Abstract
Biological nitrification/denitrification is frequently used to remove nitrogen from tannery wastewater containing high concentrations of ammonia. However, information is limited about the bacterial nitrifiers and denitrifiers and their functional genes in tannery wastewater treatment plants (WWTPs) due to the low-throughput of the previously used methods. In this study, 454 pyrosequencing and Illumina high-throughput sequencing, combined with molecular methods, were used to comprehensively characterize structures and functions of nitrification and denitrification bacterial communities in aerobic and anaerobic sludge of two full-scale tannery WWTPs. Pyrosequencing of 16S rRNA genes showed that Proteobacteria and Synergistetes dominated in the aerobic and anaerobic sludge, respectively. Ammonia-oxidizing bacteria (AOB) amoA gene cloning revealed that Nitrosomonas europaea dominated the ammonia-oxidizing community in the WWTPs. Metagenomic analysis showed that the denitrifiers mainly included the genera of Thauera, Paracoccus, Hyphomicrobium, Comamonas and Azoarcus, which may greatly contribute to the nitrogen removal in the two WWTPs. It is interesting that AOB and ammonia-oxidizing archaea had low abundance although both WWTPs demonstrated high ammonium removal efficiency. Good correlation between the qPCR and metagenomic analysis is observed for the quantification of functional genes amoA, nirK, nirS and nosZ, indicating that the metagenomic approach may be a promising method used to comprehensively investigate the abundance of functional genes of nitrifiers and denitrifiers in the environment.
Introduction
Tannery wastewater is characterized by high contents of nitrogen, organic compounds and salt ions [1],[2], and the complexity of the wastewater components can affect microbial nitrification/denitrification processes that have been frequently used for nitrogen removal in wastewater treatment plants (WWTPs). Nitrification, the biological oxidation of ammonium to nitrite and nitrate, is carried out in two sequential steps via several phylogenetically distinct groups of microorganisms: ammonia-oxidizing archaea (AOA), ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB) [3]. Denitrification consists of consecutive reactions including transformation of nitrate or nitrite into gaseous forms (N2 or N2O) by different groups of bacteria (nitrifiers and denitrifiers) [4].
Due to the technological difficulty in isolation and culture of bacterial nitrifiers and denitrifiers, molecular methods have been widely used to analyze diversity and abundance of microorganisms involved in nitrification/denitrification in various environments, such as soil [5] and activated sludge [4]. Previous studies have investigated the microbial community of activated sludge in tannery WWTPs through 16S rRNA gene amplification and DNA sequencing [1],[6]. However, information about abundance and diversity of nitrifying and denitrifying bacteria in tannery WWTPs is limited due to the low throughput of those techniques.
Recently, high-throughput sequencing techniques, such as 454 pyrosequencing and Illumina sequencing methods, have shown great advantages on microbial community analysis due to their unprecedented sequencing depth, which have been used to deeply explore microbial communities in drinking water [7], soil [8] and WWTPs [9]–[11]. However, the metagenomic methods have not been used for comprehensive insights into the communities of nitrifies and denitrifies in tannery WWTPs.
In this study, 454 pyrosequencing of 16S rRNA gene was conducted to determine the bacterial community structure of activated sludge sampled from two full-scale tannery WWTPs, and Illumina high-throughput sequencing was used to comprehensively investigate functional genes involved in the bacterial nitrification and denitrification. To confirm the metagenomic results, quantitative real time PCR (qPCR) was used to quantify the functional genes encoding subunits of the ammonia monooxygenase (amoA), nitrite reductases (nirK and nirS) and nitrous oxide reductase (nosZ). Since ammonia oxidation is a rate-limiting step in nitrification, the amoA gene was chosen for genetic diversity analysis using DNA cloning and sequencing technology. The results of this study may help to extend our knowledge about the microbial nitrification and denitrification processes and their underlying biological mechanisms for industrial wastewater treatment.
Materials and Methods
Sludge sampling and DNA extraction
In this study, activated sludge samples were separately collected from two full-scale WWTPs treating tannery wastewater of Boao Leather Industry Co., Ltd. (WWTPA geographically located in Xiangcheng City, Henan Province, China) and Yige Leather Industry Co., Ltd. (WWTPB geographically located in Zhecheng County, Henan Province, China). We would like to state that the two companies have approved this study which did not involve endangered or protected species. The two WWTPs basically involve a biological treatment system preceded by preliminary treatment (homogenization, chemical coagulation and primary settling). As described in our previous study [12], the biological treatment system of WWTPA was composed of an up-flow anaerobic sludge reactor (UASB) and an integrated anoxic/oxic (A/O) reactor, while WWTPB was composed of an oxidation ditch and an integrated anoxic/oxic (A/O) reactor. Relevant operational parameters about the two biological systems are shown in Table S1. Although the two WWTPs differed in the influent water quality and operational conditions, they both achieved high ammonia removal efficiencies (>97%). Thus, we chose the two WWTPs to explore the functional nitrifiers and denitrifiers responsible for the high ammonia removal. Four sludge samples were collected from the two WWTPs, including one anaerobic sludge sample (A-A) from the UASB and one aerobic sludge sample (A-O) from the last aerobic tank of the A/O reactor in WWTPA, and two aerobic sludge samples separately collected from the oxidation ditch (B-D) and the last aerobic tank of the A/O reactor (B-O) in WWTPB. The sludge samples were fixed with 50% ethanol (v/v) on site before transporting to laboratory for DNA extraction. The fixed sludge was centrifuged at 4,000 rpm for 10 min to collect approximately 200 mg of the pellet for total genomic DNA extraction with the FastDNA Soil Kit (MP Biomedicals, CA, USA). The concentration and quality of the extracted DNA were determined with microspectrophotometry (NanoDrop ND-1000, NanoDrop Technologies, Willmington, DE, USA).
PCR amplification, cloning, sequencing and phylogenetic analysis of amoA gene
PCR of AOA and AOB amoA genes was conducted by using the primers listed in Table S2. The 30-µL reaction mixture contained 3 µL of 10×buffer, 1.8 µL of 25 mM MgCl2, 1.2 µL of 10 mM dNTP mixture, 0.2 µL of Ex Taq polymerase (TaKaRa, Japan), 0.3 µL of each primer (10 µM) and 20–50 ng of genomic DNA. The PCR conditions were initial denaturation at 95°C for 3 min, followed by 35 cycles of 94°C for 60 s, 56°C (for AOB amoA) or 53°C (for AOA amoA) for 45 s, and 72°C for 60 s; with a final extension at 72°C for 10 min. PCR products were analyzed by gel electrophoresis using 1% (w/v) agarose in 1×TAE buffer.
PCR products were purified by using DNA Fragment Purification Kit (TaKaRa, Japan). The purified PCR products were cloned using pMD19-T Vector (TaKaRa, Japan). An AOB amoA gene clone library was constructed for each of the four sludge samples; however, AOA amoA gene library was constructed for only sample A-A since the other samples failed to generate PCR products. About 20 clones in each clone library were randomly selected for DNA sequencing. The obtained amoA gene sequences were aligned and the Jukes-Cantor distances between subsequent pairs of sequences were calculated with DNADIST from the PHYLIP package (http://www.phylip.com/), and operational taxonomic units (OTUs) were defined with a distance cut-off of 3% using the DOTUR program [13]. In order to construct the phylogenetic tree, one representative sequence in each OTU was selected and aligned with the reference sequences from National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/). The neighbor-joining phylogenetic trees of AOB and AOA amoA genes sequences were separately created by MEGA 5.1 software (http://www.megasoftware.net/).
qPCR
AOB and AOA amoA genes were used as molecular markers to determine the abundance of the nitrifiers. Meanwhile, the abundance of denitrifying bacteria was investigated by quantifying the genes nirK, nirS and nosZ. qPCR was performed in Corbett Real-Time PCR Machine with the Rotor-Gene 6000 Series Software 1.7 (QIAGEN, the Netherlands) using SYBR Green method. The 20-µL PCR mixture contained 10 µL of SYBR Premix Ex Taq Super Mix (TaKaRa Japan), 0.2 µL of each primer (10 µM) (Table S2), 8 µL of template DNA corresponding to 40 ng of total DNA and 1.6 µL of ddH2O. The reaction was initially denatured at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 20 s, annealing at the given temperatures (Table S2) for 20 s and extension at 72°C for 40 s.
To determine gene abundance in one ng of extracted DNA, all targeted genes were cloned to plasmids following the method recommended by Zhang and Zhang [14] to generate qPCR standard curves. In order to correct for potential variations in DNA extraction efficiencies, eubacterial 16S rRNA genes were also quantified using the method recommended by Lopez-Gutierrez et al. [15]. All samples and standards were analyzed in triplicate, and the specificity of qPCR products was checked by melt curves observation and agarose electrophoresis. PCR efficiency ranged from 85.9% to 105.5% with R2 values over 0.991 for all calibration curves.
454 Pyrosequencing and Illumina high-throughput sequencing
Pyrosequencing and Illumina sequencing were conducted at Beijing Genome Institute (Shenzhen, China). For pyrosequencing, the bacterial DNA was amplified with a set of primers targeting the hypervariable V3-V4 region (about 460 bp) of the 16S rRNA gene. The forward primer was V3F (5'-ACTCCTACGGGAG GCAGCAG-3') and the reverse primer was V4R (5'-TACNVGGGTATCTAATCC-3'). Equal amounts of purified amplicon products bearing individual 10 nucleotide barcode from different samples were mixed for pyrosequencing on the Roche 454 FLX Titanium platform (Roche, USA).
DNA samples (10 µg each) were subject to high-throughput sequencing using Illumina Hiseq 2000 (Illumina, USA). A library consisting of 180-bp DNA fragment sequences was constructed according to the manufacturer's instructions before DNA sequencing. The strategy “Index 101 PE” (Paired End sequencing, 101-bp reads and 8-bp index sequence) was used for the Illumina sequencing, generating nearly equal amount of data for each sample. Low quality reads were removed following the method recommended by Shi et al. [7]. The 100-bp clean reads (about 1.0 Gb per each sample) were used for subsequent metagenomic analysis.
Bioinformatic analysis on pyrosequencing datasets
After pyrosequencing, downstream sequence analyses were performed using the Ribosomal Database Project (RDP) (http://rdp.cme.msu.edu/) [16] and Mothur (http://www.mothur.org/) [17] following the method recommended by Zhang et al. [18]. All the raw reads were assigned to the designated sample based on their nucleotide barcode with the Pyrosequencing Pipeline Initial Process of the RDP, which also trimmed off the adapters and barcodes and removed sequences containing ambiguous ‘N’ or shorter than 200 bps. All samples were denoised individually using Mothur's ‘pre.cluster’ command to remove the erroneous sequences due to pyrosequencing errors. PCR chimeras were filtered out using the ‘chimera.slayer’ command in Mothur platform. The reads flagged as chimeras was extracted out using a self-written Python script, and those being assigned to any known genus with 90% confidence using the RDP Classifier were merged with the non-chimera reads.
Taxonomic assignment of the sequences was conducted individually for each sample using the RDP Classifier with a bootstrap cutoff of 50% [9],[18]. Unexpected archaeal sequences were manually removed before biodiversity analysis. After denoising, filtering out chimeras and removing the archaeal sequences, 6,471∼13,727 effective pyrosequencing reads were generated for each of the four sludge samples (Table S3). For fair comparison, the library size of each sample was normalized to the same sequencing depth (6,471 reads) by randomly removing the redundant reads. Richness and diversity indices including OTUs number and Shannon's diversity index, as well as rarefaction curves, were calculated using Mothur.Cluster analysis and principal component analysis (PCA) of the microbial community structures of the different samples were conducted with PAleontological STatistics software (PAST, version 3.01) [19] and R (version 3.0.1) [20], respectively.
Bioinformatic analysis on Illumina sequencing datasets
The Illumina sequencing datasets were functionally annotated using the Clusters of Orthologous Groups (COG) [21] database in MG-RAST [22] with default parameters. In order to quantify the abundance of functional genes involved in nitrification and denitrification in the four samples, sequences of amoA, nirK, nirS and nosZ deposited in NCBI Nucleotide database (http://www.ncbi.nlm.nih.gov/nuccore/) were downloaded to construct a local database. After removing redundancies, the local database contained 17,483 sequences of AOA amoA gene, 12,211 sequences of AOB amoA gene, 5,716 sequences of nirK gene, 7,615 sequences of nirS gene and 5,436 sequences of nosZ gene. BLASTn was used to align all the sequencing reads against the local database, and a read was identified as amoA, nirK, nirS or nosZ if the BLAST hit (E-value cutoff at 10−5) had a nucleotide sequence identity of above 90% over an alignment of at least 50 bp [10].
Additionally, the annotated reads were extracted by using a self-written Python script and then assigned to specific bacteria at the genus level by BLASTX against NCBI-nr database at an E-value of 1E-5. The BLASTX results were visualized with MEGAN (http://ab.inf.uni-tuebingen.de/software/megan/) at a threshold of bitscore>50 [10].
Accession numbers
The sequences of amoA for cloning library construction have been deposited in GenBank under accession numbers KF720406 to KF720513. The pyrosequencing datasets have been deposited into the NCBI Short Reads Archive Database (accession number: SRX396800). The Illumina metagenomic datasets are available at MG-RAST under accession numbers 4494863.3 (A-A), 4494888.3 (A-O), 4494854.3 (B-D) and 4494855.3 (B-O).
Results and Discussion
Biodiversity and functions of microbial communities of the WWTPs
OTUs and Shannon index analysis based on 16S rRNA gene pyrosequencing showed that the sample A-O from WWTPA had the richest biodiversity, followed by the anaerobic sludge sample A-A from the same WWTP (Table S3). This result is confirmed by the rarefaction curves (Figure S1) indicating that WWTPA samples had richer biodiversity than WWTPB. The biodiversity divergences between the two WWTPs may result from the differences in influent wastewater composition and biological treatment processes (Table S1). For example, WWTPB had higher levels of sodium salt than WWTPA in influent wastewater (Table S1), and it is known that that salinity is an important factor regulating and reducing bacterial diversity [23],[24]. In this study, the Good's coverage of each sample was above 0.93 and 0.96 at cutoff levels of 3% and 5%, respectively (Table S3), which indicated that the sequences generated at this sequencing depth could well represent the bacterial communities of the four sludge samples.
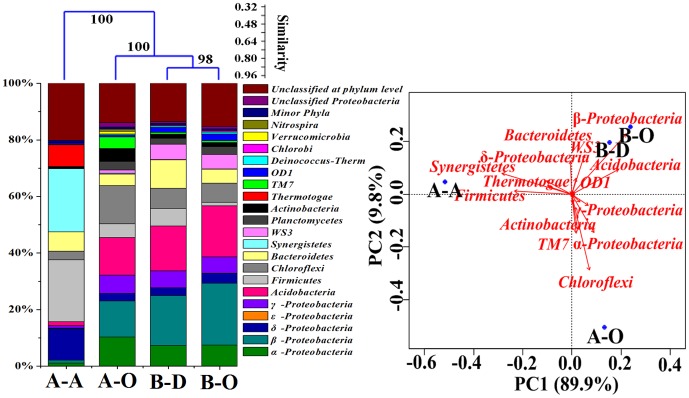
Over 30% of the pyrosequencing reads from all samples could not be assigned to any taxa at genus level (Figure S2), which is comparable to the proportions of unclassified sequences in 14 sewage sludge samples reported by Zhang et al. [18] who used V4 region for 454 pyrosequencing. Cluster analysis showed that bacterial community structure of anaerobic sludge was divergent from those of the three aerobic sludge samples, and PCA revealed that the different samples harbored characteristic bacterial communities at phylum level (Figure 1). Samples B-D and B-O, two aerobic sludge samples which collected from WWTPB, were grouped together. However, bacterial community structures of aerobic sludge (A-O) and anaerobic sludge (A-A) of WWTPA were clearly separated, both of which were dramatically different from those of WWTPB. The difference in wastewater quality may result in the divergence of the aerobic sludge communities of the two WWTPs, and available oxygen is considered a crucial factor contributing to the difference between the aerobic and anaerobic sludge samples [25],[26].
Figure 1. Abundance and distribution of different phyla and classes in Proteobacteria in the four sludge samples.
Taxa shown occurred in at least one sample with abundance over 1%. Minor phyla refer to the taxa with their maximum abundance <1% in each sample. The effective pyrosequencing reads (6,471 sequences) were classified using RDP Classifier at a confidence threshold of 50%. Cluster analysis was conducted based on a distance matrix computed with Bray–Curtis similarity of four samples. Principal component analysis was conducted based on the phylum abundance using R (version 3.0.1).
Statistical analysis showed that 22.38% and 21.90% of the sequences in anaerobic sludge sample A-A were assigned into Synergistetes and Firmicutes, respectively, while Proteobacteria were the dominant phylum in the three aerobic sludge samples, accounting for about 33.64%, 34.31%, and 39.42% in samples A-O, B-D, and B-O, respectively (Figure 1). Synergistetes is known to have low abundance in aerobic sludge, but usually dominate in UASB reactors treating brewery wastewater [27] and tannery wastewater [1].
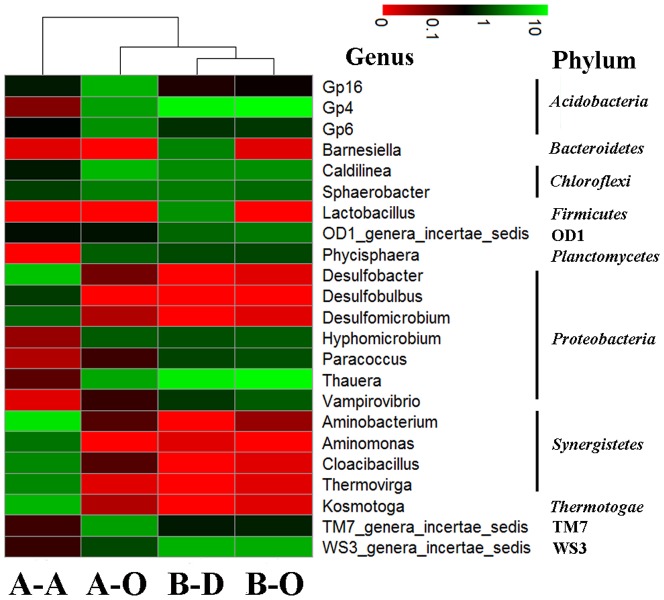
Bacterial diversity and abundance were also analyzed more specifically at the genus level (Figure 2, Table S4). Cluster analysis also showed that the bacterial community structure of the anaerobic sludge was dramatically different from those of the aerobic sludge samples (Figure 2). Similar to the results obtained at phylum level, oxygen concentration is an important factor shaping microbial community structures in WWTPs, and may make huge contributions to the difference at genus level. It was found that anaerobes such as Aminobacterium (11.47%), Desulfobacter (6.94%) and Kosmotoga (5.75%) dominated in the anaerobic sludge sample A-A, while had relative lower abundance in the three aerobic sludge samples (<0.15%). Genus Aminobacterium belongs to the phylum Synergistetes, which is known as amino acids degraders [28]. Other genera in Synergistetes, such as Aminomonas (2.21%), Thermovirga (3.00%) and Cloacibacillus (2.98%), also had higher abundance in the anaerobic sludge sample A-A. Sulfate-reducing bacteria Desulfobacter (6.94%), Desulfomicrobium (1.70%) and Desulfobulbus (0.96%) had higher abundance in the anaerobic sludge than in the aerobic samples, since sulfate is one common pollutant in tannery wastewater [29] and dissolved oxygen (DO) is limited in the UASB reactor.
Figure 2. Heat map illustrating the abundance of all the major genera (with relative abundance over 1% in at least one sample).
The color intensity (log scale) in each panel indicates the relative abundance of the genus in each sample.
In opposition, genera Thauera, Gp4 and Caldilinea dominated (>3%) in all the three aerobic samples, but had lower richness in the anaerobic sample A-A (abundance <0.60%) (Figure 2, Table S4). Thauera had extremely higher abundance in aerobic sludge samples B-D (12.95%), B-O (15.81%) and A-O (4.64%) than in anaerobic sludge sample A-A (0.11%). Thauera is known capable of denitrification and organic compounds biodegradation, which has been frequently detected in wastewater treatment bioreactors [30],[31]. Caldilinea (3.08–5.95%) consists of some filamentous species capable of flocs stabilization of activated sludge [32]. The genus Gp4 (4.20–16.74%) has not been well-described to date, but is known to dominate in the activated sludge of industrial [9] and municipal [18] WWTPs.
The functions of the four tannery activated sludge metagenomes were predicted by alignment of the Illumina sequencing reads against COG database in MG-RAST (Figure S3). Similar to the structural patterns of the microbial communities, the three aerobic sludge samples A-O, B-D and B-O showed similar functional profiles at the highest level of the COG category system (Figure S3). However, the anaerobic sludge showed different COG functional categories distribution, especially on the categories of ‘replication, recombination and repair’, ‘transcription’ and ‘translation, ribosomal structure and biogenesis’ (Figure S3). Table S5 shows that a total of nine identified COGs were responsible for denitrification (COG1140, COG2223, COG3005, COG3256, COG3420, COG4263 and COG5013) and nitrogen fixation (COG1348 and COG2710) in the aerobic or anaerobic sludge. Each category of the denitrifying functional genes had lower abundance in anaerobic sludge than in aerobic sludge. A previous study has also indicated that the abundance of denitrifying bacteria in an aerobic reactor was higher than in a nitrate-free anaerobic digester [33].
Diversity of AOB and AOA amoA genes
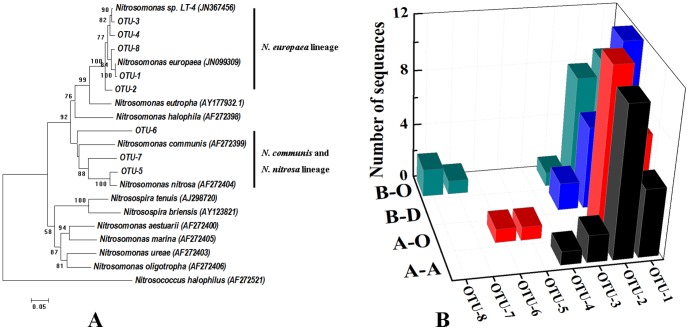
DNA cloning showed that a total of 8 OTUs were obtained at 3% distance cutoff from 81 AOB amoA gene sequences in 4 clone libraries (Figure 3A). The number of the OTUs recovered from individual libraries ranged from 3 (B-D) to 5 (B-O). OTU-1 and OTU-2 dominated in all samples, accounting for 86% of total sequences (Figure 3B). Phylogenetic analysis showed that all the bacterial amoA OTUs were affiliated to Nitrosomonas europaea, Nitrosomonas communis and Nitrosomonas nitrosa (Figure 3A), while no sequence in Nitrosospira lineage was observed. Previous studies have indicated that AOB Nitrosomonas instead of Nitrosospira dominates in WWTPs [3],[34]. Among the three species of Nitrosomonas, N. europaea dominated in all samples, and only a total of 3 sequences were affiliated to N. communis and N. nitrosa (Figure 3B). High concentration of ammonia (>170.0 mg/L) in the influent of the two WWTPs may contribute to the predominance of N. europaea, since previous studies have indicated that AOB of the N. europaea cluster are commonly found in the environments with high levels of ammonium [35]. In this study, similar AOB communities were observed in the UASB and the three aerobic bioreactors. Although DO is a limiting factor -of AOB growth in bioreactors, it has been indicated N. europaea lineage can enrich in both the high-DO and low-DO chemostats [36]. This is also supported by some previous studies revealing that N. europaea was the predominant AOB in various environments containing different levels of DO, such as laboratory-scale anaerobic ammonium-oxidizing reactors [37], full-scale modified Ludzack-Ettinger process WWTPs [38] and pilot-scale sequencing batch nitrifying reactors [39]. AOB in UASB may use the available oxygen to oxidize ammonia to nitrite, contributing to the anaerobic environments [40].
Figure 3. Neighbor-joining phylogenetic tree (A) and OTUs number (B) of AOB amoA gene in the four sludge samples.
The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values (over 50) are indicated on branch nodes. Sequences obtained from this study are shown with “OTU-” in the names, and reference sequences were obtained from GenBank.
In the present study, we attempted to amplify AOA amoA gene from all the four samples by optimizing PCR conditions, but AOA amoA gene was detectable only in the anaerobic sludge sample A-A (Figure S4). Similarly, previous studies also revealed that AOA amoA is absent in aerobic sludge of different WWTPs [3],[41]. A total of 5 OTUs were obtained from 27 AOA amoA gene sequences in A-A clone library (Figure S5). It is known that most of environmental AOA are assigned to the clusters of Group I.1a (the marine and sediment lineage) and Group I.1b (the soil lineage) [42]. This study revealed that OTU-1 occupied 78% of the total AOA amoA gene sequences, and OTU-1, OTU-3 and OTU-4 had high similarity (>98%) to those AOA detected in sediment (clustered to the Group I.1a). Four sequences of OTU-2 and OTU-5 fell in the Group I.1b cluster. This result agrees with Mußmann et al. [41] indicating that all AOA amoA clones from tannery or refinery WWTPs were affiliated with Group I.1a.Ye and Zhang [43] also reported that AOA communities in a laboratory-scale bioreactor treating saline sewage were dominated by Group I.1a. However, the majority of AOA in many municipal WWTPs belonged to Group I.1b [3],[42]. The divergence may result from the difference in wastewater salinity, an important factor in shaping AOA communities [44].
Abundance of amoA, nirK, nirS and nosZ genes
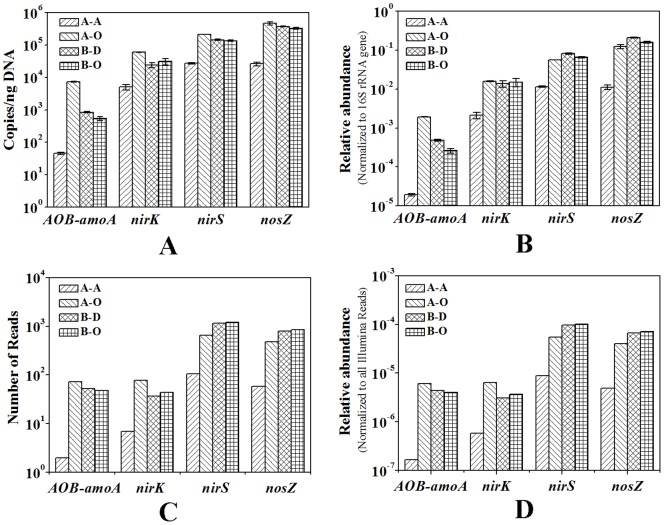
In this study, the abundance of amoA, nirK, nirS and nosZ genes was quantified using both qPCR and metagenomic (alignment) approaches, and the later method has also been frequently used to quantify functional genes in the environment, including amoA gene in soil [8] and activated sludge [10]. Correlation analysis showed that the two methods generated consistent results (Figure S6). qPCR showed that the copy numbers of bacterial amoA gene in the four samples ranged from 45.8 to 7.33×103 copies per ng DNA (Figure 4A). qPCR and metagenomic analysis consistently revealed that the relative abundance of bacterial amoA gene was higher in sample A-O than in the other sludge samples (Figure 4B and D). qPCR showed that the relative abundance of bacterial amoA gene to the overall bacterial population in the three aerobic sludge samples (0.03%–0.19%) was comparable to the previous reports of four membrane bioreactors (lower than 0.1%) capable of removing 70–95% of ammonium from different types of wastewater [45]. A previous study indicated that ammonia monooxygenase had high transcription activity in activated sludge [11]. Thus, the high ammonium removal efficiency in the two WWTPs may result from the high expression levels of ammonia monooxygenase and long hydraulic retention time (Table S1).
Figure 4. Abundance of amoA, nirK, nirS and nosZ genes in the four sludge samples revealed by qPCR (A and B) and metagenomic (alignment) approaches (C and D).
A: relative abundance of the genes normalized to the mass of the extracted DNA; B: relative abundance of the genes normalized to the abundance of 16S rRNA gene; C: Number of the Illumina sequencing reads aligned to the genes sequences in the local database at identity cutoff>90% and alignment length>50 bp; D: Relative abundance of the genes in the Illumina dataset of each sludge sample.
Nitrite reduction is usually catalyzed by two structurally different but functionally equivalent forms of nitrite reductase: copper and cytochrome cd 1 -containing nitrite reductases encoded by the genes nirK and nirS, respectively. The copy numbers of nirK and nirS ranged from 5.05×103 to 6.02×104 and from 2.72×104 to 2.14×105 gene copies per ng DNA, respectively (Figure 4A). The average abundance of nirS and nirK relative to the overall bacterial population was 1.18% and 5.38%, respectively (Figure 4B). The relative abundance of nirK was found comparable to the results reported by Geets et al. (0.16–2.75%) [4]. qPCR and metagenomic analysis consistently indicated that A-O had higher abundance of nirK than the other samples. This study showed that nirS had higher abundance than nirK in each of the four samples (The ratio of nirS/nirK was over 3.0 in each sample). Similar results were observed in some WWTPs treating hospital wastewater, papermaking wastewater or pharmaceutical wastewater [4]. However, it cannot be indicated that nirS plays a greater role than nirK in the nitrogen removal, since expression level of the genes may affect their contribution to nitrogen removal [5]. Thus, further studies based on mRNA and protein expression have to be conducted.
N2O is an important component of greenhouse gas, and reduction of N2O to N2 in the environment has been attributed exclusively to the denitrifiers carrying NosZ gene. qPCR showed that the copy number of nosZ gene in the four sludge samples ranged from 2.63×104 to 4.66×105 gene copies per ng DNA (Figure 4A) with relative abundance within the range from 1.1% to 21.0% (Figure 4B). qPCR and metagenomic analysis consistently indicated that nosZ gene preferred aerobic environment (Figure 4). In anaerobic sludge, the abundance of nosZ gene were lower than the total abundance of nirK and nirS, indicating that some denitrifiers in the anaerobic sludge might lack nosZ, which may lead to the accumulation of N2O in anaerobic environments. Previous studies also indicated that low DO concentration in WWTPs favors N2O production during nitrification/denitrification process [46],[47], although nitrous oxide reductase is the most oxygen sensitive enzyme in the denitrification pathway [48].
It was found the effluent nitrate concentration has a significantly positive correlation with the relative abundance of the denitrifying genes nirK (R = 0.977, P = 0.023), nirS (R = 0.851, P = 0.149) and nosZ (R = 0.811, P = 0.189). This is supported by a previous study revealing significant correlation between nitrate concentration and the abundance of nirK or nirS in soil [5].
Metagenomic analysis of nitrifying and denitrifying bacteria populations
16S rRNA gene pyroseqencing was conducted to investigate the abundance and diversity of AOB and NOB in the sludge samples. Results showed that Nitrosomonas and Nitrosospira, the two important genera of AOB in WWTPs [3], accounted for 0–0.34% and 0–0.20% of the total sequences of the samples, respectively (Table S6). Similarly, Zhang et al. [3] indicated that AOB occupied 0–0.64% of the total pyrosequencing sequences from six municipal WWTPs, and Nitrosomonas dominated in the WWTPs. Sample A-O had the highest abundance of AOB and NOB, followed by the other aerobic samples, and both AOB and NOB sequences were undetectable in the anaerobic sludge (A-A), which agrees with the qPCR results of amoA gene. A total of 76 AOB and 79 NOB sequences in all the samples were merged to construct phylogenetic tree to characterize the AOB and NOB communities, respectively. Similar to amoA gene diversity analysis, pyrosequencing showed that most of the AOB 16S rRNA sequences were grouped into Nitrosomonas sp., including N. europaea (5 OTUs), N. ureae (1 OTU) and Nitrosomonas-like species (3 OTUs) (Figure S7). Most of the Nitrospira-type NOB reads were grouped into N. marina (Figure S8), since tannery wastewater usually contains high concentration of salt (Table S1) [1].
Shapleigh [49] provided a list of 84 potential denitrifying bacterial genera, among which 17 genera were detectable in the four samples in this study (Table S7). The 11 types of potential denitrifying genera present in anaerobic sludge sample A-A accounted for 0.83% of the total pyrosequencing reads, which was lower than the corresponding proportions of the three aerobic sludge samples (8.22–20.52%). This agrees with the COG function alignment results, and lack of nitrate (Table S1) in UASB may be responsible for the low abundance of the denitrifiers.
Additionally, the Illumina reads annotated as amoA, nirK, nirS and nosZ genes were extracted and then assigned to specific host bacteria at genus level by BLASTX against NCBI-nr database and MEGAN. Unfortunately, most of the extracted reads had higher similarity to the corresponding genes of uncultured microorganisms. A total of 26 reads of amoA, 36 reads of nirK, 1,062 reads of nirS and 754 reads of nosZ in the samples were well assigned to specific host of 89 known genera (Figure S9). It should be noted that using the short Illumina sequencing reads in taxonomic assignment could produce unreliable results at low taxonomic levels [50], and the possible horizontal gene transfer of denitrifying genes among different taxa is likely to increase uncertainty of the results [51]. To maximize the reliability of the obtained results, only the highly abundant genera identified by both pyrosequencing and Illumina sequencing in this study were considered as the main nitrifiers or denitrifiers in the two tannery WWTPs.
All the amoA gene reads were assigned to Nitrosomonas, which agrees with amoA gene cloning results. Results showed that Nitrosomonas was the main host of nitrite reductase gene nirK in the samples A-A, B-D and B-O. This result is supported by Beaumont et al. [52] indicating presence of nirK in Nitrosomonas europaea cells. Interestingly, nirK in sample A-O was mainly carried by the bacterial cells of Rhizobium, not Nitrosomonas (Figure S9). This is consistent with the pyrosequencing results demonstrating that Rhizobium had high abundance in sample A-O (Table S4). NirS and nosZ genes were found mainly located in the bacterial cells of Thauera, Paracoccus, Hyphomicrobium, Comamonas and Azoarcus (Figure S9) dominating in the aerobic sludge (Table S4), revealing the crucial roles of the microorganisms in denitrification in aerobic environments. It is known that Thaurea mechernichensis [53] and Paracoccus denitrificans [54] can reduce nitrate even in the presence of high concentrations of O2.
In conclusion, combined use of DNA cloning, qPCR, 454 pyrosequencing and Illumina sequencing provided a comprehensive insight in microbial community structure of nitrifiers and denitrifiers in tannery WWTPs. Proteobacteria and Synergistetes phyla had highest abundance in aerobic and anaerobic sludge, respecitively. Nitrosomonas europaea dominated the ammonia-oxidizing community. Thauera, Paracoccus, Hyphomicrobium, Comamonas and Azoarcus were the predominant potential denitrifying genera, which may greatly contribute to the nitrogen removal in the WWTPs. qPCR and metagenomic approaches consistently revealed that functional genes amoA, nirK, nirS and nosZ had higher abundance in aerobic sludge than in anaerobic sludge. The results can extend our biological knowledge about nitrifiers and denitrifiers in tannery WWTPs, and might be practically useful in efficiently removing nitrogen from industrial wastewater.
Supporting Information
Rarefaction curves of 4 sludge samples at cutoff levels of 3% (solid lines) and 5% (dash lines). The rarefaction curve, plotting the number of observed OTUs as a function of the number of sequences, was computed using RDP's pyrosequencing pipeline. The error bars show 95% confidence intervals.
(DOCX)
Percentages of unclassified sequences at six taxonomic ranks for four sludge samples from two full-scale tannery wastewater treatment plants. Effective bacterial sequences were classified using RDP Classifier at a confidence threshold of 50%.
(DOCX)
Relative abundance of 21 COG functional categories in four tannery activated sludge metagenomes. Relative abundances of functional categories were estimated by normalizing to the total number of protein coding sequences assigned to each corresponding functions. Designations of functional categories: A: Replication, recombination and repair, B: RNA processing and modification, C: Transcription, D: Translation, ribosomal structure and biogenesis, E: Cell cycle control, cell division, chromosome partitioning, F: Cell wall/membrane/envelope biogenesis, G: Cytoskeleton, H: Defense mechanisms, I: Intracellular trafficking, secretion, and vesicular transport, J: Posttranslational modification, protein turnover, chaperones, K: Signal transduction mechanisms, L: Amino acid transport and metabolism, M: Carbohydrate transport and metabolism, N: Coenzyme transport and metabolism, O: Energy production and conversion, P: Inorganic ion transport and metabolism, Q: Lipid transport and metabolism, R: Nucleotide transport and metabolism, S: Secondary metabolites biosynthesis, transport and catabolism, T: Function unknown, U: General function prediction only.
(DOCX)
Gel image of PCR products of AOA amoA gene (M: DL2000 DNA Marker; 0: Negative control; 1-2: A-A; 3-4: A-O; 5-6: B-D; 7-8: B-O).
(DOCX)
Neighbor-joining phylogenetic tree of AOA amoA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values (over 50) are indicated on branch nodes.
(DOCX)
Correlation between the qPCR and metagenomic (alignment) approaches for the quantification of AOB amoA , nirK , nirS and nosZ genes. The abundance of the four genes obtained by qPCR were normalized to the total bacterial community, and the metagenomic alignment results were normalized to the total number of clean Illumina sequencing reads in each sample.
(DOCX)
Neighbor-joining phylogenetic tree of AOB 16S rRNA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values are indicated on branch nodes. Sequences obtained from 454-pyrosequencing in this study are shown with “OTU-” in the names, and the number in “| | | | ” represented the number of AOB 16S rRNA gene sequences in the samples A-O, B-D and B-O, respectively. The information of reference sequences was obtained from GenBank.
(DOCX)
Neighbor-joining phylogenetic tree of NOB 16S rRNA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values are indicated on branch nodes. Sequences obtained from 454-pyrosequencing in this study are shown with “OTU-” in the names, and the number in “| | | |” represented the number of AOB 16S rRNA gene sequences in sample A-O, B-D and B-O, respectively. The information of reference sequences was obtained from GenBank.
(DOCX)
Functional microorganisms in nitrification and denitrification processes. Based on the BLASTX against NCBI-nr database and MEGAN, the Illumina clean reads annotated as amoA, nirK, nirS or nosZ genes were assigned to specific genera, and 59 genera of bacteria with more than two hits in at least one sample are displayed in the heat map which was generated using R (version 3.01).
(DOCX)
Operational parameters of the two tannery wastewater treatment plants and concentrations of metallic elements in the influent wastewater.
(DOCX)
Primers used for PCR and qPCR in this study.
(DOCX)
Information of 16S rRNA gene pyrosequencing reads and biodiversity of the four activated sludge samples from two tannery wastewater treatment plants.
(DOCX)
Abundance of all genera in the four sludge samples. The abundance is presented in terms of percentages of the total sequences (6471 sequences) in a sample. Sorted alphabetically by phylum and genus. The bold numbers indicate the genera with relative abundance>1%.
(DOCX)
COGs related to denitrification and nitrogen fixation identified in the four activated sludge metagenomes.
(DOCX)
Composition of nitrifying bacteria (AOB and NOB) in the four sludge samples revealed by 454 pyrosequencing.
(DOCX)
Potential denitrifying genera detected in the four sludge samples through 454 pyrosequencing. The reads number was calculated by normalizing the total pyrosequencing reads to 6,471 for each sample.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The sequences of amoA for cloning library construction have been deposited in GenBank under accession numbers KF720406 to KF720513. The pyrosequencing datasets have been deposited into the NCBI Short Reads Archive Database (accession number: SRX396800). The Illumina metagenomic datasets are available at MG-RAST under accession numbers 4494863.3, 4494888.3, 4494854.3 and 4494855.3.
Funding Statement
This study was financially supported by National Natural Science Foundation of China (Grant No. 51378252, PI: BL, URL: http://www.nsfc.gov.cn), National Science and Technology Major Project of China (No. 2011ZX07210-001-1, PI: AL, URL: http://www.nmp.gov.cn/) and Technology Support Project of Jiangsu Province (Grant No. BE2011722, PI: BL; Grant No. BE2013704, PI: XXZ; URL: www.jskjjh.gov.cn). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lefebvre O, Vasudevan N, Thanasekaran K, Moletta R, Godon JJ (2006) Microbial diversity in hypersaline wastewater: the example of tanneries. Extremophiles 10:505–513. [DOI] [PubMed] [Google Scholar]
- 2. Leta S, Assefa F, Gumaelius L, Dalhammar G (2004) Biological nitrogen and organic matter removal from tannery wastewater in pilot plant operations in Ethiopia. Appl Microbiol Biotechnol 66:333–339. [DOI] [PubMed] [Google Scholar]
- 3. Zhang T, Ye L, Tong AHY, Shao MF, Lok S (2011) Ammonia-oxidizing archaea and ammonia-oxidizing bacteria in six full-scale wastewater treatment bioreactors. Appl. Microbiol. Biotechnol 91:1215–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geets J, de Cooman M, Wittebolle L, Heylen K, Vanparys B, et al. (2007) Real-time PCR assay for the simultaneous quantification of nitrifying and denitrifying bacteria in activated sludge. Appl. Microbiol. Biotechnol 75:211–221. [DOI] [PubMed] [Google Scholar]
- 5. Guo GX, Deng H, Qiao M, Yao HY, Zhu YG (2013) Effect of long-term wastewater irrigation on potential denitrification and denitrifying communities in soils at the watershed scale. Environ Sci Technol 47:3105–3113. [DOI] [PubMed] [Google Scholar]
- 6. Chen J, Tang YQ, Wu XL (2012) Bacterial community shift in two sectors of a tannery plant and its Cr (VI) removing potential. Geomicrobiol J 29:226–235. [Google Scholar]
- 7. Shi P, Jia SY, Zhang XX, Zhang T, Cheng SP, et al. (2013) Metagenomic insights into chlorination effects on microbial antibiotic resistance in drinking water. Water Res 47:111–120. [DOI] [PubMed] [Google Scholar]
- 8. Leininger S, Urich T, Schloter M, Schwark L, Qi J, et al. (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature 442:806–809. [DOI] [PubMed] [Google Scholar]
- 9. Ibarbalz FM, Figuerola ELM, Erijman L (2013) Industrial activated sludge exhibit unique bacterial community composition at high taxonomic ranks. Water Res 47:3854–3864. [DOI] [PubMed] [Google Scholar]
- 10. Ye L, Zhang T, Wang TT, Fang ZW (2012) Microbial structures, functions, and metabolic pathways in wastewater treatment bioreactors revealed using high-throughput sequencing. Environ Sci Technol 46:13244–13252. [DOI] [PubMed] [Google Scholar]
- 11. Yu K, Zhang T (2012) Metagenomic and metatranscriptomic analysis of microbial community structure and gene expression of activated sludge. PloS ONE 7:e38183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Zhang XX, Huang KL, Miao Y, Shi P, et al. (2013) Metagenomic profiling of antibiotic resistance genes and mobile genetic elements in a tannery wastewater treatment plant. PLoS ONE 8:e76079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schloss PD, Handelsman J (2005) Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol 71:1501–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang XX, Zhang T (2011) Occurrence, abundance, and diversity of tetracycline resistance genes in 15 sewage treatment olants across China and other global locations. Environ Sci Technol 45:2598–2604. [DOI] [PubMed] [Google Scholar]
- 15. Lopez-Gutierrez JC, Henry S, Hallet S, Martin-Laurent F, Catroux G, et al. (2004) Quantification of a novel group of nitrate-reducing bacteria in the environment by real-time PCR. J Microbiol Methods 57:399–407. [DOI] [PubMed] [Google Scholar]
- 16. Cole JR, Wang Q, Cardenas E, Fish J, Chai B, et al. (2009) The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res 37:D141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing Mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang T, Shao MF, Ye L (2012) 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. ISME J 6:1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4(1): 9 pp.
- 20.R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org/.
- 21. Tatusov RL, Galperin MY, Natale DA, Koonin EV (2000) The COG database: a tool for genomescale analysis of protein functions and evolution. Nucleic Acids Res 28:33–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, et al. (2008) The metagenomics RAST server - a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 9:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cortés-Lorenzo C, Sipkema D, Rodríguez-Díaz M, Fuentes S, Juárez-Jiménez B, et al. (2014) Microbial community dynamics in a submerged fixed bed bioreactor during biological treatment of saline urban wastewater. Ecol Eng 71:126–132. [Google Scholar]
- 24. Benlloch S, Lopez-Lopez A, Casamayor EO, Ovreas L, Goddard V, et al. (2002) Prokaryotic genetic diversity throughout the salinity gradient of a coastal solar saltern. Environ Microbiol 4:349–360. [DOI] [PubMed] [Google Scholar]
- 25. Wang X, Xia Y, Wen X, Yang Y, Zhou J (2014) Microbial community functional structures in wastewater treatment plants as characterized by GeoChip. PLoS ONE 9:e93422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang X, Hu M, Xia Y, Wen X, Ding K (2012) Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl Environ Microbiol 78:7042–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Novak D, Franke-Whittle IH, Pirc ET, Jerman V, Insam H, et al. (2013) Biotic and abiotic processes contribute to successful anaerobic degradation of cyanide by UASB reactor biomass treating brewery waste water. Water Res 47:3644–3653. [DOI] [PubMed] [Google Scholar]
- 28. Vartoukian SR, Palmer RM, Wade WG (2007) The division "Synergistes". Anaerobe 13:99–106. [DOI] [PubMed] [Google Scholar]
- 29. Murugananthan M, Raju GB, Prabhakar S (2004) Separation of pollutants from tannery effluents by electro flotation. Sep Purif Technol 40:69–75. [Google Scholar]
- 30. Liu B, Zhang F, Feng X, Liu Y, Yan X, et al. (2006) Thauera and Azoarcus as functionally important genera in a denitrifying quinoline-removal bioreactor as revealed by microbial community structure comparison. FEMS Microbiol Ecol 55:274–286. [DOI] [PubMed] [Google Scholar]
- 31. Zhao Y, Huang J, Zhao H, Yang H (2013) Microbial community and N removal of aerobic granular sludge at high COD and N loading rates. Bioresour Technol 143:439–446. [DOI] [PubMed] [Google Scholar]
- 32. Yoon DN, Park SJ, Kim SJ, Jeon CO, Chae JC, et al. (2010) Isolation, characterization, and abundance of filamentous members of Caldilineae in activated sludge. J Microbiol 48:275–283. [DOI] [PubMed] [Google Scholar]
- 33.Reyes M, Borrás L, Seco A, Ferrer J (2014) Identification and quantification of microbial populations in activated sludge and anaerobic digestion processes. Environ Techno DOI: 10.1080/09593330.2014.934745. [DOI] [PubMed]
- 34. Figuerola ELM, Erijman L (2010) Diversity of nitrifying bacteria in a full-scale petroleum refinery wastewater treatment plant experiencing unstable nitrification. J Hazard Mater 181:281–288. [DOI] [PubMed] [Google Scholar]
- 35. Limpiyakorn T, Kurisu F, Sakamoto Y, Yagi O (2007) Effects of ammonium and nitrite on communities and populations of ammonia-oxidizing bacteria in laboratory-scale continuous-flow reactors. FEMS Microbiol Ecol 60:501–512. [DOI] [PubMed] [Google Scholar]
- 36. Park HD, Noguera DR (2004) Evaluating the effect of dissolved oxygen on ammonia-oxidizing bacterial communities in activated sludge. Water Res 38:3275–3286. [DOI] [PubMed] [Google Scholar]
- 37. Quan ZX, Rhee SK, Zuo JE, Yang Y, Bae JW, et al. (2008) Diversity of ammonium-oxidizing bacteria in a granular sludge anaerobic ammonium-oxidizing (anammox) reactor. Environ Microbiol 10:3130–3139. [DOI] [PubMed] [Google Scholar]
- 38. Figuerola EL, Erijman L (2010) Diversity of nitrifying bacteria in a full-scale petroleum refinery wastewater treatment plant experiencing unstable nitrification. J Hazard Mater 181:281–288. [DOI] [PubMed] [Google Scholar]
- 39. Gieseke A, Bjerrum L, Wagner M, Amann R (2003) Structure and activity of multiple nitrifying bacterial populations co-existing in a biofilm. Environ Microbiol 5:355–369. [DOI] [PubMed] [Google Scholar]
- 40. Ma B, Peng Y, Zhang S, Wang J, Gan Y, et al. (2013) Performance of anammox UASB reactor treating low strength wastewater under moderate and low temperatures. Bioresour Technol 129:606–611. [DOI] [PubMed] [Google Scholar]
- 41. Mußmann M, Brito I, Pitcher A, Damste JSS, Hatzenpichler R, et al. (2011) Thaumarchaeotes abundant in refinery nitrifying sludges express amoA but are not obligate autotrophic ammonia oxidizers. Proc Natl Acad Sci USA 108:16771–16776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Limpiyakorn T, Sonthiphand P, Rongsayamanont C, Polprasert C (2011) Abundance of amoA genes of ammonia-oxidizing archaea and bacteria in activated sludge of full-scale wastewater treatment plants. Bioresource Technol 102:3694–3701. [DOI] [PubMed] [Google Scholar]
- 43. Ye L, Zhang T (2011) Ammonia-oxidizing bacteria dominates over ammonia-oxidizing archaea in a saline nitrification reactor under low DO and high nitrogen loading. Biotechnol Bioeng 108:2544–2552. [DOI] [PubMed] [Google Scholar]
- 44. Mosier AC, Francis CA (2008) Relative abundance and diversity of ammonia-oxidizing archaea and bacteria in the San Francisco Bay estuary. Environ Microbiol 10:3002–3016. [DOI] [PubMed] [Google Scholar]
- 45. Zhang B, Sun BS, Ji M, Liu HN, Liu XH (2010) Quantification and comparison of ammonia-oxidizing bacterial communities in MBRs treating various types of wastewater. Bioresource Technol 101:3054–3059. [DOI] [PubMed] [Google Scholar]
- 46. Jia W, Liang S, Zhang J, Ngo HH, Guo W, et al. (2013) Nitrous oxide emission in low-oxygen simultaneous nitrification and denitrification process: sources and mechanisms. Bioresource Technol 136:444–451. [DOI] [PubMed] [Google Scholar]
- 47. Tallec G, Garnier J, Billen G, Gousailles M (2006) Nitrous oxide emissions from secondary activated sludge in nitrifying conditions of urban wastewater treatment plants: effect of oxygenation level. Water Res 40:2972–2980. [DOI] [PubMed] [Google Scholar]
- 48. Bonin P, Gilewicz M, Bertrand J (1989) Effects of oxygen on each step of denitrification on Pseudomonas nautica . Can J Microbiol 35:1061–1064. [Google Scholar]
- 49.Shapleigh JP (2006) The Denitrifying Prokaryotes. Springer, New York, NY: 769–792.
- 50. Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Heylen K, Gevers D, Vanparys B, Wittebolle L, Geets J, et al. (2006) The incidence of nirS and nirK and their genetic heterogeneity in cultivated denitrifiers. Environ Microbiol 8:2012–2021. [DOI] [PubMed] [Google Scholar]
- 52. Beaumont HJ, Lens SI, Westerhoff HV, van Spanning RJ (2005) Novel nirK cluster genes in Nitrosomonas europaea are required for NirK-dependent tolerance to nitrite. J Bacteriol 187:6849–6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Scholten E, Lukow T, Auling G, Kroppenstedt RM, Rainey FA, et al. (1999) Thauera mechernichensis sp nov., an aerobic denitrifier from a leachate treatment plant. Int J Syst Bacteriol 49:1045–1051. [DOI] [PubMed] [Google Scholar]
- 54. Baumann B, Snozzi M, Zehnder AJ, Van Der MeerJR (1996) Dynamics of denitrification activity of Paracoccus denitrificans in continuous culture during aerobic-anaerobic changes. J Bacteriol 178:4367–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction curves of 4 sludge samples at cutoff levels of 3% (solid lines) and 5% (dash lines). The rarefaction curve, plotting the number of observed OTUs as a function of the number of sequences, was computed using RDP's pyrosequencing pipeline. The error bars show 95% confidence intervals.
(DOCX)
Percentages of unclassified sequences at six taxonomic ranks for four sludge samples from two full-scale tannery wastewater treatment plants. Effective bacterial sequences were classified using RDP Classifier at a confidence threshold of 50%.
(DOCX)
Relative abundance of 21 COG functional categories in four tannery activated sludge metagenomes. Relative abundances of functional categories were estimated by normalizing to the total number of protein coding sequences assigned to each corresponding functions. Designations of functional categories: A: Replication, recombination and repair, B: RNA processing and modification, C: Transcription, D: Translation, ribosomal structure and biogenesis, E: Cell cycle control, cell division, chromosome partitioning, F: Cell wall/membrane/envelope biogenesis, G: Cytoskeleton, H: Defense mechanisms, I: Intracellular trafficking, secretion, and vesicular transport, J: Posttranslational modification, protein turnover, chaperones, K: Signal transduction mechanisms, L: Amino acid transport and metabolism, M: Carbohydrate transport and metabolism, N: Coenzyme transport and metabolism, O: Energy production and conversion, P: Inorganic ion transport and metabolism, Q: Lipid transport and metabolism, R: Nucleotide transport and metabolism, S: Secondary metabolites biosynthesis, transport and catabolism, T: Function unknown, U: General function prediction only.
(DOCX)
Gel image of PCR products of AOA amoA gene (M: DL2000 DNA Marker; 0: Negative control; 1-2: A-A; 3-4: A-O; 5-6: B-D; 7-8: B-O).
(DOCX)
Neighbor-joining phylogenetic tree of AOA amoA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values (over 50) are indicated on branch nodes.
(DOCX)
Correlation between the qPCR and metagenomic (alignment) approaches for the quantification of AOB amoA , nirK , nirS and nosZ genes. The abundance of the four genes obtained by qPCR were normalized to the total bacterial community, and the metagenomic alignment results were normalized to the total number of clean Illumina sequencing reads in each sample.
(DOCX)
Neighbor-joining phylogenetic tree of AOB 16S rRNA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values are indicated on branch nodes. Sequences obtained from 454-pyrosequencing in this study are shown with “OTU-” in the names, and the number in “| | | | ” represented the number of AOB 16S rRNA gene sequences in the samples A-O, B-D and B-O, respectively. The information of reference sequences was obtained from GenBank.
(DOCX)
Neighbor-joining phylogenetic tree of NOB 16S rRNA gene sequences. The evolutionary distances were computed using the Jukes–Cantor method. Bootstrap values are indicated on branch nodes. Sequences obtained from 454-pyrosequencing in this study are shown with “OTU-” in the names, and the number in “| | | |” represented the number of AOB 16S rRNA gene sequences in sample A-O, B-D and B-O, respectively. The information of reference sequences was obtained from GenBank.
(DOCX)
Functional microorganisms in nitrification and denitrification processes. Based on the BLASTX against NCBI-nr database and MEGAN, the Illumina clean reads annotated as amoA, nirK, nirS or nosZ genes were assigned to specific genera, and 59 genera of bacteria with more than two hits in at least one sample are displayed in the heat map which was generated using R (version 3.01).
(DOCX)
Operational parameters of the two tannery wastewater treatment plants and concentrations of metallic elements in the influent wastewater.
(DOCX)
Primers used for PCR and qPCR in this study.
(DOCX)
Information of 16S rRNA gene pyrosequencing reads and biodiversity of the four activated sludge samples from two tannery wastewater treatment plants.
(DOCX)
Abundance of all genera in the four sludge samples. The abundance is presented in terms of percentages of the total sequences (6471 sequences) in a sample. Sorted alphabetically by phylum and genus. The bold numbers indicate the genera with relative abundance>1%.
(DOCX)
COGs related to denitrification and nitrogen fixation identified in the four activated sludge metagenomes.
(DOCX)
Composition of nitrifying bacteria (AOB and NOB) in the four sludge samples revealed by 454 pyrosequencing.
(DOCX)
Potential denitrifying genera detected in the four sludge samples through 454 pyrosequencing. The reads number was calculated by normalizing the total pyrosequencing reads to 6,471 for each sample.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The sequences of amoA for cloning library construction have been deposited in GenBank under accession numbers KF720406 to KF720513. The pyrosequencing datasets have been deposited into the NCBI Short Reads Archive Database (accession number: SRX396800). The Illumina metagenomic datasets are available at MG-RAST under accession numbers 4494863.3, 4494888.3, 4494854.3 and 4494855.3.