Abstract
In winter 2009/10, a sudden under-ice bloom of heterotrophic bacteria occurred in the seasonally ice-covered, temperate, deep, oligotrophic Lake Stechlin (Germany). Extraordinarily high bacterial abundance and biomass were fueled by the breakdown of a massive bloom of Aphanizomenon flos-aquae after ice formation. A reduction in light resulting from snow coverage exerted a pronounced physiological stress on the cyanobacteria. Consequently, these were rapidly colonized, leading to a sudden proliferation of attached and subsequently of free-living heterotrophic bacteria. Total bacterial protein production reached 201 µg C L−1 d−1, ca. five times higher than spring-peak values that year. Fluorescence in situ hybridization and denaturing gradient gel electrophoresis at high temporal resolution showed pronounced changes in bacterial community structure coinciding with changes in the physiology of the cyanobacteria. Pyrosequencing of 16S rRNA genes revealed that during breakdown of the cyanobacterial population, the diversity of attached and free-living bacterial communities were reduced to a few dominant families. Some of these were not detectable during the early stages of the cyanobacterial bloom indicating that only specific, well adapted bacterial communities can colonize senescent cyanobacteria. Our study suggests that in winter, unlike commonly postulated, carbon rather than temperature is the limiting factor for bacterial growth. Frequent phytoplankton blooms in ice-covered systems highlight the need for year-round studies of aquatic ecosystems including the winter season to correctly understand element and energy cycling through aquatic food webs, particularly the microbial loop. On a global scale, such knowledge is required to determine climate change induced alterations in carbon budgets in polar and temperate aquatic systems.
Introduction
On a global scale ice covers, either seasonally or permanently, about 8% of saline and freshwater bodies (not including the Southern hemisphere) [1], [2]. While under-ice phytoplankton blooms are common phenomena (e.g. [3], [4]), little is known about the activity of heterotrophic bacteria during and following such blooms [5]. In the absence of ice coverage, cyanobacterial blooms are known to be accompanied by an increase in bacterial counts and bacterial activity [6]. For example, in four Swedish lakes (two of which had Aphanizomenon spp. blooms) bacterial numbers increased during the cyanobacterial bloom to 1010 –1011 cells L−1. At that time the bacterial protein production was between 25 and 350 µg C d−1, respectively [6].
It is believed that as a result of low temperatures and reduced nutrient remineralization due to low protozoan grazing, microorganisms under ice are killed, harmed or exist in a dormant state [5], [7], [8]. However, estimated biomass turnover for the overall bacterial communities from these studies usually ranges between 4–10 days indicating that winter microbial communities are actively growing and not just dormant. Although seasonally ice-covered lakes are greatly understudied in the cold season, there is increasing evidence that winter dynamics of the lake's microbiome (prokaryotic and eukaryotic microorganisms) is important for understanding bacterial community succession in the following ice-free period [5], [9].
Powerful molecular tools enable systematic explorations of the diverse and largely uncultivable bacterioplankton communities [10] including the winter season (e.g. [11]). So far, only a few studies [12]–[14] have focused on the bacterial community composition of temporary ice-covered lakes during the winter season. These studies reveal a typical winter bacterioplankton community, which is distinct from that during the warm season indicating specific bacterial adaptation to environmental conditions in winter, e.g. little light, low temperatures and organic matter (OM) concentrations, and high inorganic nutrient availability during winter mixing and early ice cover stages.
Although lakes cover a little more than 3% of the Earth surface as compared to 71% by oceans and seas, they have an equal contribution to CO2 emissions [15] and a larger contribution to carbon burial [16]. Therefore, it is surprising that annual studies of freshwater systems often neglect the entire period of ice coverage. Using a well-studied limnic system (Lake Stechlin, Germany), we present evidence for the quantitative significance of heterotrophic bacterial activity due to rapid mineralization of phytoplankton biomass in ice-covered aquatic systems. Our findings thus confirm and extend recent findings on the rapid sinking of under-ice phytoplankton blooms in the Arctic Ocean [4].
In this study we followed a sudden and massive under-ice proliferation of heterotrophic bacteria in oligotrophic Lake Stechlin following the breakdown of an under-ice bloom of the low light-adapted cyanobacterium Aphanizomenon flos-aquae in March 2010. Based on this phenomenon during which OM became suddenly available at high concentrations in the cold season, we hypothesize that once winter conditions have reached a steady state, bacterial activity and community composition are mainly regulated by OM availability and subsequent protozoan grazing, and only to a lesser extent by low water temperatures as has been traditionally suggested.
Materials and Methods
Field permit was granted to the Leibniz institute by the stechlin natural park authorities on a permanent basis.
Description of study site
Lake Stechlin (53′09 N, 13′02 E) is a seasonally ice-covered meso-oligotrophic freshwater lake formed after the last glacial period in northern Europe (Weichselian). With an area of 4.23 km2 and a maximum depth of 69.5 m, this relatively small, lake is one of the deepest lakes of the Northeastern German Lowlands [17]. Similar to other temperate deep lakes, Lake Stechlin overturns in spring and autumn and thus is classified as a dimictic lake [18].
Sampling procedure
Our main focus was the ice-covered period and the microbial community residing beneath the ice. Samples were collected between 10th December 2009 and 21st April 2010 from the epilimnion (integrated from 0–10 m depth) and the hypolimnion (distinct depth of 20 m) (Table S1). Temperature, oxygen, pH and conductivity were measured with probes (WTW, Weilheim, Germany) to monitor thermal stratification and determine the potential cyanobacterial bloom period. Bacterial protein production (BPP) was determined by [14C] - leucine incorporation in triplicates at in situ incubation for 1 h as described in Rösel & Grossart [19]. Samples were processed for microscopy, BPP, catalyzed reporter deposition fluorescence in situ hybridization (CARD-FISH), denaturing gradient gel electrophoresis (DGGE) and 454 tag pyrosequencing.
For CARD-FISH, water samples were fixed for 24 h at 4°C with formaldehyde (1% final concentration). Subsequently, aliquots were separated to determine particle-attached (PA) and free-living (FL) bacteria by sequential filtration through polycarbonate filters (5 and 0.2 µm pore size, respectively [Millipore, Eschborn, Germany]) as previously described in Rösel et al. [12]. CARD-FISH was done according to Pernthaler et al. [20] with minor modifications as described in Pizzetti et al. [21]. All probes used in this study are given in Table S2. Automated image acquisition and analysis was done as previously described in Zeder et al. [22]. Shortly, analysis of samples of FL bacteria was performed in a fully automated manner using a multi-purpose imaging system (MPISYS, modified after Zeder et al. [22] based on a fully motorized epifluorescence microscope (AxioImager.Z2m, Zeiss, Jena, Germany). To analyze PA bacteria the MPISYS was ran in a semi-automated mode as the density of particles varied from sample to sample. Upon selection, the system acquired a z-stack of 25 images around the focused position with an interval of 0.3 µm for each channel. For cell quantification, a maximum intensity projection was calculated on each channel. Following acquisition FL and PA sample images were analyzed using ACMEtool2 (www.technobiology.ch).
Samples for DNA extraction were size-fractionated immediately after sampling and stored at −80°C for further processing. DNA from 20 epilimnic and 20 hypolimnic samples (10 PA and 10 FL per strata) (Table S1) was extracted as described by Ionescu et al. [23]. To reveal the temporal resolution of the bacterial community composition (BCC), a segment of the 16S rRNA gene was amplified from the DNA extracts using the primer set 341F – GC and 907R [24], after which the amplicons were analyzed by DGGE as detailed in Rösel et al. [12]. The DGGE gels were analyzed using the Phoretix 1D version 11.4 (TotalLab) software and cluster analysis was done with PAST3 [25] using the Bray-Curtis similarity measure. Samples for 454 tag pyrosequencing were then selected based on changes in BCC during the ice-coverage detected by DGGE analysis. A total of 32 epilimnic and hypolimnic samples of PA and FL bacteria were pooled into 8 representative samples (Table S1). 454 tag pyrosequencing for bacterial diversity, using primer sets 28F and 519R [26], was done by MrDNA (Shallowater, Texas), using a Roche 454 FLX Genome Sequencer system equipped with titanium technology.
Sequence Analysis
Sequences were analyzed as previously described by Ionescu et al. [23], using the bioinformatics pipeline of the SILVA rRNA gene database project and the SILVA SSURef dataset (release 111; [27]). The SILVA databases include all high quality published sequences regardless of their marine, limnic or terrestrial origin. Furthermore, it follows the approved list of bacterial species (www.bacterio.net) and the bacterial code of nomenclature; therefore, differences in nomenclature may occur when compared to uncurated databases. Diversity and community structure analyses were performed based on >132,000 bacterial sequences. A detailed summary of the analysis process of the pyrosequencing data of each sample, including the total number of reads and length distribution, as well as the results of quality management, dereplication and clustering, can be found in the supplement (Table S3). A detailed list of the final taxonomic affiliation of all analyzed sequences together with their relative abundances within the amplicon pool is given in Table (S4) (Bacteria). The sequence data presented in this study can be freely obtained from the European Bioinformatics Institute (Study number PRJEB5107).
Results
Environmental conditions
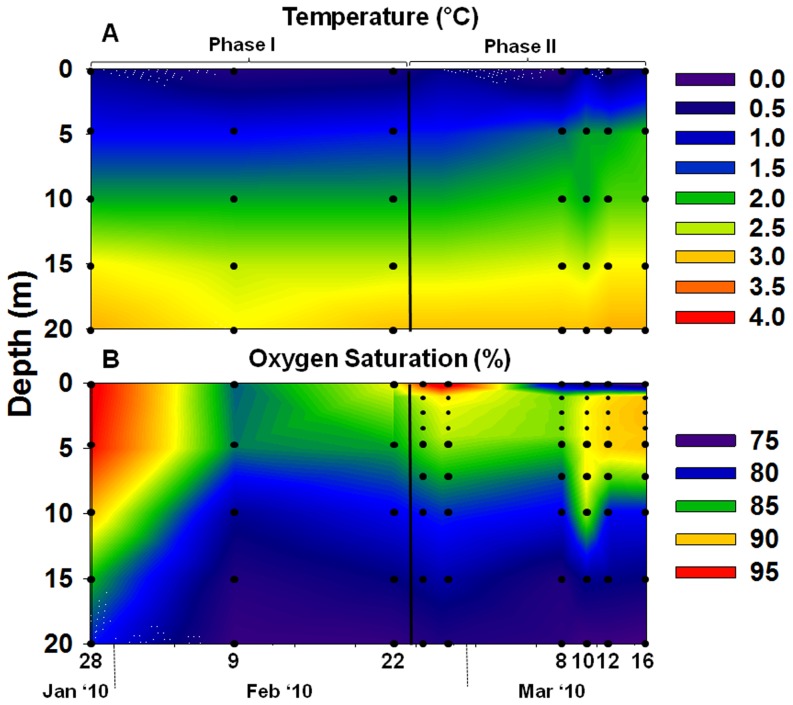
In winter 2009/10, ice on the surface of Lake Stechlin first appeared at the end of December. Throughout January, the lake was covered by a thickening layer of clear ice. The ice (∼30 cm thick) remained clear until mid-February, but thereafter the ice sheet was covered by >50 cm of snow. Thermal stratification under the ice was inverted as compared to the summer stratification. In the upper 5 m the water was <1°C while between 5 and 20 m the temperature increased to 3.1°C (Fig. 1A). Below 20 m the temperature increased gradually, but remained below 4°C. The ice-off occurred in the second half of March.
Figure 1. Temperature (A), and oxygen saturation (B) profiles during Phase I and Phase II of the bloom.
Black circles represent sampling points. Data are available at a higher spatial resolution only during Phase II of the bloom (small black circles).
Oxygen was overall below 100% saturation (450 and 405 µmol L−1 in surface and bottom water, respectively) throughout the ice-coverage period with a background of 75% saturation (Fig. 1B, S1A). Until the end of February 2010, O2 saturation level in the upper 4–5 m increased to maximal 95% following a surface bloom of the low light-adapted cyanobacterium Aphanizomenon flos-aquae [28]. Thereafter, the O2 saturation levels in the same water stratum decreased to 75% in early March (Fig. 1B) when the ice was covered by a thick layer of snow. The increase and decrease in O2 saturation levels during February to March 2010 are accompanied by a respective increase and decrease in pH values (Fig. S1B).
Cyanobacteria dynamics
Two cyanobacterial blooms were observed in Lake Stechlin in 2009, during September (A. flos-aquae and Anabaena macrospora) and December (A. flos-aquae), respectively [28]. The second bloom, initiated by the mixing of the water column in late fall, started in November and peaked during December. Although A. flos-aquae in Lake Stechlin forms heterocysts in summer, no heterocysts were visible in any of the analyzed filaments collected in winter (n>200 filaments), consistent with data on available combined nitrogen (20 µmol L−1 NO3; [28]). The second bloom persisted during the clear-ice period of the lake (December to mid-February). As the ice became covered by snow reducing the incident photosynthetically active radiation to 40 and 5 µmol photon m−2 s−1 at 1 and 5 m, respectively [28]. Due to the combination of low light and low temperature, the cyanobacteria became physiologically stressed [28] and a rapid bloom (>3 fold in 8 days) of heterotrophic particle-attached (PA) and subsequently free-living (FL) bacteria occurred.
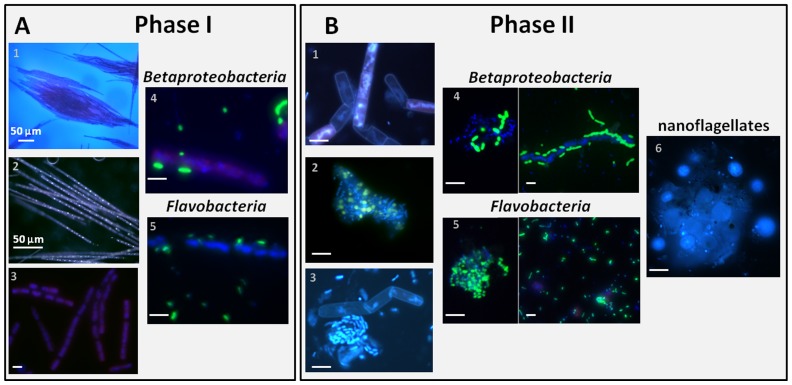
Therefore, we divided the sampling period during lake ice coverage into two phases. Phase I (January 28th-February 22nd) comprises the late bloom of A. flos-aquae. Phase II (February 23rd-March 16th) depicts the breakdown of the cyanobacterial bloom and the rapid rise of heterotrophic bacteria. Differences between Phases I and II are evident from micrographs of DAPI and FISH stained cyanobacterial filaments collected from the epilimnion (Fig. 2). First, the bloom of A. flos-aquae consists of intact filaments that contain poly-P granules and are rarely colonized by heterotrophic bacteria (Fig. 2A, S2). Subsequently, in Phase II damaged filaments with “ghost cells” are often aggregated and the poly-P granules are no longer associated with the filaments. These aggregates are heavily colonized by heterotrophic bacteria (Fig. 2B, S2).
Figure 2. Micrographs of DAPI and FISH stained Aphanizomenon flos-aquae filaments and heterotrophic bacteria, respectively; collected from the epilimnion during Phase I (A) and Phase II (B).
Cyanobacteria in Phase I are found in tufts (A1), the filaments contain poly-P granules (A2) and are hardly colonized by heterotrophic bacteria (A3). In Phase II single filaments with ghost cells are visible (B1). These are often aggregated with poly-P granules spilled onto the aggregate (B2) and heavily colonized by heterotrophic bacteria (B3). Significant increase in colonization between Betaproteobacteria and Flavobacteria in Phase I (A4–5) and Phase II (B4–5) is visible. End of Phase II is characterized by an increase in nanoflagellates (B6).
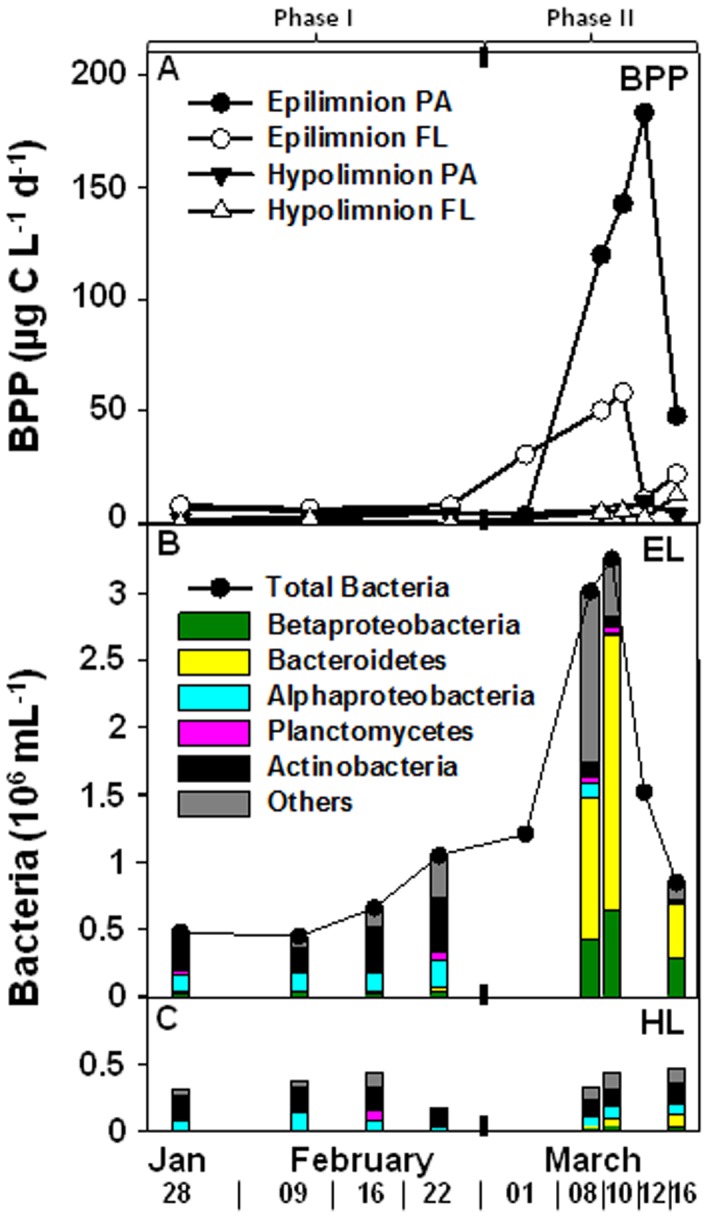
In the epilimnion (0–10 m), BPP of PA and FL bacteria, increased abruptly from Phase I to Phase II (5 to 183 and 8 to 58 µg C L−1 d−1, respectively) (Fig. 3A). This indicates that BPP can be very high at temperatures <1°C as compared to the rest of the year in Lake Stechlin or to other aquatic ecosystems [29]. The increase in BPP in Phase II was first seen in the FL fraction, but was followed by a larger increase in BPP of the PA fraction. The subsequent breakdown of the A. flos-aquae bloom led to a sudden rise in abundance of heterotrophic bacteria from 1×106 to 3.25×106 cells mL−1 (Fig. 3B). At the end of Phase II, BPP in the PA and FL fraction rapidly decreased to 48 and 22 µg C L−1 d−1, respectively; still several folds higher than at the end of January when the lake surface was covered by clear ice. BPP eventually decreased as the number of bacterial cells was reduced to 0.85×106 cells mL−1, parallel to the increase in abundance of heterotrophic flagellates.
Figure 3. Bacterial protein production (BPP) of particle-attached (PA) and free-living (FL) bacteria in the epilimnion and hypolimnion (A) and bacterial abundance of total bacteria in the epilimnion (B) and hypolimnion (C) during the two phases of the bloom.
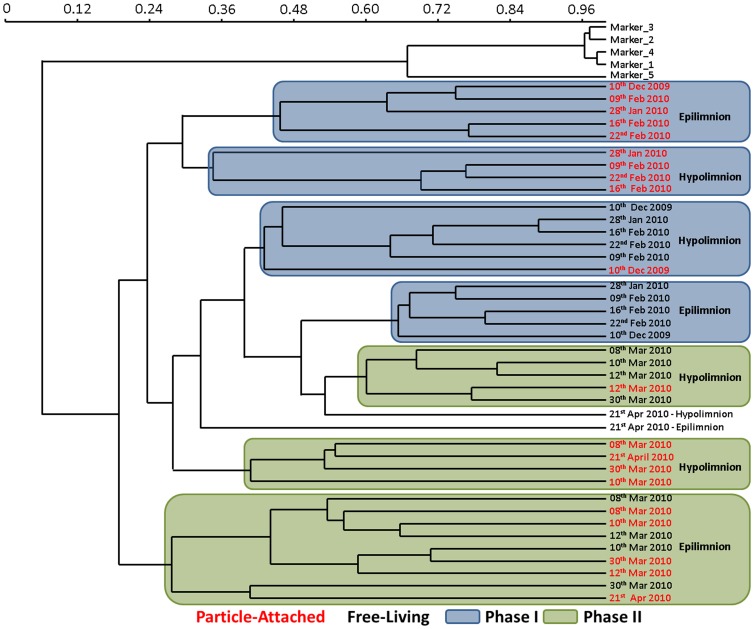
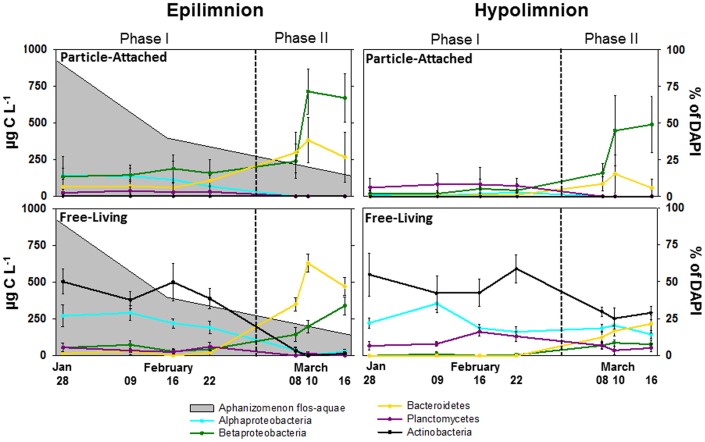
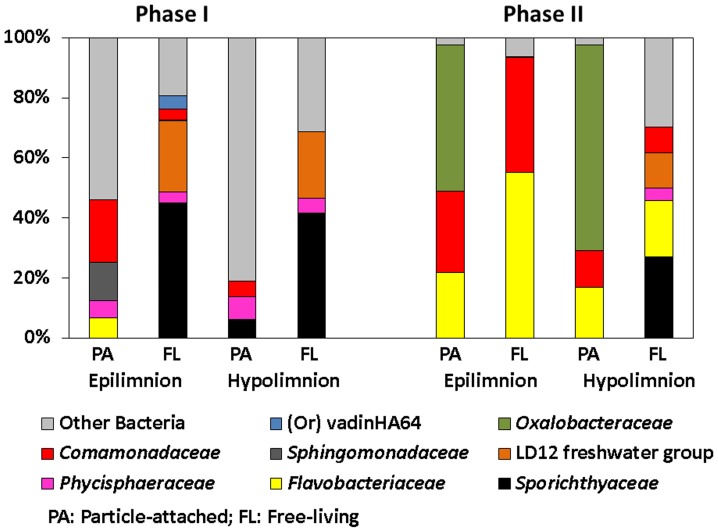
The sudden increase in bacterial abundance and activity were accompanied by a similarly rapid shift in Bacterial Community Composition (BCC) as evident from Denaturing Gradient Gel Electrophoresis (DGGE), Catalyzed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH) and 454 tag pyrosequencing results (Fig. 4 & S3; 3B & 5; 6 & S4 respectively). The swiftness of the change is best observed by high temporal resolution DGGE fingerprinting, showing the presence of two, stable, yet different communities in Phase I and II of the bloom (Fig. 4; Fig. S3). In Phase I, members of Alphaproteobacteria, Betaproteobacteria, Bacteroidetes and Planctomycetes (11% (0–40%), 16% (0–33%), 7% (0–19%) and 3% (0–28%), respectively) were associated with A. flos-aquae cells (Fig. 5). In Phase II, we observed a simultaneously strong increase in relative abundances of Betaproteobacteria and Bacteroidetes (54% (4–88%) and 31% (4–63%), respectively). These were also the only two bacterial groups associated with A. flos-aquae at that time. Alphaproteobacteria and Actinobacteria (on average 24% and 44%, respectively) dominated the FL fraction in Phase I, but were replaced in Phase II by a dominance of Bacteroidetes and Betaproteobacteria (on average 49% and 23%, respectively) (Fig. 3B; 5). Comparative 16S rRNA gene sequencing of pooled PA and FL fractions of both the epi- and hypolimnion showed that the transition between Phase I and II resulted not only from a change in the bacterial groups, as already implied by CARD-FISH (Fig. 3B; 4), but also from a strong decrease in the number of dominant bacterial families, and the expansion of a few groups of specialist bacteria (Fig. 6 & S4). In Phase I, 50% of PA bacteria in the epilimnion belonged to 4 major families while the rest was distributed among 44 others. In contrast, in Phase II, 96% of the PA bacteria were dominated by 3 families alone: Flavobacteriaceae (21%), Comamonadaceae (27%) and Oxalobacteraceae (48%). While the first two families dominated the PA fraction in the epilimnion during Phase I, Oxalobacteraceae contributed to <0.5% of the BCC (Table S4). During the transition from Phase I to Phase II, FL bacteria in the epilimnion showed a similar succession as PA bacteria resulting in a decreased bacterial diversity. These changes in PA and FL BCC did not occur simultaneously. A succession between the PA and FL fractions is obvious from the DGGE fingerprints, as several bands appeared first on particles and only later in the surrounding water (Fig. S3). Cluster analyses further shows the strong link between the PA and FL fractions in Phase II of the bloom where in contrast to Phase I PA and FL communities cluster together (Fig. 4).
Figure 4. Denaturing gradient gel electrophoresis dendrogram showing the relationship between particle-attached and free-living bacterial communities from epilimnion and hypolimnion of Lake Stechlin during Phase I and Phase II of the bloom.
The gels were analyzed using the Phoretix 1D version 11.4 (TotalLab) software and cluster analysis was done with PAST3 [25] using the Bray-Curtis similarity measure.
Figure 5. Distribution of relative abundances of major phylogenetic groups (% of DAPI - right axis): Alphaproteobacteria, Betaproteobacteria, Bacteroidetes, Planctomycetes and Actinobacteria as assessed by CARD-FISH in the particle-attached and free-living fractions in both epilimnion and hypolimnion.
Phase I and II of the bloom are separated by the dashed line. The abundance of Aphanizomenon flos-aquae (gray field) is presented during both phases of the bloom (left axis).
Figure 6. Sequence frequencies of major particle-attached (PA) and free-living (FL) bacterial families comprising to >5% of total sequences in at least one sample in the epilimnion and hypolimnion of Lake Stechlin during Phase I and Phase II of the under-ice bloom.
Our results indicate that the hypolimnion (20 m) was affected by the events in the epilimnion only towards the end of Phase II. Total bacterial numbers (FL) in the hypolimnion (20 m) were <0.5×106 cells mL−1 throughout the entire period. Accordingly, BPP was much lower than in the epilimnion (1–9 and 0.1–12 µg C L−1 d−1 in PA and FL, respectively) during the whole period and only slightly increased at the end of Phase II (Fig. 3A). This, together with our microscopic observations, indicates that solely a small fraction of cyanobacterial cell debris reached this depth, and that the cyanobacterial biomass was efficiently remineralized in the epilimnion.
Discussion
In temperate lakes, persistent cyanobacterial blooms frequently occur in winter under the ice (e.g. [30]) (Table S5). However, most ice-covered (meso-) oligotrophic lakes such as Lake Stechlin have been considered to be low carbon environments in winter [5]. This is mainly because of two factors: 1) Primary productivity is usually low due to decreased light availability under the ice; 2) Input of atmospheric and terrestrial OM is blocked by the ice coverage and the available internal OM is rapidly consumed by the residual heterotrophic bacterial community. Accordingly, a low BPP was measured during Phase I of the cyanobacterial bloom. Coverage of Lake Stechlin with clear ice, however, allowed for penetration of sufficient light which together with a stable inverse (as compared to the summer) stratification of the water column sustained growth of low temperature- and low light-adapted A. flos-aquae [28] in the cold epilimnion (<4°C).
Bacterial numbers and BPP remained low in Lake Stechlin in Phase I. At this time inorganic nutrients necessary for the blooming of A. flos-aquae were abundant after the mixing in early winter, including nitrogen as indicated by the lack of heterocysts and phosphorus by storage in form of poly-P granules. The high growth potential of the winter bacterioplankton - irrespective of temperature - became evident when A. flos-aquae became light limited [28] due to low irradiance under the snow-covered ice in Phase II. The sudden increase in bacterial abundance and BPP in the epilimnion during the transition from Phase I to Phase II in parallel to the relatively high availability of inorganic nutrients support the hypothesis that the winter bacterioplankton in Lake Stechlin is controlled by carbon availability rather than temperature. The physiologically stressed cyanobacteria were rapidly colonized by heterotrophic bacteria that alone, or in combination with cyanophages degraded the A. flos-aquae filaments, leading to an extremely high bacterial abundance and BPP in Phase II. The degradation of cyanobacterial filaments resulted in the release of the protoplasmic content into the surrounding water leaving empty “ghost cells” (Fig. 2B), thus increasing OM availability also for FL bacteria. Although temperature remained <4°C in the epilimnion in Phase II, high inorganic nutrient availability coupled to the relief from carbon limitation led to an extreme increase in BPP, which was >5 times higher than measured during the peak of the subsequent spring bloom [19]. Moreover, BPP under the ice is in the same range as measured during summer blooms in four Swedish lakes despite the fact that the bacterial abundance under the ice was 1–2 orders of magnitude lower [6]. From this notion it is evident that periodical events such as the under ice A. flos-aquae bloom in Lake Stechlin lead to high microbial OM turnover in winter when concentrations of herbivorous zooplankton are low [5]. This suggests that availability of OM rather than temperature triggered BPP leading to a rapid mineralization of the phytoplankton biomass in ice-covered Lake Stechlin counter-acting its sinking and burial in the sediment. Thus, the rapid sinking of under-ice phytoplankton blooms in cold environments [4] should be seen in an environmental context and should not be considered as a general rule.
It can be assumed that the high heterotrophic activities measured in Phase II have the potential to strongly affect C cycling, particularly the release of CO2 from the lake into the atmosphere after ice-off. CO2 concentrations under the ice gas-barrier can exceed summer values [31] reaching concentrations of up to 650 µmol L−1 and often 2–3 folds higher than during the ice-free period [32]. Hence, the resultant CO2 buildup can be rapidly released to the atmosphere upon ice-off. So far, under ice blooms of heterotrophic bacteria have been neglected for modeling of metabolism and element cycling in lakes. However, Karlsson et al. [33] suggest that up to 55% of the annual gas emission from freshwater systems occurs during ice-off. To better understand and predict environmental consequences of lake biogeochemistry, year-round studies are required to also include massive under ice blooms of photoautotrophic and heterotrophic microorganisms. These may also set the frame for the subsequent food web development during the growing season [5].
High availability of OM during bacterial colonization and lysis of A. flos-aquae cells also dramatically affected biodiversity reflected by a temporal shift in BCC. Our DGGE fingerprints suggest a sudden change of BCC between Phase I and II. The uniformity in quality of the available OM in Phase II of the bloom was indicated by the dominance of solely a small group of specialized bacteria different from those in Phase I. These bacteria first appeared in the epilimnic PA fraction and later in the FL fraction of the epilimnion and in the PA fraction of the hypolimnion. This suggests that a) bacterial colonization of senescent cyanobacteria resulted in detachment of bacteria and release of substrates into the surrounding water supporting the proliferation of these communities; b) a small fraction of colonized A. flos-aquae aggregates sank into the hypolimnion. The latter process was reflected by significant changes in BCC of hypolimnic PA bacteria greatly reducing microbial diversity in this fraction.
These shifts in microbial communities present a limnic under-ice analogue to the substrate-driven succession of marine bacterioplankton populations induced by phytoplankton blooms recently described by Teeling et al. [34]. Although temperatures and salt content greatly differed between the two studies, the Flavobacteriaceae family, known to degrade high-molecular-weight OM [35], were among the first to colonize the A. flos-aquae cells. Nevertheless, while the marine event harbored numerous genera from the Flavobacteriaceae, Flavobacterium was the sole genus detected under the ice in Lake Stechlin (Fig. S4). In the marine analogue Gammaproteobacteria and to some extent Alphaproteobacteria succeeded the Flavobacteriaceae. In contrast, in our study, Betaproteobacteria (Fig. 6), specifically members of the genera Undibacterium and Massilia as well as an unclassified group of Comamonadaceae (Fig. S4) followed the initial colonization and rapidly exceeded the abundance of Flavobacterium (Fig. 5). We propose that the betaproteobacterial clades detected under the ice in Lake Stechlin fill in similar ecological niches in freshwater as the marine Gammaproteobacteria associated with a decaying algal bloom in Teeling et al. [34]. Interestingly, both blooms independent of water temperature had a similar carrying capacity, leading to an increase in FL bacterial abundance from 0.5×106 to 3×106 cells mL−1. This maximal carrying capacity could be a result of a protozoan top-down control similar to that observed in Lake Stechlin.
In our study, available OM affects the BCC and BPP. While the temperature in the epilimnion remains constant <1°C throughout the period, and, therefore, can be excluded as an influencing factor. OM increases dramatically in Phase II due to cyanobacterial decay. Based on our DGGE results, the BCC remained stable during Phase I (for at least 2 months) in parallel to a rather stable BPP. Nevertheless, during the rapid transition between Phase I and II of the bloom, while temperature remained constant, we observed a major change in BCC accompanied by a large increase in activity. The initially low abundant Flavobacteriaceae in Phase I appeared in larger numbers in Phase II and the previously diverse Comamonadaceae were reduced to 3 genera solely (Fig. S4). These phylotypes included cold adapted species such as Flavobacterium psychrolimnae, F. fryxellicola, F. limicola, F. omnivorum, F. frigidarium that are often reported from ice-covered aquatic systems [36]–[39]. The bacterial phylotypes in Phase II grew rapidly and were able to degrade the cyanobacterial cells to a large extent. This suggests that availability of OM not only triggered BPP, but also succession in BCC.
Under-ice blooms of phytoplankton are not sporadic events since, during the last decades, they have been regularly described from limnic environments and more recently also from marine systems (Table S5). Using Lake Stechlin as an example, we show that temperate lakes bear the potential for high biological activity even during the cold winter season. Despite the low temperature (<4°C), carbon flow from primary production and, particularly, from cyanobacteria biomass during bloom breakdown has the potential to fuel massive blooms of heterotrophic microorganisms. These were soon top-down controlled in the epilimnion by heterotrophic nanoflagellates (Fig. 2B), demonstrating that also protozoa growth was less dependent on water temperatures than on prey availability. Consequently, under ice blooms of photoautotrophic and heterotrophic microorganisms greatly affect the lake's biogeochemistry and thus set the frame for further food web dynamics in the ice-free season.
Current climate change leads to a reduced intensity of ice coverage and hence to dramatic environmental changes with so far unknown ecological consequences, e.g., for biogeochemical processes such as greenhouse gas fluxes across the water-atmosphere boundary. Limnic systems are known to be super-saturated with greenhouse gases [40]. While global CO2 emissions from freshwater have been estimated to 0.75 Pg y−1 [41] (equal to oceanic emissions [15]), these estimates do not include emissions following ice-off. Sediments of freshwater systems store ca. 3 times more carbon (0.3 Pg y−1) than marine ones (0.1 Pg y−1) (∼150 times more when normalized to area) [16]. Yet, this figure does not include the unresolved fate of massive under-ice blooms in seasonally ice covered lakes. With the exception of extremely oligotrophic lakes, most of the OM in limnic systems is of autochthonous origin [16]. Therefore, the response of the heterotrophic bacterial community to massive under ice phytoplankton blooms potentially determines whether the lake acts as a C sink or a source. To better understand the role of lakes in global biogeochemical cycles and biodiversity throughout the whole season, it will be crucial to perform year-round studies with a greater focus on the winter season, which - independent of the low temperature - has the potential for high biological activities as long as phototrophic OM remains available for heterotrophic processing. Such studies should have a high temporal sampling resolution (as can be achieved by automatic sampling or monitoring) since as demonstrated in our study the entire bloom event can last less than two weeks. The short duration of such dynamic events points to the fact that monthly routine sampling is very likely to miss such events and that even in winter, boosts in biological activities and OM turnover occur. Extending earlier studies, e.g. those from Lake Baikal [42], usually covered by clear ice, we here suggest that energy and substrate availability rather than temperature are the key factors controlling dynamics of aquatic microorganisms. The dimensions of these carbon driven heterotrophic blooms are important for understanding their present ecological role in aquatic ecosystems, particularly when taking into account that in the future sea ice will rapidly reside [43] and perennially ice-covered aquatic ecosystems may fully disappear [15].
Supporting Information
Oxygen concentration (A) and PH (B) profiles measured during winter 2010 in Lake Stechlin.
(TIF)
Colonization density of particle-attached bacteria and abundance of free living cells. Since particles vary in size, the cell number (DAPI signal) per particle (a cyanobacteria segment) was normalized according to the particle's area to 100 µm2. Median and average values are shown by full and dashed lines, respectively.
(TIFF)
DGGE analysis of the bacterial diversity of the two phases of the bloom separated into particle-attached (PA) and free-living (FL) bacterial communities of epilimnion (EL) and hypolimnion (HL).
(TIF)
Sequence frequencies of major particle-attached and free-living bacterial genera in the epilimnion and hypolimnion of Lake Stechlin during Phase I (A) and Phase II (B) of the under-ice bloom. Genera and clades of not yet cultured bacteria (e.g. Ac1, LD12) are detailed only within families making up over 2% of the total sequences in at least one sample. Numbers in the gray fields refer to all the families that made less than 2% of the total sequences each.
(TIFF)
List of samples analyzed for Bacterial Protein Production (BPP), Denaturing Gradient Gel Electrophoresis (DGGE), Tag Pyrosequencing (454) and Fluorescence in situ hybridization (FISH).
(DOCX)
Oligonucleotide probes used in this study.
(DOCX)
Sequence quality and diversity data for each sample.
(DOCX)
List of bacterial tax paths.
(PDF)
Under-ice phytoplankton blooms in limnic and marine systems.
(DOC)
Acknowledgments
We thank Timothy G. Ferdelman and Danny Ionescu for providing many helpful comments. We acknowledge the scientific and technical support of Judit Padisak, Elke Mach and Solvig Pinnow.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The sequence data presented in this study can be obtained from the European Bioinformatics Institute (Study number PRJEB5107).
Funding Statement
Funding provided by the Leibniz Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Benson B, Magnuson J (2007) Global lake and river ice phenology database. National Snow and Ice Data Center/World Data Center for Glaciology, Boulder, CO. Digital media. http//nsidc.org/data/g01377.html.
- 2.Chapman WL, Walsh JE (1991) Updated 1996. Arctic and Southern Ocean Sea Ice Concentrations. Boulder, Colorado USA.
- 3. Dokulil MT, Herzig A (2009) An analysis of long-term winter data on phytoplankton and zooplankton in Neusiedler See, a shallow temperate lake, Austria. Aquat Ecol 43:715–725 10.1007/s10452-009-9282-3 [DOI] [Google Scholar]
- 4. Boetius A, Albrecht S, Bakker K, Bienhold C, Felden J, et al. (2013) Export of algal biomass from the melting Arctic sea ice. Science 339:1430–1432 10.1126/science.1231346 [DOI] [PubMed] [Google Scholar]
- 5. Bertilsson S, Burgin A, Carey CC, Fey SB, Grossart H-P, et al. (2013) The under-ice microbiome of seasonally frozen lakes. Limnol Oceanogr 58:1998–2012 10.4319/lo.2013.58.6.1998 [DOI] [Google Scholar]
- 6. Eiler A, Bertilsson S (2004) Composition of freshwater bacterial communities associated with cyanobacterial blooms in four Swedish lakes. Environ Microbiol 6:1228–1243 10.1111/j.1462-2920.2004.00657.x [DOI] [PubMed] [Google Scholar]
- 7. Greenbank J (1945) Limnological conditions in ice-covered lakes, especially as related to winter-kill of fish. Ecol Monogr 15:343–392. [Google Scholar]
- 8. Tulonen T, Kankaala P (1994) Factors controlling production of phytoplankton and bacteria under ice in a humic, boreal lake. J Plankton Res 16:1411–1432. [Google Scholar]
- 9. Twiss MR, McKay RML, Bourbonniere RA, Bullerjahn GS, Carrick HJ, et al. (2012) Diatoms abound in ice-covered Lake Erie: An investigation of offshore winter limnology in Lake Erie over the period 2007 to 2010. J Gt Lakes 38:18–30. [Google Scholar]
- 10. Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49 10.1128/MMBR.00028-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tada Y, Grossart H-P (2014) Community shifts of actively growing lake bacteria after N-acetyl-glucosamine addition: improving the BrdU-FACS method. ISME J 8:441–454 10.1038/ismej.2013.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rösel S, Allgaier M, Grossart H-P (2012) Long-term characterization of free-living and particle-associated bacterial communities in Lake Tiefwaren reveals distinct seasonal patterns. Microb Ecol 64:571–583 10.1007/s00248-012-0049-3 [DOI] [PubMed] [Google Scholar]
- 13. Llorens-Marès T (2012) Winter to spring changes in the slush bacterial community composition of a high mountain lake (Lake Redon, Pyrenees). Environ Microbiol Rep 4:50–56. [DOI] [PubMed] [Google Scholar]
- 14. Glatz R, Lepp P, Ward B, Francis C (2006) Planktonic microbial community composition across steep physical/chemical gradients in permanently ice-covered Lake Bonney, Antarctica. Geobiology 4:53–67. [Google Scholar]
- 15.IPCC 2007 (n.d.) Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Parry ML, Canziani OF, Palutikof JP, van der Linden PJ, Hanson CEeditors. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA [Google Scholar]
- 16. Dean WE, Gorham E (1998) Magnitude and significance of carbon burial in lakes, reservoirs, and peatlands. Geology 26:535–538 doi:10.1130/0091-7613(1998)026<0535:MASOCB>2.3.CO;2 [Google Scholar]
- 17. Koschel R, Adams DD (2003) An approach to understanding a temperate oligotrophic lowland lake (Lake Stechlin, Germany). Adv Limnol 58:1–9. [Google Scholar]
- 18.Ruttner F (1968) Fundamentals of limnology. 3rd ed. Univ of Toronto Press, Toronto.
- 19. Rösel S, Grossart H-P (2012) Contrasting dynamics in activity and community composition of free-living and particle-associated bacteria in spring. Aquat Microb Ecol 66:169–181 10.3354/ame01568 [DOI] [Google Scholar]
- 20.Pernthaler A, Pernthaler J, Amann R (2004) Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms. In:Kowalchuk G, de Bruijn FJ, Head IM, Akkermans AD, van Elsas JDeditors. Molecular Microbial Ecology Manual. Dodrecht, the Netherlands: Kluwer Academic Publishers, Vol. 2nd edditi. pp.711–726. doi: 10.1007/978-1-4020-2177-0_311.
- 21. Pizzetti I, Fuchs BM, Gerdts G, Wichels A, Wiltshire KH, et al. (2011) Temporal variability of coastal Planctomycetes clades at Kabeltonne station, North Sea. Appl Environ Microbiol 77:5009–5017 10.1128/AEM.02931-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeder M, Ellrott A, Amann R (2011) Automated sample area definition for high-throughput microscopy. Cytom Part A 79:306–310 10.1002/cyto.a.21034 [DOI] [PubMed] [Google Scholar]
- 23. Ionescu D, Siebert C, Polerecky L, Munwes YY, Lott C, et al. (2012) Microbial and chemical characterization of underwater fresh water springs in the Dead Sea. PLoS One 7:e38319 10.1371/journal.pone.0038319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muyzer G, Waal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammer Ø, Harper DAT, Ryan PD (2001) PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron 4:1–9. [Google Scholar]
- 26.Lane D (1991) 16S/23S rRNA sequencing. Nucleic acid techniques in bacterial systematics. John Wiley and Sons, New York, NY. pp.115–175. [Google Scholar]
- 27. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, et al. (2013) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–6 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Üveges V, Tapolczai K, Krienitz L, Padisák J (2012) Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 698:263–272 10.1007/s10750-012-1103-3 [DOI] [Google Scholar]
- 29. Cole JJ, Findlay S, Pace ML (1988) Bacterial production in fresh and saltwater ecosystems: a cross-system overview. Mar Ecol Prog Ser 43:1–10. [Google Scholar]
- 30. Keto J, Tallberg P (2000) The recovery of Vesijarvi, a lake in southern Finland: water quality and phytoplankton interpretations. Boreal Environ Res 5:15–26. [Google Scholar]
- 31. Kling G, Kipphut G, Miller M (1992) The flux of CO2 and CH4 from lakes and rivers in arctic Alaska. Hydrobiologia 240:23–36. [Google Scholar]
- 32. Kankaala P, Huotari J, Tulonen T, Ojala A (2013) Lake-size dependent physical forcing of carbon dioxide and methane effluxes from lakes in a boreal landscape. Limnol Oceanogr 58:1915–1930 10.4319/lo.2013.58.6.1915 [DOI] [Google Scholar]
- 33. Karlsson J, Giesler R, Persson J, Lundin E (2013) High emission of carbon dioxide and methane during ice thaw in high latitude lakes. Geophys Res Lett 40:1123–1127 10.1002/grl.50152 [DOI] [Google Scholar]
- 34. Teeling H, Fuchs BM, Becher D, Klockow C, Gardebrecht A, et al. (2012) Substrate-controlled succession of marine bacterioplankton populations induced by a phytoplankton bloom. Science 336:608–611 10.1126/science.1218344 [DOI] [PubMed] [Google Scholar]
- 35. Kirchman DL (2002) The ecology of Cytophaga-Flavobacteria in aquatic environments. FEMS Microbiol Ecol 39:91–100 10.1111/j.1574-6941.2002.tb00910.x [DOI] [PubMed] [Google Scholar]
- 36. Van Trappen S, Vandecandelaere I, Mergaert J, Swings J (2005) Flavobacterium fryxellicola sp. nov. and Flavobacterium psychrolimnae sp. nov., novel psychrophilic bacteria isolated from microbial mats in Antarctic lakes. Int J Syst Evol Microbiol 55:769–772 10.1099/ijs.0.03056-0 [DOI] [PubMed] [Google Scholar]
- 37. Tamaki H (2003) Flavobacterium limicola sp. nov., a psychrophilic, organic-polymer-degrading bacterium isolated from freshwater sediments. Int J Syst Evol Microbiol 53:519–526 10.1099/ijs.0.02369-0 [DOI] [PubMed] [Google Scholar]
- 38. Zhu F, Wang S, Zhou P (2003) Flavobacterium xinjiangense sp. nov. and Flavobacterium omnivorum sp. nov., novel psychrophiles from the China No. 1 glacier. Int J Syst Evol Microbiol 53:853–857 10.1099/ijs.0.02310-0 [DOI] [PubMed] [Google Scholar]
- 39. Humphry DR, George A, Black GW, Cummings SP (2001) Flavobacterium frigidarium sp. nov., an aerobic, psychrophilic, xylanolytic and laminarinolytic bacterium from Antarctica. Int J Syst Evol Microbiol 51:1235–1243. [DOI] [PubMed] [Google Scholar]
- 40. Cole JJ, Caraco NF, Kling G, Kratz T (1994) Carbon dioxide supersaturation in the surface waters of lakes. Science (80-) 265:1568–1570. [DOI] [PubMed] [Google Scholar]
- 41. Cole JJ, Prairie YT, Caraco NF, McDowell WH, Tranvik LJ, et al. (2007) Plumbing the Global Carbon Cycle: Integrating Inland Waters into the Terrestrial Carbon Budget. Ecosystems 10:172–185 10.1007/s10021-006-9013-8 [DOI] [Google Scholar]
- 42. Straškrábová V, Izmest'yeva LR, Maksimova EA, Fietz S, Nedoma J, et al. (2005) Primary production and microbial activity in the euphotic zone of Lake Baikal (Southern Basin) during late winter. Glob Planet Change 46:57–73 10.1016/j.gloplacha.2004.11.006 [DOI] [Google Scholar]
- 43. Levasseur M (2013) Impact of Arctic meltdown on the microbial cycling of sulphur. Nat Geosci 6:691–700 10.1038/ngeo1910 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Oxygen concentration (A) and PH (B) profiles measured during winter 2010 in Lake Stechlin.
(TIF)
Colonization density of particle-attached bacteria and abundance of free living cells. Since particles vary in size, the cell number (DAPI signal) per particle (a cyanobacteria segment) was normalized according to the particle's area to 100 µm2. Median and average values are shown by full and dashed lines, respectively.
(TIFF)
DGGE analysis of the bacterial diversity of the two phases of the bloom separated into particle-attached (PA) and free-living (FL) bacterial communities of epilimnion (EL) and hypolimnion (HL).
(TIF)
Sequence frequencies of major particle-attached and free-living bacterial genera in the epilimnion and hypolimnion of Lake Stechlin during Phase I (A) and Phase II (B) of the under-ice bloom. Genera and clades of not yet cultured bacteria (e.g. Ac1, LD12) are detailed only within families making up over 2% of the total sequences in at least one sample. Numbers in the gray fields refer to all the families that made less than 2% of the total sequences each.
(TIFF)
List of samples analyzed for Bacterial Protein Production (BPP), Denaturing Gradient Gel Electrophoresis (DGGE), Tag Pyrosequencing (454) and Fluorescence in situ hybridization (FISH).
(DOCX)
Oligonucleotide probes used in this study.
(DOCX)
Sequence quality and diversity data for each sample.
(DOCX)
List of bacterial tax paths.
(PDF)
Under-ice phytoplankton blooms in limnic and marine systems.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The sequence data presented in this study can be obtained from the European Bioinformatics Institute (Study number PRJEB5107).