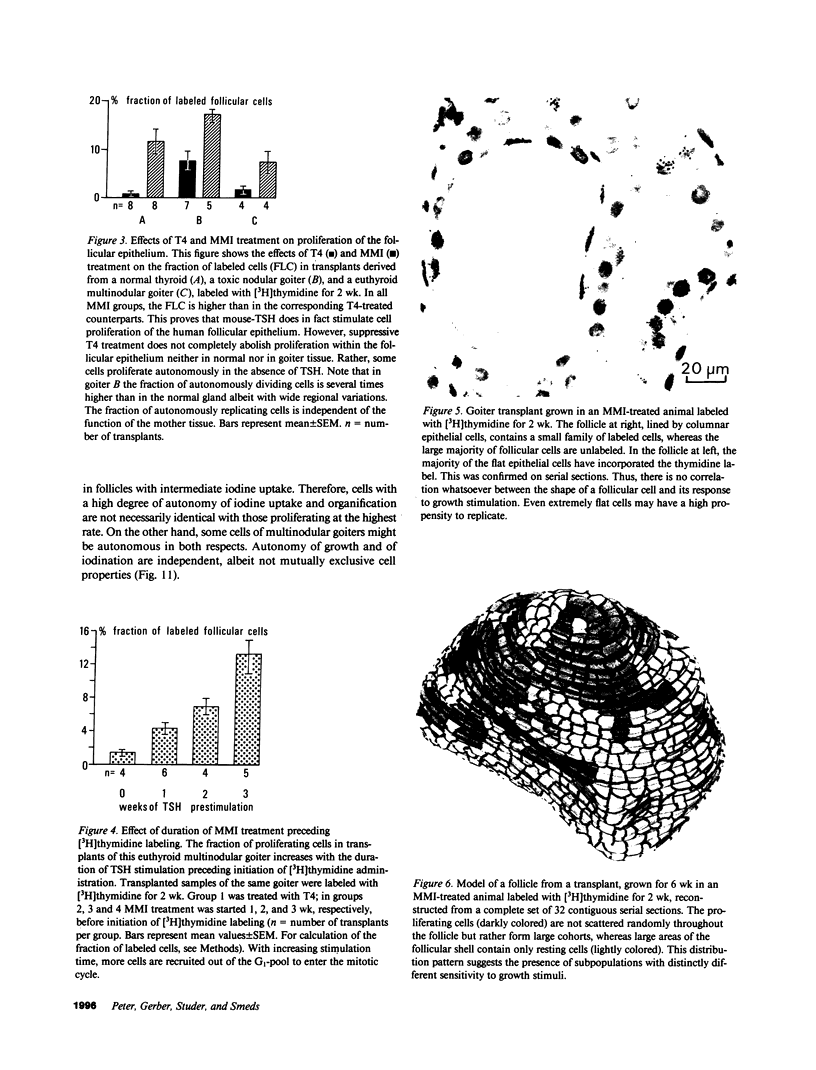
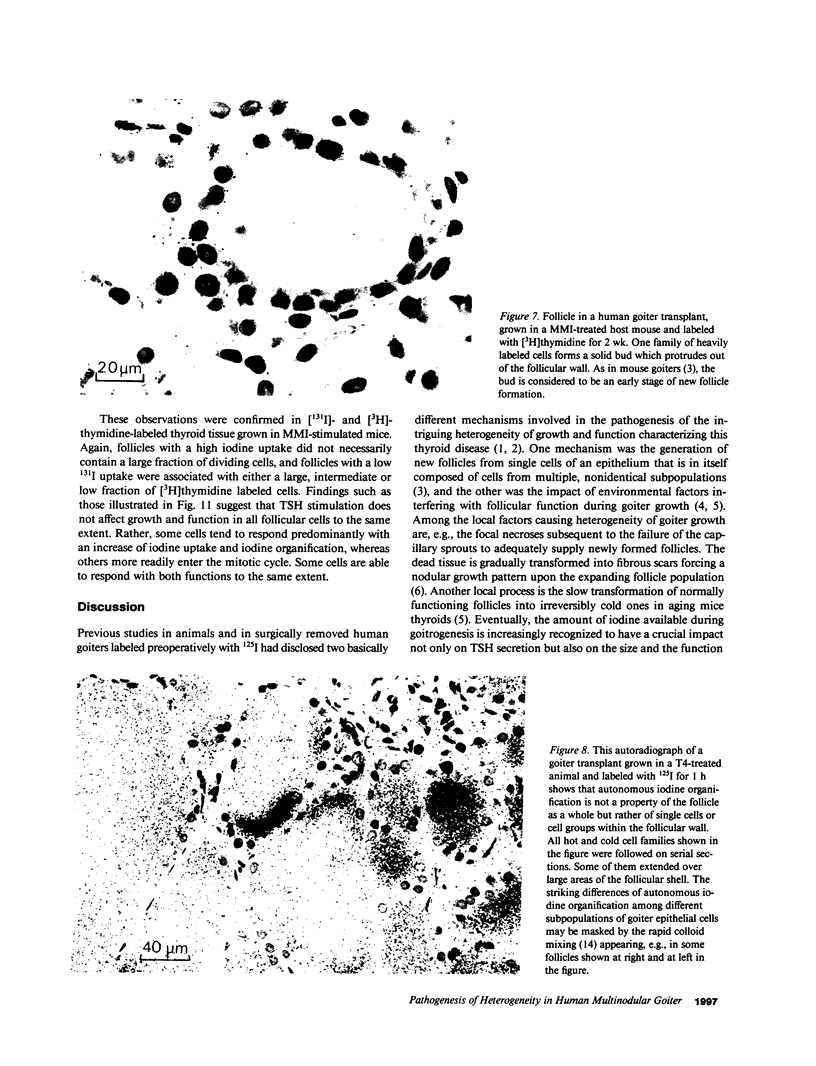
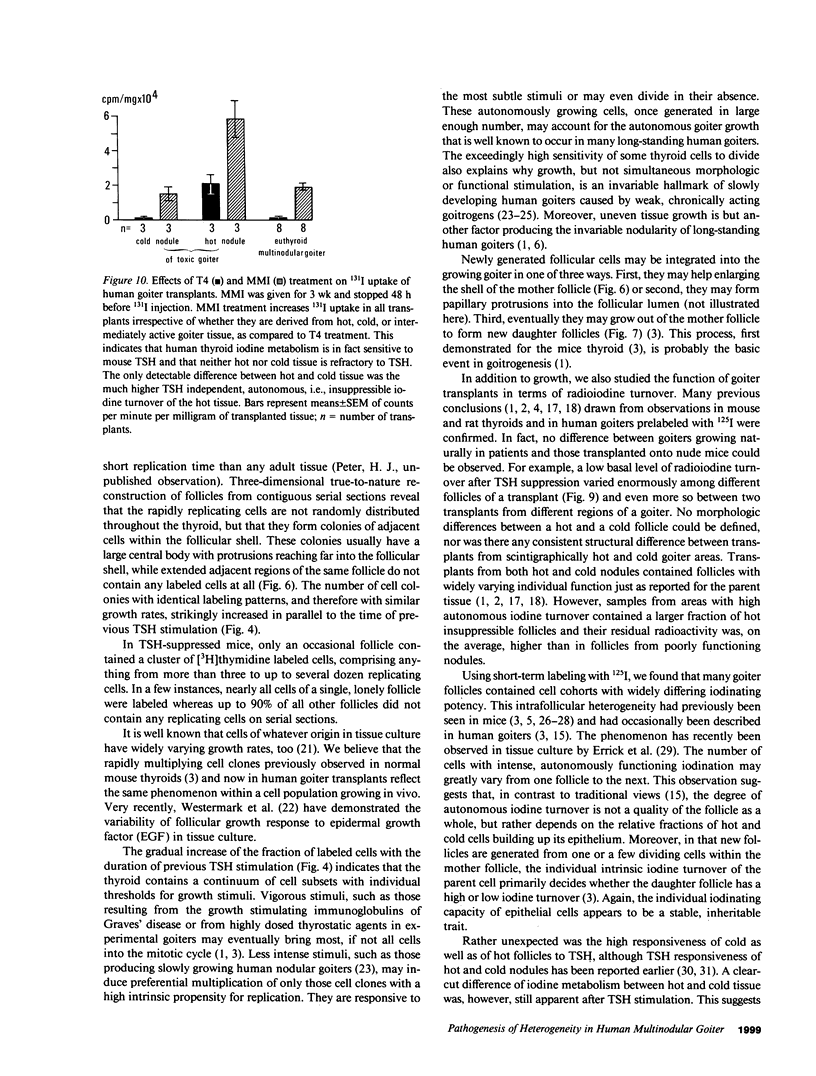
Functional and morphologic heterogeneity of human multinodular goiters was investigated in 300 samples from "cold" and "hot" regions of 20 goiters transplanted onto nude mice. Transplants were labeled with [3H]thymidine and radioiodine, while the host's thyroid-stimulating hormone (TSH) secretion was either stimulated or suppressed. Proliferation and function of follicular cells were assessed in whole follicles reconstructed from autoradiographs of serial sections. Hot transplants had a higher autonomous iodine uptake than those of cold tissue in TSH-suppressed hosts. Functional autonomy widely varied among the follicles, but even more so among individual cells. Hot grafts differed from cold ones only by a comparatively larger fraction of autonomous cells. Intercellular differences of iodinating activity were not abolished by TSH. Grafts faithfully reproduced the individual growth pattern of the original tissue. Between 0.5% and 7% of all follicular cells replicated despite suppression of TSH. Up to 70% of these cells were clustered, forming scattered foci of autonomously growing tissue. Other cells only started replicating after long-term TSH stimulation. Thus, goiters contained subsets of cells with high and others with low growth response. Progenies of replicating cells remained clustered, sometimes budding outwards to form new follicles. Autonomy of growth and autonomy of function are independent traits of epithelial cells. Epithelial cells have their individual growth pattern, replication rate, and functional capacity. These traits are passed on from a mother cell to its progeny during follicle neogenesis. To this main mechanism accounting for the morphologic and functional heterogeneity of human goiters, inheritable modifications of gene expression must probably be added.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht-Buehler G. Daughter 3T3 cells. Are they mirror images of each other? J Cell Biol. 1977 Mar;72(3):595–603. doi: 10.1083/jcb.72.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aufderheide K. J., Frankel J., Williams N. E. Formation and positioning of surface-related structures in protozoa. Microbiol Rev. 1980 Jun;44(2):252–302. doi: 10.1128/mr.44.2.252-302.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown R. S., Jackson I. M., Pohl S. L., Reichlin S. Do thyroid-stimulating immunoglobulins cause non-toxic and toxic multinodular goitre? Lancet. 1978 Apr 29;1(8070):904–906. doi: 10.1016/s0140-6736(78)90682-7. [DOI] [PubMed] [Google Scholar]
- Cairns J. Mutation selection and the natural history of cancer. Nature. 1975 May 15;255(5505):197–200. doi: 10.1038/255197a0. [DOI] [PubMed] [Google Scholar]
- DeRubertis F., Yamashita K., Dekker A., Larsen P. R., Field J. B. Effects of thyroid-stimulating hormone on adenyl cyclase activity and intermediary metabolism of "cold" thyroid nodules and normal human thyroid tissue. J Clin Invest. 1972 May;51(5):1109–1117. doi: 10.1172/JCI106903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dralle H., Böcker W., Döhler K. D., Schröder S., Haindl H., Geerlings H., Schwarzrock R., Pichlmayr R. Growth and function of thirty-four human benign and malignant thyroid xenografts in untreated nude mice. Cancer Res. 1985 Mar;45(3):1239–1245. [PubMed] [Google Scholar]
- Emrich D., Bähre M. Autonomy in euthyroid goitre: maladaptation to iodine deficiency. Clin Endocrinol (Oxf) 1978 Mar;8(3):257–265. doi: 10.1111/j.1365-2265.1978.tb01502.x. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. The homeo box: a key to the understanding of development? Cell. 1985 Jan;40(1):3–5. doi: 10.1016/0092-8674(85)90300-9. [DOI] [PubMed] [Google Scholar]
- Gerber H., Studer H., Conti A., Engler H., Kohler H., Haeberli A. Reaccumulation of thyroglobulin and colloid in rat and mouse thyroid follicles during intense thyrotropin stimulation. A clue to the pathogenesis of colloid goiters. J Clin Invest. 1981 Nov;68(5):1338–1347. doi: 10.1172/JCI110381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber H., Studer H., von Grünigen C. Paradoxical effects of thyrotropin on diffusion of thyroglobulin in the colloid of rat thyroid follicles after long term thyroxine treatment. Endocrinology. 1985 Jan;116(1):303–310. doi: 10.1210/endo-116-1-303. [DOI] [PubMed] [Google Scholar]
- Grubeck-Loebenstein B., Derfler K., Kassal H., Knapp W., Krisch K., Liszka K., Smyth P. P., Waldhäusl W. Immunological features of nonimmunogenic hyperthyroidism. J Clin Endocrinol Metab. 1985 Jan;60(1):150–155. doi: 10.1210/jcem-60-1-150. [DOI] [PubMed] [Google Scholar]
- Heppner G. H. Tumor heterogeneity. Cancer Res. 1984 Jun;44(6):2259–2265. [PubMed] [Google Scholar]
- Larsen P. R., Yamashita K., Dekker A., Field J. B. Biochemical observations in functioning human thyroid adenomas. J Clin Endocrinol Metab. 1973 May;36(5):1009–1018. doi: 10.1210/jcem-36-5-1009. [DOI] [PubMed] [Google Scholar]
- Leclere J., Bene M. C., Duprez A., Faure G., Thomas J. L., Vignaud J. M., Burlet C. Behaviour of thyroid tissue from patients with Graves' disease in nude mice. J Clin Endocrinol Metab. 1984 Jul;59(1):175–177. doi: 10.1210/jcem-59-1-175. [DOI] [PubMed] [Google Scholar]
- MCCLINTOCK B. Controlling elements and the gene. Cold Spring Harb Symp Quant Biol. 1956;21:197–216. doi: 10.1101/sqb.1956.021.01.017. [DOI] [PubMed] [Google Scholar]
- MILLER J. M., HORN R. C., BLOCK M. A. THE EVOLUTION OF TOXIC NODULAR GOITER. Arch Intern Med. 1964 Jan;113:72–88. doi: 10.1001/archinte.1964.00280070074014. [DOI] [PubMed] [Google Scholar]
- Miller J. M., Horn R. C., Block M. A. The autonomous functioning thyroid nodule in the evolution of nodular goiter. J Clin Endocrinol Metab. 1967 Sep;27(9):1264–1274. doi: 10.1210/jcem-27-9-1264. [DOI] [PubMed] [Google Scholar]
- Miller J. M. Plummer's disease. Med Clin North Am. 1975 Sep;59(5):1203–1216. doi: 10.1016/s0025-7125(16)31968-x. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Peter H. J., Studer H., Forster R., Gerber H. The pathogenesis of "hot" and "cold" follicles in multinodular goiters. J Clin Endocrinol Metab. 1982 Nov;55(5):941–946. doi: 10.1210/jcem-55-5-941. [DOI] [PubMed] [Google Scholar]
- Ramelli F., Studer H., Bruggisser D. Pathogenesis of thyroid nodules in multinodular goiter. Am J Pathol. 1982 Nov;109(2):215–223. [PMC free article] [PubMed] [Google Scholar]
- Razin A., Cedar H. DNA methylation in eukaryotic cells. Int Rev Cytol. 1984;92:159–185. doi: 10.1016/s0074-7696(08)61327-3. [DOI] [PubMed] [Google Scholar]
- Rentsch H., Studer H., Frauchiger B., Siebenhüner L. Topographical heterogeneity of basal and thyrotropin-stimulated adenosine 3'5'-monophosphate in human nodular goiter. J Clin Endocrinol Metab. 1981 Sep;53(3):514–521. doi: 10.1210/jcem-53-3-514. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Farabaugh P. J., Chaleff D. T., Fink G. R. The origins of gene instability in yeast. Science. 1980 Sep 19;209(4463):1375–1380. doi: 10.1126/science.6251544. [DOI] [PubMed] [Google Scholar]
- Roger P. P., Dumont J. E. Thyrotrophin and the differential expression of proliferation and differentiation in dog thyroid cells in primary culture. J Endocrinol. 1983 Feb;96(2):241–249. doi: 10.1677/joe.0.0960241. [DOI] [PubMed] [Google Scholar]
- Smeds S., Anderberg B., Boeryd B., Ericson L. E., Gillquist J., Persliden J. The nude mouse. A possible experimental model for investigation of human thyroid tissue. J Endocrinol Invest. 1981 Jan-Mar;4(1):11–15. doi: 10.1007/BF03349407. [DOI] [PubMed] [Google Scholar]
- Studer H., Forster R., Conti A., Kohler H., Haeberli A., Engler H. Transformation of normal follicles into thyrotropin-refractory "cold" follicles in the aging mouse thyroid gland. Endocrinology. 1978 May;102(5):1576–1586. doi: 10.1210/endo-102-5-1576. [DOI] [PubMed] [Google Scholar]
- Studer H., Ramelli F. Simple goiter and its variants: euthyroid and hyperthyroid multinodular goiters. Endocr Rev. 1982 Winter;3(1):40–61. doi: 10.1210/edrv-3-1-40. [DOI] [PubMed] [Google Scholar]
- WOLLMAN S. H., WODINSKY I. Localization of protein-bound I131 in the thyroid gland of the mouse. Endocrinology. 1955 Jan;56(1):9–20. doi: 10.1210/endo-56-1-9. [DOI] [PubMed] [Google Scholar]