Abstract
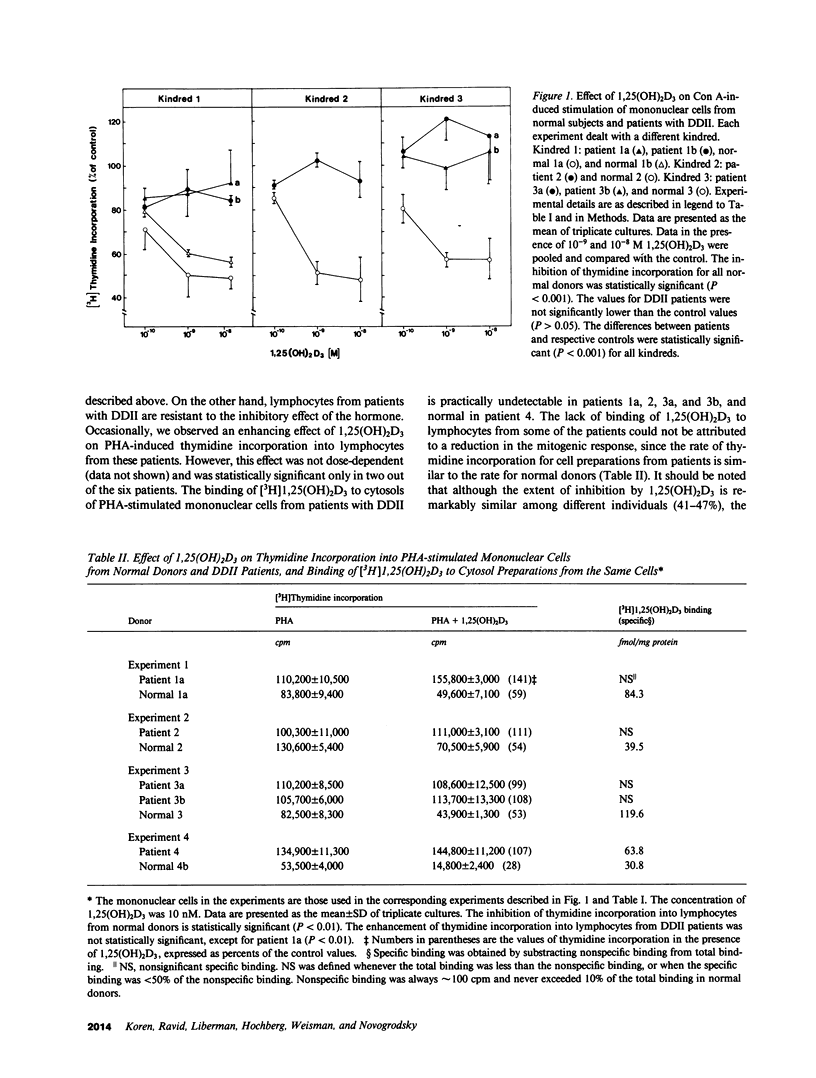
Lectin-induced DNA synthesis by peripheral mononuclear cells from 17 normal donors was inhibited (40-60%) by 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) at physiological concentrations (10(-10)-10(-9) M). The lymphocytes acquire specific receptors for 1,25(OH)2D3 upon activation by the lectins. This process precedes the inhibitory effect of 1,25(OH)2D3. We studied lymphocytes from six patients from four different kindreds with the syndrome of hereditary end-organ resistance to 1,25(OH)2D (the so-called vitamin D-dependent rickets type II). In five patients (three kindreds) peripheral blood mononuclear cells did not acquire receptors for 1,25(OH)2D3 upon phytohemagglutinin-induced activation. Moreover, in contrast to normal lymphocytes, the mitogenic stimulation of these patients' lymphocytes by phytohemagglutinin and concanavalin A was not inhibited by 1,25(OH)2D3. Activated lymphocytes of the sixth patient from a fourth kindred exhibited normal binding of [3H]1,25(OH)2D3 but the hormone failed to inhibit the mitogenic stimulation. A similar pattern of the vitamin D effector system was previously observed in fibroblasts cultured from skin biopsies of the same group of patients. The conclusions from these findings are: (a) the inhibition of mitogenic stimulation by 1,25(OH)2D3 is mediated by specific functional receptors to the hormone; and (b) the receptors for 1,25(OH)2D3 in mononuclear cells are probably controlled genetically by the same mechanisms as the effector system in well-characterized target organs of the hormone, such as intestine and kidney.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhalla A. K., Amento E. P., Clemens T. L., Holick M. F., Krane S. M. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983 Dec;57(6):1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Hirst M. A., Cone C. M., Hochberg Z., Tietze H. U., Feldman D. 1,25-dihydroxyvitamin D resistance, rickets, and alopecia: analysis of receptors and bioresponse in cultured fibroblasts from patients and parents. J Clin Endocrinol Metab. 1984 Sep;59(3):383–388. doi: 10.1210/jcem-59-3-383. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. Subcellular mechanisms involving vitamin D. Subcell Biochem. 1981;8:251–272. doi: 10.1007/978-1-4615-7951-9_5. [DOI] [PubMed] [Google Scholar]
- Feldman D., Chen T., Hirst M., Colston K., Karasek M., Cone C. Demonstration of 1,25-dihydroxyvitamin D3 receptors in human skin biopsies. J Clin Endocrinol Metab. 1980 Dec;51(6):1463–1465. doi: 10.1210/jcem-51-6-1463. [DOI] [PubMed] [Google Scholar]
- Grody W. W., Schrader W. T., O'Malley B. W. Activation, transformation, and subunit structure of steroid hormone receptors. Endocr Rev. 1982 Spring;3(2):141–163. doi: 10.1210/edrv-3-2-141. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liberman U. A., Eil C., Marx S. J. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest. 1983 Feb;71(2):192–200. doi: 10.1172/JCI110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman U. A., Samuel R., Halabe A., Kauli R., Edelstein S., Weisman Y., Papapoulos S. E., Clemens T. L., Fraher L. J., O'Riordan J. L. End-organ resistance to 1,25-dihydroxycholecalciferol. Lancet. 1980 Mar 8;1(8167):504–506. doi: 10.1016/s0140-6736(80)92763-4. [DOI] [PubMed] [Google Scholar]
- Manolagas S. C., Deftos L. J. The vitamin D endocrine system and the hematolymphopoietic tissue. Ann Intern Med. 1984 Jan;100(1):144–146. doi: 10.7326/0003-4819-100-1-144. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Roth J., Orci L. The vitamin D endocrine system: steroid metabolism, hormone receptors, and biological response (calcium binding proteins). Endocr Rev. 1982 Fall;3(4):331–366. doi: 10.1210/edrv-3-4-331. [DOI] [PubMed] [Google Scholar]
- Provvedini D. M., Tsoukas C. D., Deftos L. J., Manolagas S. C. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983 Sep 16;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- Ravid A., Koren R., Novogrodsky A., Liberman U. A. 1,25-Dihydroxyvitamin D3 inhibits selectively the mitogenic stimulation of mouse medullary thymocytes. Biochem Biophys Res Commun. 1984 Aug 30;123(1):163–169. doi: 10.1016/0006-291x(84)90394-2. [DOI] [PubMed] [Google Scholar]
- Tsoukas C. D., Provvedini D. M., Manolagas S. C. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984 Jun 29;224(4656):1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]



