Abstract
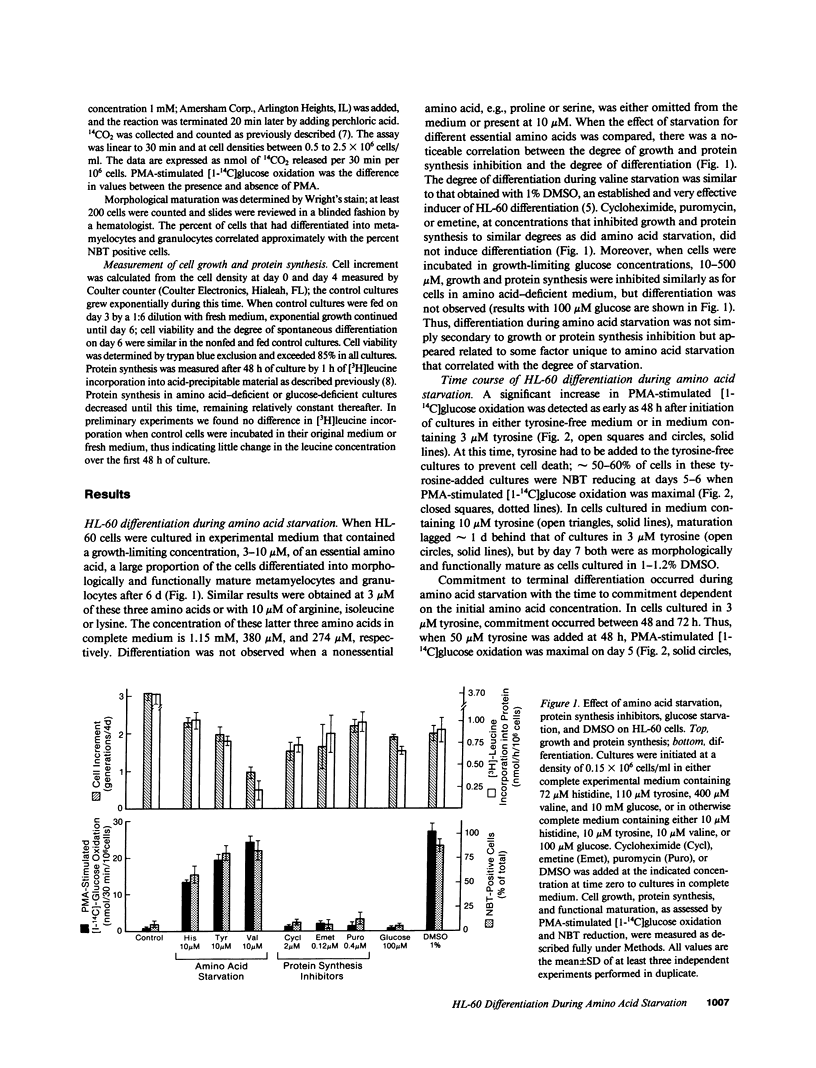
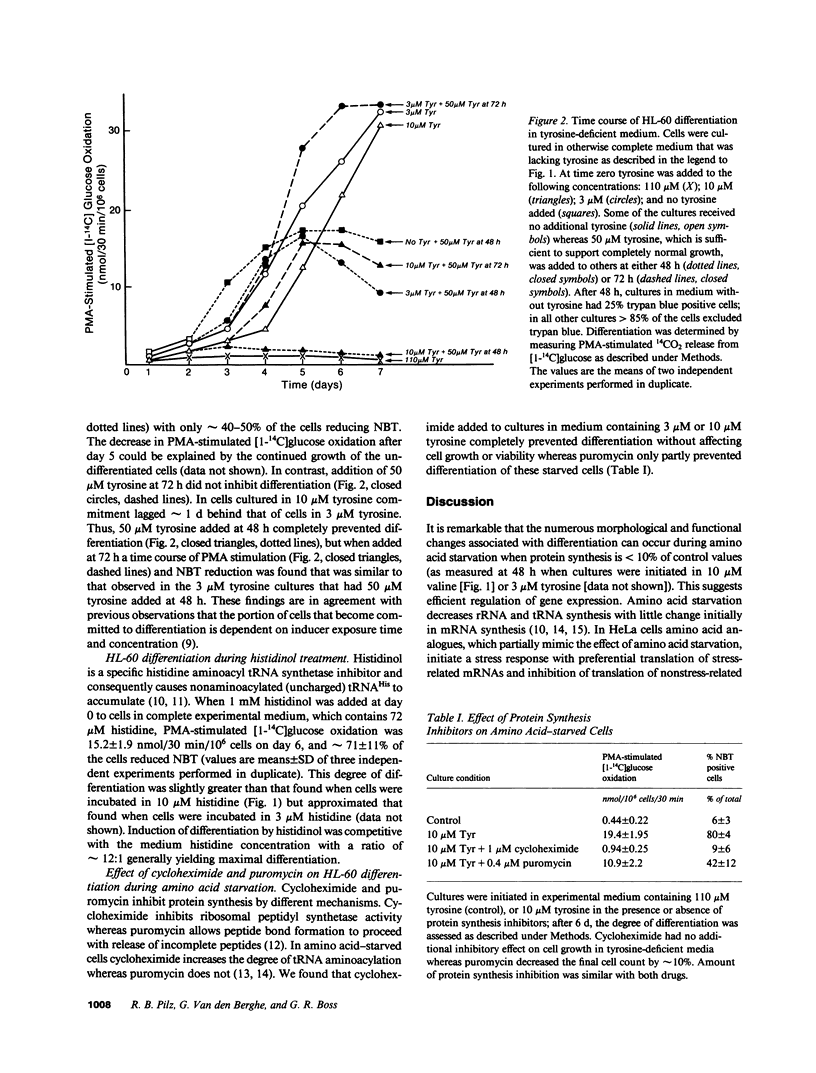
Starvation for a single essential amino acid induced differentiation of the human promyelocytic leukemia line HL-60 into morphologically and functionally mature granulocytes. Differentiation occurred when protein synthesis was inhibited up to 90% but was not simply secondary to growth arrest or protein synthesis inhibition, because neither glucose starvation nor treatment with protein synthesis inhibitors induced differentiation. Induction of differentiation by an aminoacyl tRNA synthetase inhibitor and the effect of cycloheximide and puromycin on amino acid-starved cells suggested an important regulatory role of tRNA molecules during differentiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boss G. R. Decreased phosphoribosylpyrophosphate as the basis for decreased purine synthesis during amino acid starvation of human lymphoblasts. J Biol Chem. 1984 Mar 10;259(5):2936–2941. [PubMed] [Google Scholar]
- Boss G. R., Erbe R. W. Decreased purine synthesis during amino acid starvation of human lymphoblasts. J Biol Chem. 1982 Apr 25;257(8):4242–4247. [PubMed] [Google Scholar]
- Boss G. R., Pilz R. B. Phosphoribosylpyrophosphate synthesis from glucose decreases during amino acid starvation of human lymphoblasts. J Biol Chem. 1985 May 25;260(10):6054–6059. [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis A., Christner P., Rosenbloom J. Preferential usage of tRNA isoaccepting species in collagen synthesis. J Biol Chem. 1977 Nov 25;252(22):8023–8026. [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlinger P. J., Hamilton T. A., Litt M. Amino acid control of stable RNA synthesis in Friend leukemia cells in relation to intracellular purine nucleoside triphosphate levels. Eur J Biochem. 1977 Aug 1;77(3):495–499. doi: 10.1111/j.1432-1033.1977.tb11691.x. [DOI] [PubMed] [Google Scholar]
- Fournier M. J., Peterkofsky A. Formation of chromatographically unique species of transfer ribonucleic acid during amino acid starvation of relaxed-control Escherichia coli. J Bacteriol. 1975 May;122(2):538–548. doi: 10.1128/jb.122.2.538-548.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt F., Grummt I. Studies on the role of uncharged tRNA in pleiotypic response of animal cells. Eur J Biochem. 1976 Apr 15;64(1):307–312. doi: 10.1111/j.1432-1033.1976.tb10302.x. [DOI] [PubMed] [Google Scholar]
- Lin V. K., Agris P. F. Alterations in tRNA isoaccepting species during erythroid differentiation of the Friend leukemia cell. Nucleic Acids Res. 1980 Aug 11;8(15):3467–3480. doi: 10.1093/nar/8.15.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. The pathway of eukaryotic mRNA formation. Annu Rev Biochem. 1983;52:441–466. doi: 10.1146/annurev.bi.52.070183.002301. [DOI] [PubMed] [Google Scholar]
- Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J Cell Biol. 1979 Aug;82(2):315–322. doi: 10.1083/jcb.82.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie A., Huschka U., Kersten W. Control of protein synthesis in mammalian cells by aminoacylation of transfer ribonucleic acid. Biochim Biophys Acta. 1979 Dec 17;565(2):293–304. doi: 10.1016/0005-2787(79)90206-5. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Smulson M. E., Thomas J. Ribonucleic acid biosynthesis of human cells during amino acid deprivation. J Biol Chem. 1969 Oct 10;244(19):5309–5312. [PubMed] [Google Scholar]
- Sueoka N., Kano-Sueoka T. Transfer RNA and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1970;10:23–55. doi: 10.1016/s0079-6603(08)60560-7. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. Alterations of transcription and translation in HeLa cells exposed to amino acid analogs. Mol Cell Biol. 1984 Jun;4(6):1063–1072. doi: 10.1128/mcb.4.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan M. H., Hansen B. S. Control of initiation of protein synthesis in human cells. Evidence for a role of uncharged transfer ribonucleic acid. J Biol Chem. 1973 Oct 25;248(20):7087–7096. [PubMed] [Google Scholar]



