Abstract
Bergamot (Citrus bergamia, Risso et Poiteau) essential oil (BEO) is a well characterized, widely used plant extract. BEO exerts anxiolytic, analgesic and neuroprotective activities in rodents through mechanisms that are only partly known and need to be further investigated. To gain more insight into the biological effects of this essential oil, we tested the ability of BEO (0.005–0.03%) to modulate autophagic pathways in human SH-SY5Y neuroblastoma cells. BEO-treated cells show increased LC3II levels and appearance of dot-like formations of endogenous LC3 protein that colocalize with the lysosome marker LAMP-1. Autophagic flux assay using bafilomycin A1 and degradation of the specific autophagy substrate p62 confirmed that the observed increase of LC3II levels in BEO-exposed cells is due to autophagy induction rather than to a decreased autophagosomal turnover. Induction of autophagy is an early and not cell-line specific response to BEO. Beside basal autophagy, BEO also enhanced autophagy triggered by serum starvation and rapamycin indicating that the underlying mechanism is mTOR independent. Accordingly, BEO did not affect the phosphorylation of ULK1 (Ser757) and p70S6K (Thr389), two downstream targets of mTOR. Furthermore, induction of autophagy by BEO is beclin-1 independent, occurs in a concentration-dependent manner and is unrelated to the ability of BEO to induce cell death. In order to identify the active constituents responsible for these effects, the two most abundant monoterpenes found in the essential oil, d-limonene (125–750 µM) and linalyl acetate (62.5–375 µM), were individually tested at concentrations comparable to those found in 0.005–0.03% BEO. The same features of stimulated autophagy elicited by BEO were reproduced by d-limonene, which rapidly increases LC3II and reduces p62 levels in a concentration-dependent manner. Linalyl acetate was ineffective in replicating BEO effects; however, it greatly enhanced LC3 lipidation triggered by d-limonene.
Introduction
Autophagy is an intracellular catabolic process by which cytosolic materials are enclosed by a double-membraned structure, forming an autophagosome that is delivered to, and fuses with lysosomes for proteolytic degradation [1]. This process acts as an adaptive metabolic response by recycling cellular components under conditions of nutrient limitations, and as an intracellular quality control system by promoting turnover of long-lived proteins, removal of damaged organelles, degradation of misfolded and aggregate-prone proteins, elimination of intracellular bacteria [1]. In recent years, converging data have been accumulated linking dysregulated autophagy to diverse pathologies, from cancer to muscle, liver, cardiac, infectious, immune, inflammatory, and neurodegenerative diseases [2]. Autophagy defects have been implicated in the accumulation of misfolded disease-causing proteins in amyotrophic lateral sclerosis, Alzheimer's, Parkinson's and Huntington's disease [3]. Moreover, perturbation of basal autophagy has been involved in neuronal dysfunctions of other central nervous system-related pathologies including glaucoma [4] and neuropathic pain [5]. Defective autophagy has been reported in human tumors with monoallelic deletions of the autophagy gene beclin1 and in cancer cells in which gain-of-function mutations or somatic amplifications in the oncogenes PI3K and Akt lead to constitutive activation of the autophagy-suppressing PI3K/mTOR pathway [2].
While the evidence accumulated so far indicates targeting autophagy as an attractive therapeutic approach for different pathologic conditions, this also raises the need for chemical modulators of autophagy to validate this treatment strategy in experimental and clinical settings.
Here we evaluated the ability of bergamot essential oil (BEO) to modulate autophagy in vitro. Bergamot essential oil (BEO) is a well-known plant extract, obtained by cold pressing of the epicarp and, partly, of the mesocarp of the fresh fruit of bergamot (Citrus bergamia Risso et Poiteau). BEO comprises a volatile fraction (93–96% of total) containing monoterpene and sesquiterpene hydrocarbons (such as limonene, γ-terpinene, α- and ß-pinene, ß-myrcene, sabinene, ß-bisabolene) and oxygenated derivatives (such as linalool, linalyl acetate, neral, geranial, neryl acetate, geranyl acetate), and a non volatile fraction (4–7% of total) characterized by coumarins and furocoumarins, such as bergapten (5-methoxypsoralen) [6], [7]. The most abundant compounds found in BEO are the monoterpene hydrocarbon d-limonene and the monoterpene ester linalyl acetate; among these, d-limonene accounts for about 40% of the whole oil [8]–[10].
The essential oil is widely used in perfumery, cosmetic, pharmaceutical and food industries. In the past, BEO has been used as antiseptic and to facilitate wound healing; consistent with this, experimental data have later shown that it possesses antifungal [11], [12] and antimicrobial [13] activities and it increases oxidative metabolism in human polimorphonuclear leukocytes [14]. However, recent years have seen an increasing use of this essential oil in aromatherapy for the relief of pain and symptoms associated with anxiety and depression [15], [16]. Well designed clinical trials are needed to conclusively ascertain efficacy and tolerability of BEO in these conditions along with basic research to elucidate its pharmacodynamic profile. The latter point has been addressed by a number of studies which, indeed, documented that BEO may affect synaptic transmission in rodents. In fact, BEO modulates release of specific amino acid neurotransmitters in discrete brain regions under both basal [17] and pathological conditions [18], produces a dose-related sequence of sedative and stimulatory behavioural effects in normal rats [19], exerts anxiolytic effects in the elevated plus-maze and hole-board tests [20] and neuroprotective effects against exicitotoxic [18], nociceptive [21] and allodynic stimuli [15], yet the underlying molecular mechanisms have not been conclusively established and need to be further investigated.
Here, to gain more insight into the biological activity of BEO we tested the ability of this essential oil to modulate autophagy in vitro. Experiments were performed in human neuroblastoma SH-SY5Y cells because we recently characterized the sensitivity of this cell line to BEO-induced cell death [22] and this would, indeed, facilitate unrevealing a connection between modulation of autophagy, if any, and cell death.
The results demonstrate that BEO rapidly modulates, in a concentration-dependent manner, biochemical and morphological markers of autophagy. Features of stimulated autophagy are observed before appearance of nuclear alterations on treatment with a cytotoxic concentration of BEO, yet they are shared by SH-SY5Y cells exposed to a concentration devoid of cytotoxicity. Importantly, here we identified d-limonene as involved in modulation of autophagic markers induced by BEO.
Materials and Methods
Reagents
BEO was kindly provided by CAPUA s.r.l. (Reggio Calabria, Italy; www.webcapua.com). BEO contained 39.76% limonene, 29.59% linalyl acetate, 8.09% γ-terpinene, 7.32% ß-pinene, 6.71% linalool, 1.28% α-pinene, 1.23% sabinene, 1.00% myrcene, 0.45% ß-bisabolene, 0.35% terpinolene, 0.34% neryl acetate, 0.33% α-thujene, 0.32% geranyl acetate, 0.31% ß-caryophyllene, 0.31% trans-α-bergamotene, 0.25% geranial, 0.17% phellandrene, 0.16% neral, 0.13% p-cimene, 0.12% decanal, 0.03% camphene, 0.02% o-cimene as determined by GC-FID analysis, and 2.331 g/kg bergapten (HPLC/UV). BEO was diluted 1∶1 in a 1∶9 water/ethanol solution and then further diluted in culture medium to obtain final concentrations of 0.005–0.03%; ethanol was added to the medium of parallel control cultures to obtain concentrations ranging from 0.0045 to 0.027% (vehicle-treated cells).
d-Limonene (CAS No 5989-27-5) and linalyl acetate (CAS No 115-95-7) were obtained from Sigma-Aldrich (Milan, Italy). Stock solution (10%) of limonene in dimethyl sulfoxide (DMSO; Sigma-Aldrich) and linalyl acetate in 90% ethanol were further diluted in culture medium to obtain final concentrations of 125–750 µM limonene and 62.5–375 µM linalyl acetate. DMSO or ethanol were added to the medium of parallel control cultures (vehicle-treated cultures) to obtain concentrations equal to those present in drug-treated cultures and these never exceeded 0.108% DMSO or 0.066% ethanol.
Cells, culture conditions and treatments
Adherent SH-SY5Y human neuroblastoma cells were obtained from ICLC-IST (Genoa, Italy); MCF7 human breast cancer cells were purchased from American Type Culture Collection (Manassas, VA, USA). Cell lines were routinely grown in RPMI medium (Invitrogen, Carlsband, CA) cointaining 10% foetal bovine serum (FBS: Invitrogen, Carlsband, CA). Cells were seeded onto 6-well plates; 24 h after plating, the growth medium was replaced with fresh normal medium (control cells) or medium supplemented with vehicle or different compounds. Cells were exposed to BEO (0.005–0.03%), d-limonene (Limo; 125–750 µM) or linalyl acetate (LinAc; 62.5–375 µM) for the indicated period of time. Stock solution of bafilomycin A1 (BafA1, 100 µM; Sigma-Aldrich) or rapamycin (2.74 mM; Sigma Aldrich) in DMSO were further diluted in culture medium. BafA1 or rapamycin were applied to SH-SY5Y cells 2 h and 48 h respectively before exposure to BEO or Limo for 1 h. Where indicated cells were subjected to starvation in serum-free media for 24 h and exposed to BEO (0.01% and 0.02%) for 1 h.
Flow cytometric analysis of cell viability
After treatment, cells were collected, co-labelled with 2 µg/ml propidium iodide (PI) and 0.1 µM fluorescein diacetate (FDA) and analyzed by a FACSCalibur flow cytometer (Becton Dickinson, CA, USA) to detect the proportion of viable (FDA+/PI-), apoptotic (FDA-/PI-) and necrotic cells (FDA-/PI+) by quadrant analysis as previously described [22], [23]. Ten thousands events/sample were acquired. Data acquisition and analysis were performed with CellQuest Software (Becton Dickinson).
Immunocytochemistry
SH-SY5Y cells were plated on coverslips, treated with BEO for the indicated time and fixed with formalin solution, containing 4% paraformaldehyde (Sigma-Aldrich). After blocking with 10% donkey serum for 30 min, coverslips were incubated with primary antibodies against microtubule-associated protein 1 light chain 3 (LC3; code PD036, MBL International Corporation, Nagoya, Japan; 1∶500 dilution) and/or lysosomal-associated membrane protein 1 (LAMP1; Developmental Studies Hybridoma Bank, Iowa City, IA, USA; 1∶400 dilution) overnight at 4°C followed by 1h incubation with Alexa Fluor secondary antibodies (Alexa Fluor 488 donkey anti-mouse and Alexa Fluor 594 donkey anti-rabbit, Molecular Probes, Life Technologies Italia, Monza, Italy; 1∶500 dilution). Coverslips were mounted with Vectashield solution containing 1.5 µg/ml 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, CA, USA) to visualize nuclei. Images were acquired under a 63X objective using a confocal microscope (Leica TC-SP2 Confocal System; Leica Microsystems Srl, Milan, Italy).
Protein extraction and western blot analysis
SH-SY5Y cells were lysed in ice-cold RIPA buffer (50 mM Tris-HCl, pH 8, 150 mM NaCl, 1 mM EDTA, 0.1% SDS, 1% IGEPAL and 0.5% sodium deoxycholate) containing a protease inhibitor cocktail (code P8349; Sigma-Aldrich). For detection of phosphoproteins, SH-SY5Y cells were lysed in ice-cold buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton, 1 nM okadaic acid, protease (code P8349, Sigma-Aldrich) and phosphatase (code 524625, Calbiochem) inhibitor cocktails.
Lysates were centrifuged for 15 min at 20.000 g at 4°C and supernatants were assayed for protein content by Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Milan, Italy). Equal amount of total proteins was resolved by 12% SDS-polyacrylamide gel electrophoresis and transferred onto PVDF membranes (Immobilon-P, Sigma-Aldrich). Membranes were blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween 20 for 1 h at room temperature. Primary antibodies were incubated overnight at 4°C, followed by a horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. Protein bands were visualized with the ECL Western Blotting Detection kit (ECL, Amersham Biosciences, GE Healthcare, Milan, Italy), and the chemiluminescence signal detected using X-ray films (Hyperfilm ECL, Amersham Biosciences). Exposure time to obtain the optimal density of the images varied from 5 s to 20 min. Autoradiographic films were scanned, digitalized at 300 dpi and band quantification was performed using ImageJ software (NIH, Bethesda, MD, USA).
The following primary antibodies and dilutions were used: anti-LC3 1∶1000 (code PD036; MBL), anti-p62/SQSTM1 1∶2000 (code PM045; MBL), anti-Beclin-1 1∶2000 (code PD017; MBL), anti-phospho p70S6K (Thr389) 1∶1000 (code 9234; Cell Signalling Technology), anti-p70S6K 1∶2000 (code 2708; Cell Signalling Technology), anti-phospho ULK (Ser757) 1∶1000 (code 6888; Cell Signalling Technology), anti-ULK 1∶1000 (code 8054, Cell Signalling Technology), anti-actin 1∶1000 (clone AC-40; Sigma-Aldrich), anti-β-tubulin 1∶30000 (clone B-5-1-2; Sigma-Aldrich), anti-GAPDH 1∶40000 (clone 6C5; Applied Biosystems, Carlsbad, CA, USA). HRP-conjugated goat anti-rabbit or anti-mouse IgG (Pierce Biotechnology, Rockford, IL, U.S.A) were used as secondary antibodies.
Knockdown of Beclin-1 expression by siRNA
Cells were transfected with small interfering RNA (siRNA) targeting Beclin-1 or scramble control (Dharmacon RNAi Technologies; Chicago, IL, USA) at a final concentration of 50 nM, 48 h before BEO exposure, by using Lipofectamine 2000 transfection reagent (Invitrogen, Life Technologies) according to the manufacturer's instruction.
Statistical analysis
Data are expressed as the mean ± s.e.m. of the indicated number of independent experiments and evaluated statistically for difference by ANOVA followed by Tukey-Kramer test for multiple comparisons. Where indicated, Student's t test was used to evaluate differences between two means. A value of P less than 0.05 was considered to be significant.
Results
Effects of BEO on basal and stimulated autophagy
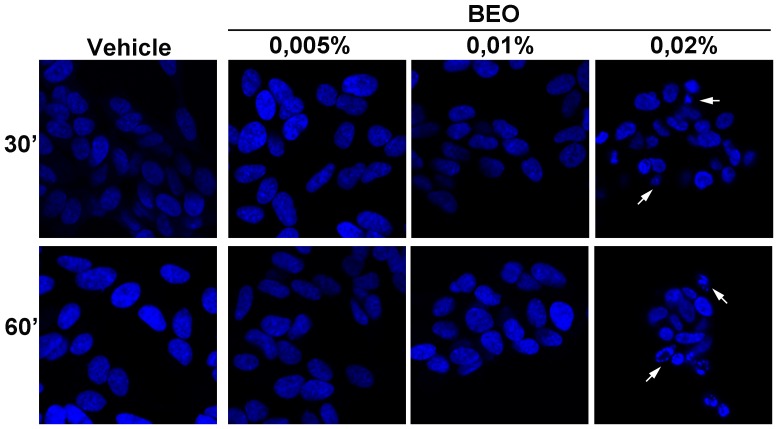
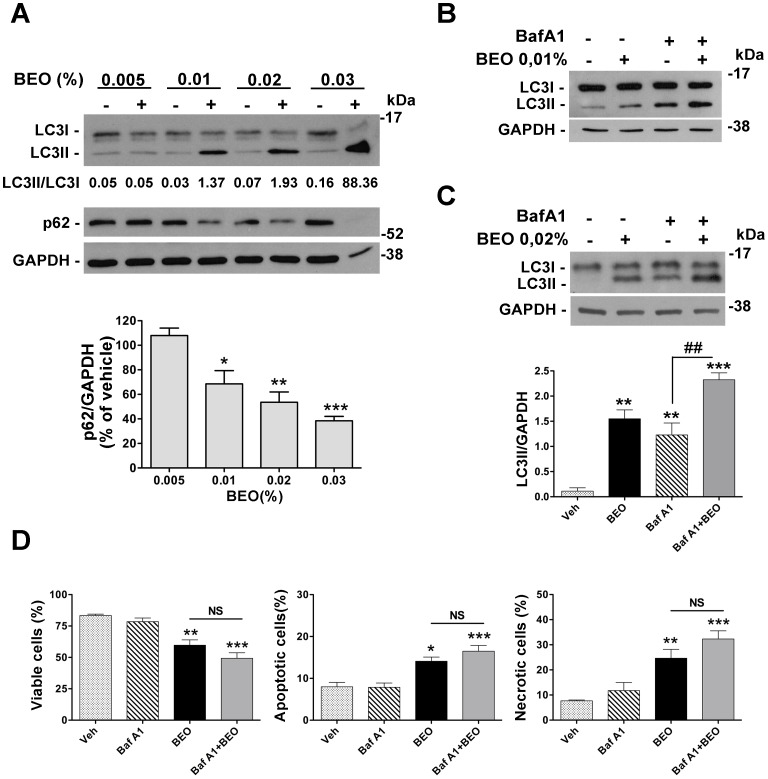
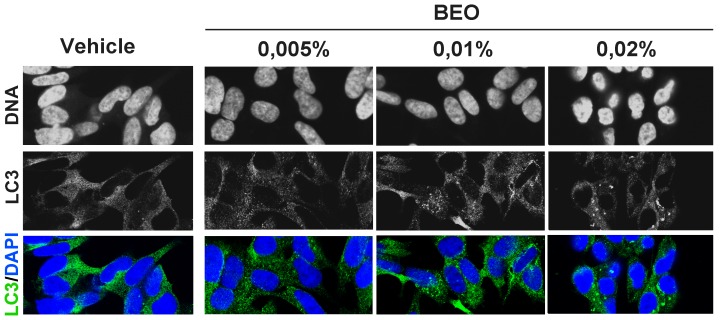
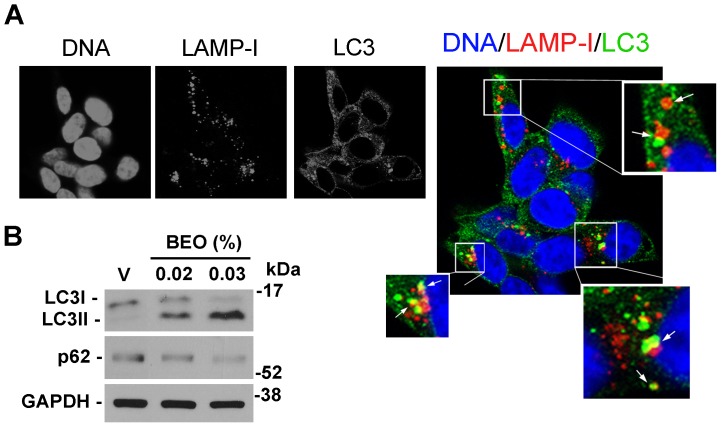
Our previous data show that a significant percentage of apoptotic and necrotic cell death occurs within 1 h exposure to 0.02% BEO and this dramatically increases in SH-SY5Y cells incubated for the same time period with 0.03% BEO; conversely, no cytotoxic effects are observed following incubation with lower concentrations (0.005–0.01%) of BEO for 1 h and up to 24 h [22]–[24]. Accordingly, immunofluorescence analysis here revealed DNA fragmentation, chromatin marginalization, and nuclear shrinkage and condensation in a significant proportion of cells exposed to 0.02% BEO for 1 h (Figure 1); signs of nuclear alterations, such as nuclear condensation, were also detected at an earlier time (30 min; Figure 1), though less pronounced. Nuclear morphological alterations were absent in cells treated for up to 1 h with lower concentrations (0.005–0.01%) of BEO (Figure 1). Based on these and previous observations, biochemical assessment of autophagy was initially performed following 1 h exposure to 0.005–0.03% BEO, i.e. a dilution range encompassing both non cytotoxic and cytotoxic concentrations. As shown in Figure 2A, treatment with BEO resulted in a concentration-dependent conversion of the non-lipidated form of LC3, LC3I, to the lipidated form, LC3II, that specifically associates with the membrane of expanding autophagosomes [25]. As compared to vehicle-treated cells, enhanced LC3I to LC3II conversion, measured as the LC3II/LC3I ratio, was detected in cells exposed to 0.01–0.03% BEO but not to a lower concentration (0.005%) (Figure 2A). Changes in LC3II levels were paralleled by a concentration-dependent reduction of the selective autophagy substrate p62 [26], [27] (Figure 2A), indicative of an increased autophagy. To analyze the autophagic flux, the effects of BEO were studied in the presence of the lysosomal inhibitor bafilomycin A1 (BafA1), which blocks autophagosome-lysosome fusion [28], thus preventing LC3II degradation [29]. In SH-SY5Y cells pretreated with BafA1, BEO further enhanced LC3II levels as compared with BafA1 alone (Figure 2 B and C) which indicates that BEO induces autophagosome formation. In fact, immunofluorescence analysis of intracellular distribution of endogenous LC3 protein showed that the latter was evenly distributed in vehicle-treated cells, with scattered LC3 punta (autophagosomes) reflecting ongoing (basal) autophagy, whereas a gradual increase in LC3 punctal pattern occurred in cells treated 30 min before with increasing concentrations of BEO (Figure 3). To determine whether autophagy modulation precedes the occurrence of nuclear alterations on treatment with 0.02% BEO, biochemical and morphological assessment of autophagy was carried out 15 min after BEO exposure. The results show that, at this time, BEO has stimulated the formation of autophagosomes, yet nuclei maintain a normal morphology; further, most of the LC3 puncta colocalize with the lysosomal protein LAMP-1, indicating that autophagosome-lysosome fusion occurred (Figure 4A). Coincident with this, enhanced LC3II/LC3I ratio and reduced levels of the autophagy substrate p62 were, indeed, revealed by western blotting analysis (Figure 4B). Altogether, these results indicate that 0.02% BEO rapidly stimulates autophagic flux in SH-SY5Y cells and this temporally precedes the profound alterations of nuclear morphology detected at later time points (Figure 1). To ascertain whether autophagy might have a causative role in cell death triggered by BEO, the latter was evaluated in cells pretreated with the autophagy inhibitor, BafA1. We found that BafA1 given alone did not affected neuroblastoma cell viability nor it protected from cell death induced by BEO; rather, BafA1 caused a trend toward an increase in the percentages of both necrotic and apoptotic cells induced by 0.02% BEO (Figure 2D).
Figure 1. Confocal microscopy of DAPI-stained SH-SY5Y cells treated for up to 1 h with BEO.
SH-SY5Y cells were exposed to the indicated concentration of BEO (0.005–0.02%) for 30 or 60 minutes. Apoptotic nuclear morphological changes, such as chromatin condensation and marginalization (arrows), are evident in cells exposed to BEO 0.02%, whereas no nuclear alterations are present in cells exposed to lower concentrations (0.005–0.01%). Images are representative of three independent experiments.
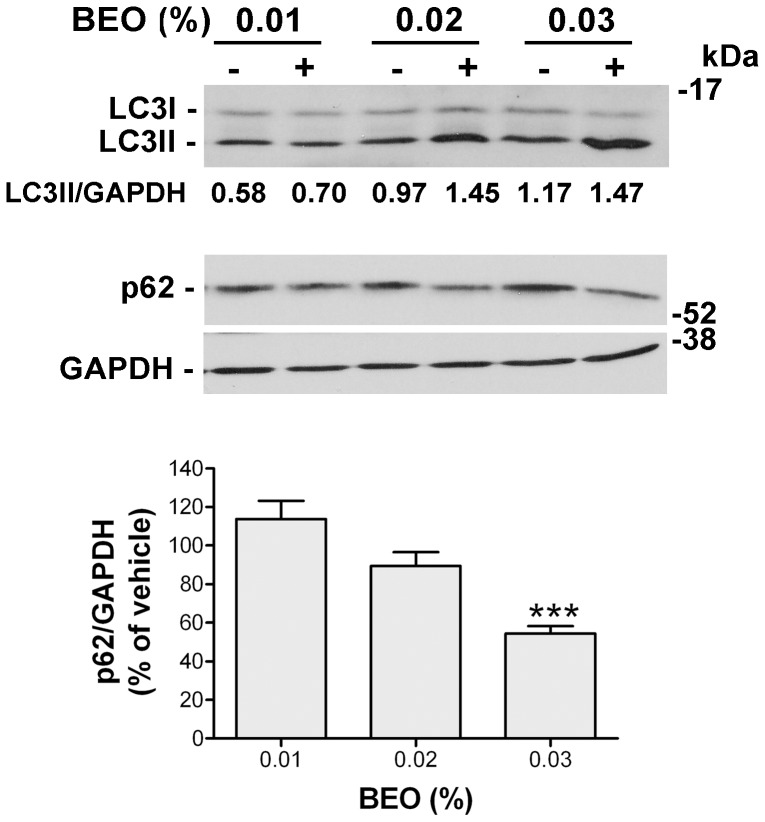
Figure 2. BEO activates autophagy in SH-SY5Y cells.
(A) Concentration-dependent induction of autophagy by BEO. Representative immunoblot showing LC3I to LC3II conversion and reduced p62 levels following exposure of SH-SY5Y to increasing concentrations of BEO. Cells were incubated with medium containing either vehicle (ethanol, 0.0045–0.027%) or BEO (0.005–0.03%) for 1 h. GAPDH was used as loading control. LC3II/LC3I optical density (OD) ratio for the reported blot is shown. Histogram shows the results of densitometric analysis of p62 levels normalized on GAPDH values and expressed as percentage of vehicle from three independent experiments (mean ± s.e.m.). *P<0.05,**P<0.01, P<0.001*** vs 0.005% BEO (ANOVA followed by Tukey-Kramer multiple comparisons test). (B, C) Effect of BafA1 pretreatment on LC3II accumulation in SH-SY5Y exposed to BEO. Cells were preincubated for 2 h with BafA1 (100 nM) and then treated with BEO 0.01% (B) or 0.02% (C) for 1 h. LC3II levels were detected by western blotting. Histogram in (C) shows the results of densitometric analysis of LC3II relative to internal control and reported as mean ± s.e.m of three independent experiments. **P<0.01, ***P<0.001 vs vehicle treated cells, ## P<0.01 vs BafA1 given alone (ANOVA followed by Tukey-Kramer multiple comparisons test). (D) Effect of autophagy inhibition by BafA1 on the percentage of viable, apoptotic and necrotic cells induced by BEO. Cells were incubated with BafA1 for 2 h and cell viability was assessed by cytofluorimetric analysis of FDA/PI stained cells 1 h after the addition of 0.02% BEO. Data are the mean ± s.e.m. of four independent experiments. *P<0.05, **P<0.01, ***P.001 vs vehicle treated cells (ANOVA followed by Tukey-Kramer multiple comparisons test).
Figure 3. Accumulation of endogenous LC3 positive structures in cells exposed to increasing concentrations of BEO.
SH-SY5Y cells were exposed to vehicle (ethanol 0.018%) or BEO (0.005–0.02%) for 30 minutes and immunostained with anti-LC3 antibody; nuclei were counterstained with DAPI. Confocal analysis of endogenous LC3 intracellular distribution shows a dose-dependent increase of LC3 puncta (autophagosomes) in cells treated with BEO 0.01% and 0.02% as compared to vehicle or BEO 0.005% treated cells. The described patter of LC3 distribution was observed in three independent experiments.
Figure 4. Fusion of autophagosomes and lysosomes in BEO-treated cells.
(A) SH-SY5Y were treated with BEO 0.02% for 15 min, fixed and double stained with LC3 and LAMP-1 antibodies. Nuclei were counterstained with DAPI. Representative images were taken with a confocal microscopy and higher magnification views of the boxed area from the merged image are shown. White arrows indicate areas of fusion (yellow signal) between LC3 positive autophagosomes (green) and LAMP1 positive endosomes and/or lysosomes (red) indicative of a functional autophagic maturation. (B) Immunoblot showing the early conversion of LC3I into LC3II and the reduction of p62 following 15 min incubation with BEO. GAPDH was used as loading control. Images are representative of three independent experiments. (V = vehicle, 0.018% ethanol).
The ability of BEO to modulate autophagic markers is not cell-line specific because enhanced LC3-II expression and reduced p62 levels were also observed following 1 h exposure of human breast cancer MCF7 cells to increasing concentrations of BEO (Figure 5).
Figure 5. BEO activates autophagy in MCF7 cells.
Representative immunoblot showing LC3I to LC3II conversion and reduced p62 levels following exposure of MCF7 cells to increasing concentrations of BEO. Cells were incubated with medium containing either vehicle (ethanol, 0.009–0.027%) or BEO (0.01–0.03%) for 1 h. GAPDH was used as loading control. LC3II/GAPDH optical density (OD) ratio for the reported blot is shown. Histogram shows the results of densitometric analysis of p62 levels normalized on GAPDH values and expressed as percentage of vehicle from three independent experiments (mean ± s.e.m.). ***P<0.001 vs vehicle (Student's t test).
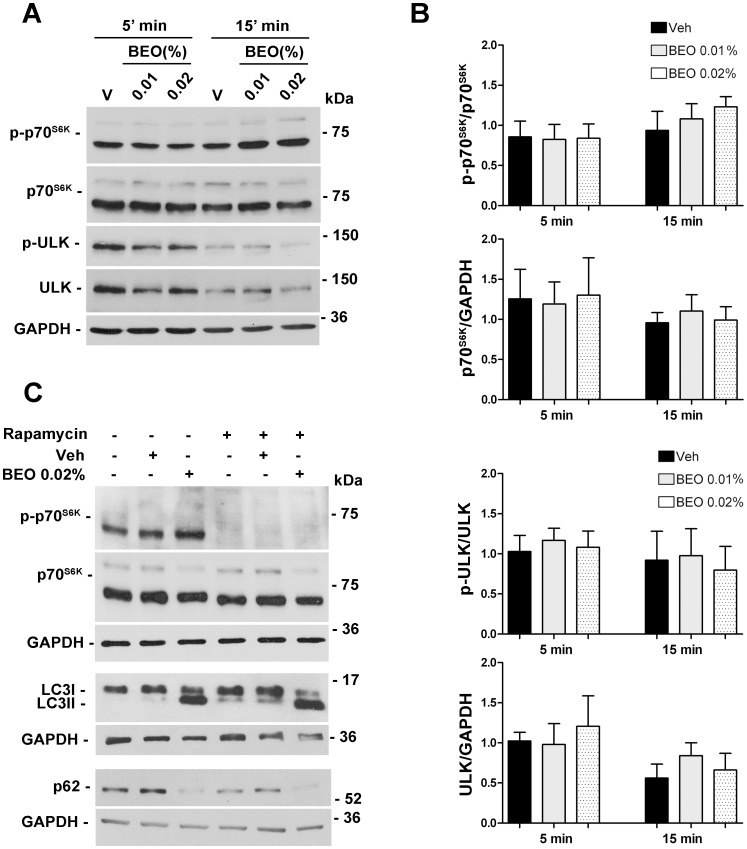
The above reported results demonstrated that BEO enhances basal autophagy. Further experiments were then performed to investigate the effect of BEO on stimulated autophagy. To this end, autophagy was induced by serum starvation [29]. SH-SY5Y cells exposed to serum deprivation for 24 h showed an increase in LC3I/LC3II conversion and a reduction of p62 levels when compared to not starved cells (Figure 6A). Treatment of serum deprived cells with BEO induced further accumulation of LC3II and reduction of p62 levels (Figure 6A). These results indicate that BEO enhances basal autophagy and autophagy induced by serum starvation. Furthermore, they suggest that signalling events in BEO-induced autophagy might be partially distinct from those induced by starvation known to stimulate autophagy through inhibition of mTOR kinase [2]. To establish whether or not BEO activates autophagy by repressessing mTOR, the phosphorylation level of p70S6K (Thr389) and ULK1 (Ser 757), two downstream targets of mTOR kinase [30], [31], was examined at 5 and 15 min after addition of 0.01 and 0.02% BEO. As shown in Figure 7A and B, no change in the level of phosphorylation of p70S6K (Thr389) and ULK1 (Ser 757) was observed indicating that induction of autophagy by BEO is mTOR independent. Accordingly, BEO further enhanced LC3 lipidation and reduction of p62 triggered by the mTOR inhibitor, rapamycin [32], [33]; as expected, the latter caused loss of p70S6K phosphorylation at Thr389 (Figure 7C). Under these same experimental conditions, no change in p70S6K phosphorylation accompanied modulation of autophagic markers caused by 1 hour exposure to BEO (Figure 7C).
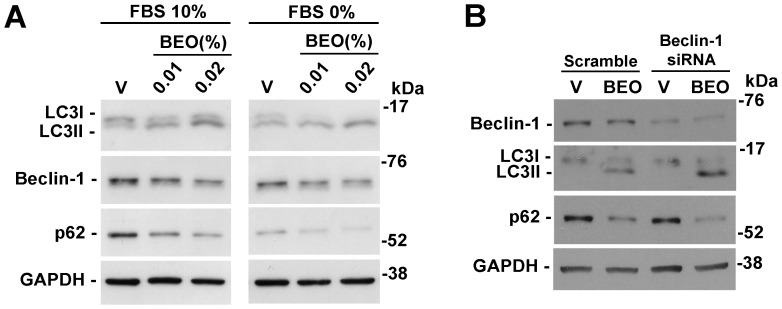
Figure 6. Stimulatory effects of BEO on autophagy still occur in serum-starved cells and overcome beclin-1 knockdown.
(A) BEO enhances serum starvation-induced autophagy in SH-SY5Y cells. Neuroblastoma cells serum-starved for 24 h (FBS 0%) were exposed to BEO (0.01–0.02%) or vehicle (V, 0.018% ethanol) for 1 h. Protein extracts were analyzed by western blotting for LC3, Beclin-1 and p62 levels. GAPDH was used as internal control. Serum starvation induced autophagy in SH-SY5Y cells as demonstrated by increased LC3II/LC3I ratio and reduced p62 levels (V FBS 0% vs V FBS 10%); further lipidation of LC3 and p62 reduction was evident in BEO-treated starved cells (FBS 0%) as compared to cells maintained in normal culture conditions (FBS 10%). Images from normal and starved cells are from the same immunoblot that has been cut to remove irrelevant lanes. (B) BEO induces autophagy through a beclin-1 independent mechanism. SH-SY5Y cells were transiently transfected with specific beclin-1 siRNA or scramble non targeting sequence 48 h before treatment with BEO (0.02%; 1 h). Effective knockdown of the protein was confirmed by western blotting. Silencing of beclin-1 did not prevent LC3I/LC3II conversion and p62 reduction induced by BEO exposure (0.02%, 1 h). Immunoblot is representative of three independent experiments (V = vehicle, 0.018% ethanol).
Figure 7. BEO-induced autophagy is mTOR independent.
(A, B) Treatment with BEO does not affect phosphorylation status of p70S6K and ULK. (A) Representative immunoblots showing the levels of phospho p70S6K (Thr 389; p-p70S6K) and phospho ULK (Ser 757; p-ULK) following treatment with BEO 0.01% and 0.02% for 5 and 15 min. Total protein extracts were analyzed by western blotting for phospho p70S6K and pULK and subsequently for total p70S6K and total ULK. GAPDH was used as internal control. (B) Histograms show the results of densitometric analysis of autoradiographic bands from three independent experiments (mean ± s.e.m.) (V = vehicle, 0.0018% ethanol). (C) BEO enhances autophagy induced by rapamycin. Neuroblastoma cells treated with rapamycin for 48h (3 µM) were exposed to BEO 0.02% or vehicle (Veh, 0.018% ethanol) for 1h. Protein extracts were analyzed by western blotting for phospho p70S6K, p70S6K, LC3 and p62. GAPDH was used as internal control. Treatment with rapamycin significantly reduced the phosphorylation status of p70S6K and induced autophagy in SH-SY5Y cells as demonstrated by increased LC3II and reduced p62 levels; no change of p70S6K phosphorylation accompanied autophagy induced by BEO; further enhancement of LC3 lipidation and p62 reduction was evident in rapamycin-treated cells exposed to BEO 0.02% for 1 h. Immunoblots are representative of three independent experiments.
BEO-induced autophagy is beclin-1-independent
Autophagy modulation by BEO is associated to a trend toward a reduction of beclin-1 (Figure 6A), a protein involved in vesicle nucleation step of autophagosome formation [34]. There are evidence that certain pro-apoptotic stimuli may induce a non-canonical type of autophagosomal formation which is independent from beclin-1 [35]. To study the relevance of the upstream protein beclin-1 in LC3II and p62 modulation induced by BEO, SH-SY5Y cells were transiently transfected with beclin-1 siRNA; as control, cells were transfected with scramble non-targeting sequence. Western blot analyses confirmed the effective reduction of beclin-1 expression and the tendency of BEO to decrease the expression of beclin-1 (Figure 6B); however, silencing of beclin-1 failed to prevent the increase in LC3II and the reduction of p62 levels induced by BEO (Figure 6B) thus suggesting that BEO-induced autophagy can bypass the beclin-1-dependent nucleation of autophagosomal precursor.
Role for d-limonene in autophagy induction by BEO
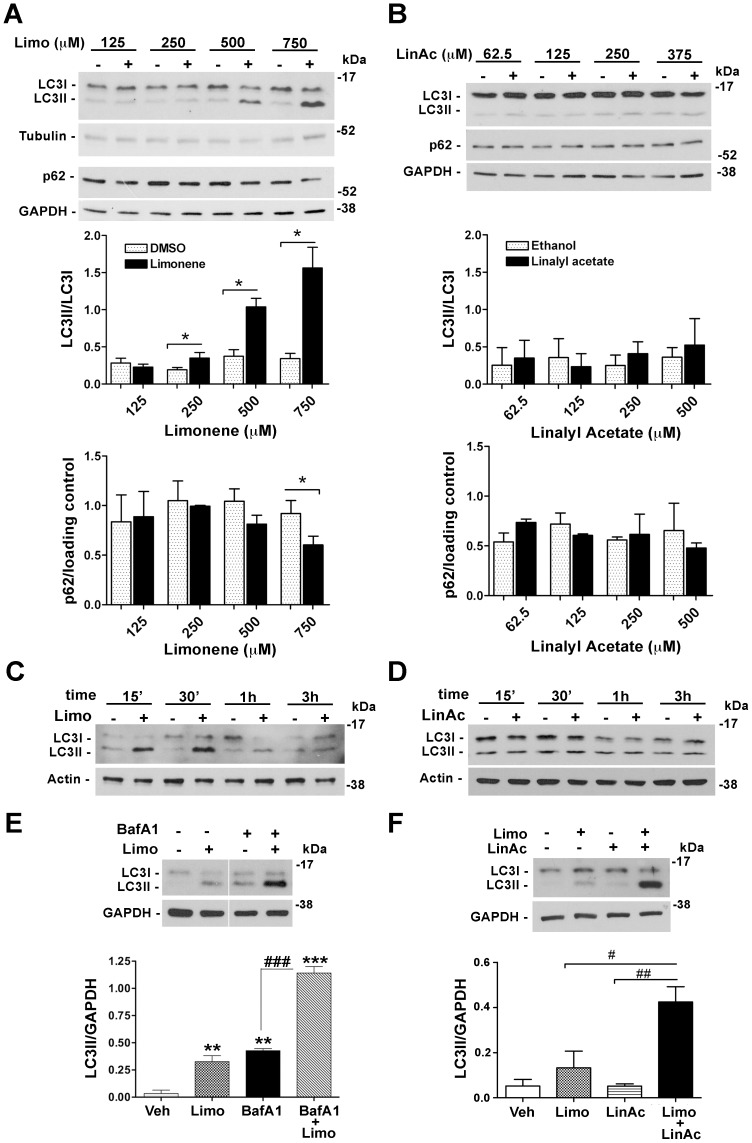
To identify the active constituent responsible for the modulation of autophagic markers induced by BEO, we focussed on the monoterpene hydrocarbon d-limonene and the monoterpene ester linalyl acetate, which altogether account for about 70% of the whole oil. SH-SY5Y cells were treated with d-limonene (125–750 µM) or linalyl acetate (62.5–375 µM) at concentrations comparable with those found in 0.005–0.03% BEO. As compared to vehicle-treated cells, a significant (P<0.05), concentration-dependent increase in LC3I to LC3II conversion occurred in neuroblastoma cells exposed for 30 min to 250–750 µM limonene, whereas a lower concentration was ineffective (Figure 8A). Enhanced LC3II expression was paralleled by a graded reduction of p62 levels, which resulted statistically significant (P<0.05) in cells treated with 750 µM d-limonene (Figure 8A). Changes in LC3II expression were time-related and indicative of a rapid autophagic flux induction by d-limonene (Figure 6C). Moreover, in cells pretreated with BafA1, d-limonene further enhanced LC3II levels as compared with BafA1 alone (P<0.001; Figure 8E), thus suggesting that it induces autophagosome formation.
Figure 8. d-limonene is implicated in autophagy induced by BEO.
Concentration-dependent changes of LC3 lipidation and p62 expression following treatment with (A) d-limonene, (B) linalyl acetate and the corresponding vehicles for 30 min. Tubulin or GAPDH were used as loading control. Histograms show the results of densitometric analysis from three independent experiments (mean ± s.e.m.) *P<0.05 vs vehicle (Student's t test). (C, D) Representative immunoblots showing the time-dependent LC3I to LC3II conversion following exposure of SH-SY5Y cells to (C) d-limonene (500 µM) but not (D) linalyl acetate (250 µM). (E) Effects of BafA1 pretreatment on LC3 levels in SH-SY5Y exposed to d-limonene. Cells preincubated for 2 h with BafA1 (100 nM) were treated with d-limonene (750 µM) for 1 h. LC3II levels were detected by western blotting. Image from the autoradiographic film has been cut to remove irrelevant lanes. Histograms show the result of densitometric analysis from three independent experiments (mean ± s.e.m). **P<0.01, ***P<0.001 vs vehicle; ### P<0.001 (ANOVA followed by Tukey-Kramer multiple comparisons test). (F) Linalyl acetate enhances LC3 lipidation induced by d-limonene. Cells were incubated with vehicle or exposed to d-limonene (Limo, 500 µM) and linalyl acetate (LinAc, 250 µM), given individually or in combination. After 1 h incubation protein cell extracts were analyzed by western blot for LC3 levels. GAPDH was used as internal control. Histograms show the result of densitometric analysis from three independent experiments (mean ± s.e.m). # P<0.05, ## P<0.01 (ANOVA followed by Tukey-Kramer multiple comparisons test).
Exposure of SH-SY5Y cells for 30 min to linalyl acetate, given at concentrations (62.5–375 µM) found in 0.005–0.03% BEO, did not elicit LC3II accumulation (Figure 8B). Further, no changes in LC3II could be noticed on treatment with linalyl acetate (250 µM) for 15 min to 3 hours (Figure 8D). Collectively, these findings indicate that d-limonene, but not linalyl acetate, plays a key role in modulation of autophagic markers triggered by BEO; interestingly, however, linalyl acetate significantly (P<0.05) enhanced LC3 lipidation triggered by d-limonene (Figure 8F).
Discussion
The experimental data we have provided here demonstrate that BEO modulates autophagic markers in human SH-SY5Y neuroblastoma cells. The rise in LC3II levels, the appearance of dot-like formations of endogenous LC3 protein and its colocalization with LAMP-1 protein, along with the reduction of the selective autophagy substrate p62 following BEO exposure are indicative of autophagy induction. In addition, BEO further increased LC3II levels in BafA1-treated cells confirming that it enhanced the formation of autophagosomes rather than inhibiting their degradation.
Stimulation of autophagy occurs rapidly following incubation with BEO. In fact, LC3I/LC3II conversion, autophagosome-lysosome fusion and reduced p62 levels were detected as early as 15 min after exposure to 0.02% BEO. At this time the cells still have an apparently normal nuclear morphology and do not manifest the dramatic alterations occurring at later times. These findings demonstrate that autophagy induction is an early response to 0.02% BEO but they are not sufficient to ascribe a causative role for autophagy in cell death induced by the essential oil. This concentration of BEO affects multiple death pathways in SH-SY5Y cells, causing cytoskeletal alteration, mitochondrial dysfunction, caspase-3 activation, DNA fragmentation, plasma membrane damage and cleavage of pro-survival proteins [22]; therefore, stimulated autophagy might either fail in coping with this stress or aggravate it by non-specific degradation of large amounts of cytoplasmic contents. On the other hand, the trend towards an increase in the percentages of necrotic and apoptotic cells reported following autophagy inhibition by BafA1 is not sufficient to assign to the observed autophagy a protective role against cellular stress triggered by BEO.
However, enhanced LC3 lipidation, reduced p62 levels and the appearance of LC3-positive round structure (i.e. autophagosomes) were also observed following incubation with 0.01% BEO, a concentration which does not induce cytotoxicity. Indeed, here we reported the absence of nuclear morphology alterations following exposure to BEO 0.01% and this is consistent with our previous observations that 0.01% BEO does not affect cell viability following 1 h or longer incubation [22]–[24]. These findings indicates that modulation of autophagy is a concentration-related effect induced by both non cytotoxic and cytotoxic concentrations of the essential oil and would also exclude BEO-stimulated autophagy as having merely a role as a death effector mechanism or a death accomplice.
The autophagic process triggered by BEO does not seem to involve the canonical autophagy pathway dependent on beclin-1; silencing of beclin-1, indeed, failed to prevent the increase in LC3II and the reduction of p62 levels induced by BEO. Moreover, the upstream signalling events in BEO-induced autophagy do not converge on mTOR kinase[2]; in fact, BEO did not affect mTOR kinase activity and further enhanced autophagy triggered by stimuli that inhibit mTOR (i.e. serum starvation and rapamycin) [29].
These findings could be of relevance as they suggest that active components in BEO could be exploited to overcome downregulation of autophagy in autophagy defective cells [2].
Further experiments are needed to elucidate the mechanisms through which BEO modulates autophagy and to assign a potential role for this process in the pharmacological effects of BEO reported in animal models of diseases, including anxiety [20], cerebral ischemia [18] or pain [15], [21].
Here, considering the inherent difficulties in dissecting the molecular mechanisms underlying biological effects of complex mixtures, we focused on the identification of the active constituent/s responsible for BEO-induced effects; this led us to identify limonene as involved in modulation of autophagic markers. In fact, d-limonene, but not linalyl acetate, rapidly enhanced LC3II and reduced p62 levels in a concentration-dependent manner. Autophagic flux assay by using bafilomycin A1 showed that limonene enhanced autophagosome formation. Time-course experiments to monitor LC3 turnover confirmed that limonene enhanced autophagic flux. In fact, limonene induced a dramatic but transient increase in LC3II levels a finding consistent with the notion that LC3 itself is degraded by autophagy and its levels are reduced after a period of sustained autophagy. Under our experimental conditions, modulation of autophagic markers by 250–750 µM d-limonene is not associated to nor it precedes cell death; in fact, we recently showed that incubation of SH-SY5Y cells with these concentrations of d-limonene for 1 h and 24 h does not affect SH-SY5Y cell viability [23]. The latter findings are consistent with results obtained by other research groups showing that relatively high concentrations of limonene are required to affect proliferation of diverse cancer cell lines [36]–[41].
The mechanisms underlying in vitro antiproliferative effects of limonene are not clearly defined and several mechanisms have been called into question including inhibition of protein isoprenylation and possibly alteration of Ras signaling [42], Ras-independent-mechanisms [40], increase in nitric oxide levels [43], ERK pathway activation [44], inactivation of Akt [39]. Here we provide further insight into the biological activities of this naturally occurring monoterpene by showing that it rapidly modulates autophagic markers in vitro and this occurs at concentrations that can be found in breast tissue of women with early-stage breast cancer taking 2 grams of limonene daily for 2–6 weeks (41.3±49.9 µg/g tissue; 332.3±336.1 µM) [45]. Our present observations provide the basis for further investigations to characterize the mechanisms underlying modulation of autophagy by d-limonene. These mechanisms might be involved in its biological effects, including antinflammatory and chemopreventive activities, reported in preclinical animal models [46]–[48]. In particular, autophagy might play a role in chemoprevention by limonene, since this pathway acts as a tumor suppressor in the early phases of tumorigenesis [49].
The finding that linalyl acetate significantly enhanced LC3 lipidation triggered by d-limonene, highlights the inherent difficulties in dissecting the molecular mechanisms underlying the effects of herbal drugs. Each constituent can contribute indeed to the overall effect of a phytocomplex and the latter might be not completely mirrored by any of each single ingredient given alone [23].
Collectively, here we have provided evidence that BEO modulates autophagy in vitro and that d-limonene is implicated in this effect; these observations may stimulate further studies to identify the mechanisms involved and their relevance for the biological activities of these natural products. Such efforts might also be expected to lead to the identification of new targets/lead compounds for drug development.
Acknowledgments
The LAMP-1 monoclonal antibody developed by J.T. August and J.E.K. Hildreth was obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee R, Beal MF, Thomas B (2010) Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci 33:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, et al. (2011) Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis 2:e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Berliocchi L, Russo R, Maiaru M, Levato A, Bagetta G, et al. (2011) Autophagy impairment in a mouse model of neuropathic pain. Mol Pain 7:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dugo P, Mondello L, Dugo L, Stancanelli R, Dugo G (2000) LC-MS for the identification of oxygen heterocyclic compounds in citrus essential oils. J Pharm Biomed Anal 24:147–154. [DOI] [PubMed] [Google Scholar]
- 7. Mondello L, Stagno d'Alcontres, I., Del Duce, R. & Crispo. F (1993) On the genuineness of citrus essential oils. Part XL. The composition of the coumarins and psoralens of calabrian bergamot essential oil (Citrus bergamia Risso).. Flavour Fragr J 8:17–24. [Google Scholar]
- 8. Mondello L, Dugo P, Bartle KD, Dugo, G. & Cotroneo. A (1995) Automated HPLC-HRGC: a powerful method for essential oils analysis. Part V. Identification of terpene hydrocarbons of bergamot, lemon, mandarin, sweet orange, bitter orange, grapefruit, clementine and mexican lime oils by coupled HPLC-HRGC-MS(ITD). Flavour FragrJ 10:33–42. [Google Scholar]
- 9. Verzera A, Lamonica G, Mondello L, Trozzi, A. & Dugo. G (1996) The composition of bergamot oil. Perfumer & Flavorist 21:19–34. [Google Scholar]
- 10. Verzera A, Trozzi A, Gazea F, Cicciarello G, Cotroneo A (2003) Effects of rootstock on the composition of bergamot (Citrus bergamia Risso et Poiteau) essential oil. J Agric Food Chem 51:206–210. [DOI] [PubMed] [Google Scholar]
- 11. Romano L, Battaglia F, Masucci L, Sanguinetti M, Posteraro B, et al. (2005) In vitro activity of bergamot natural essence and furocoumarin-free and distilled extracts, and their associations with boric acid, against clinical yeast isolates. J Antimicrob Chemother 55:110–114. [DOI] [PubMed] [Google Scholar]
- 12. Sanguinetti M, Posteraro B, Romano L, Battaglia F, Lopizzo T, et al. (2007) In vitro activity of Citrus bergamia (bergamot) oil against clinical isolates of dermatophytes. J Antimicrob Chemother 59:305–308. [DOI] [PubMed] [Google Scholar]
- 13. Laird K, Armitage D, Phillips C (2012) Reduction of surface contamination and biofilms of Enterococcus sp. and Staphylococcus aureus using a citrus-based vapour. J Hosp Infect 80:61–66. [DOI] [PubMed] [Google Scholar]
- 14.Cosentino M, Luini A, Bombelli R, Corasaniti MT, Bagetta G, et al. (2014) The Essential Oil of Bergamot Stimulates Reactive Oxygen Species Production in Human Polymorphonuclear Leukocytes. Phytother Res. [DOI] [PubMed]
- 15. Bagetta G, Morrone LA, Rombola L, Amantea D, Russo R, et al. (2010) Neuropharmacology of the essential oil of bergamot. Fitoterapia 81:453–461. [DOI] [PubMed] [Google Scholar]
- 16. Ndao DH, Ladas EJ, Cheng B, Sands SA, Snyder KT, et al. (2012) Inhalation aromatherapy in children and adolescents undergoing stem cell infusion: results of a placebo-controlled double-blind trial. Psychooncology 21:247–254. [DOI] [PubMed] [Google Scholar]
- 17. Morrone LA, Rombola L, Pelle C, Corasaniti MT, Zappettini S, et al. (2007) The essential oil of bergamot enhances the levels of amino acid neurotransmitters in the hippocampus of rat: implication of monoterpene hydrocarbons. Pharmacol Res 55:255–262. [DOI] [PubMed] [Google Scholar]
- 18. Amantea D, Fratto V, Maida S, Rotiroti D, Ragusa S, et al. (2009) Prevention of Glutamate Accumulation and Upregulation of Phospho-Akt may Account for Neuroprotection Afforded by Bergamot Essential Oil against Brain Injury Induced by Focal Cerebral Ischemia in Rat. Int Rev Neurobiol 85:389–405. [DOI] [PubMed] [Google Scholar]
- 19. Rombola L, Corasaniti MT, Rotiroti D, Tassorelli C, Sakurada S, et al. (2009) Effects of systemic administration of the essential oil of bergamot (BEO) on gross behaviour and EEG power spectra recorded from the rat hippocampus and cerebral cortex. Funct Neurol 24:107–112. [PubMed] [Google Scholar]
- 20. Saiyudthong S, Marsden CA (2011) Acute effects of bergamot oil on anxiety-related behaviour and corticosterone level in rats. Phytother Res 25:858–862. [DOI] [PubMed] [Google Scholar]
- 21. Sakurada T, Kuwahata H, Katsuyama S, Komatsu T, Morrone LA, et al. (2009) Intraplantar injection of bergamot essential oil into the mouse hindpaw: effects on capsaicin-induced nociceptive behaviors. Int Rev Neurobiol 85:237–248. [DOI] [PubMed] [Google Scholar]
- 22. Berliocchi L, Ciociaro A, Russo R, Cassiano MG, Blandini F, et al. (2011) Toxic profile of bergamot essential oil on survival and proliferation of SH-SY5Y neuroblastoma cells. Food Chem Toxicol 49:2780–2792. [DOI] [PubMed] [Google Scholar]
- 23. Russo R, Ciociaro A, Berliocchi L, Cassiano MG, Rombola L, et al. (2013) Implication of limonene and linalyl acetate in cytotoxicity induced by bergamot essential oil in human neuroblastoma cells. Fitoterapia 89:48–57. [DOI] [PubMed] [Google Scholar]
- 24. Corasaniti MT, Maiuolo J, Maida S, Fratto V, Navarra M, et al. (2007) Cell signaling pathways in the mechanisms of neuroprotection afforded by bergamot essential oil against NMDA-induced cell death in vitro. Br J Pharmacol 151:518–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, et al. (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bjorkoy G, Lamark T, Brech A, Outzen H, Perander M, et al. (2005) p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol 171:603–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Komatsu M, Waguri S, Koike M, Sou YS, Ueno T, et al. (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, et al. (1998) Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23:33–42. [DOI] [PubMed] [Google Scholar]
- 29. Mizushima N, Yoshimori T, Levine B (2010) Methods in mammalian autophagy research. Cell 140:313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wong PM, Puente C, Ganley IG, Jiang X (2013) The ULK1 complex: sensing nutrient signals for autophagy activation. Autophagy 9:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, et al. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175. [DOI] [PubMed] [Google Scholar]
- 33. Johnsen JI, Segerstrom L, Orrego A, Elfman L, Henriksson M, et al. (2008) Inhibitors of mammalian target of rapamycin downregulate MYCN protein expression and inhibit neuroblastoma growth in vitro and in vivo. Oncogene 27:2910–2922. [DOI] [PubMed] [Google Scholar]
- 34. He C, Levine B (2010) The Beclin 1 interactome. Curr Opin Cell Biol 22:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Codogno P, Mehrpour M, Proikas-Cezanne T (2012) Canonical and non-canonical autophagy: variations on a common theme of self-eating? Nat Rev Mol Cell Biol 13:7–12. [DOI] [PubMed] [Google Scholar]
- 36. Bicas JL, Neri-Numa IA, Ruiz AL, De Carvalho JE, Pastore GM (2011) Evaluation of the antioxidant and antiproliferative potential of bioflavors. Food Chem Toxicol 49:1610–1615. [DOI] [PubMed] [Google Scholar]
- 37. Chidambara Murthy KN, Jayaprakasha GK, Patil BS (2012) D-limonene rich volatile oil from blood oranges inhibits angiogenesis, metastasis and cell death in human colon cancer cells. Life Sci 91:429–439. [DOI] [PubMed] [Google Scholar]
- 38. Itani WS, El-Banna SH, Hassan SB, Larsson RL, Bazarbachi A, et al. (2008) Anti colon cancer components from Lebanese sage (Salvia libanotica) essential oil: Mechanistic basis. Cancer Biol Ther 7:1765–1773. [DOI] [PubMed] [Google Scholar]
- 39. Jia SS, Xi GP, Zhang M, Chen YB, Lei B, et al. (2013) Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol Rep 29:349–354. [DOI] [PubMed] [Google Scholar]
- 40. Karlson J, Borg-Karlson AK, Unelius R, Shoshan MC, Wilking N, et al. (1996) Inhibition of tumor cell growth by monoterpenes in vitro: evidence of a Ras-independent mechanism of action. Anticancer Drugs 7:422–429. [DOI] [PubMed] [Google Scholar]
- 41. Rabi T, Bishayee A (2009) d -Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J Carcinog 8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Crowell PL, Chang RR, Ren ZB, Elson CE, Gould MN (1991) Selective inhibition of isoprenylation of 21-26-kDa proteins by the anticarcinogen d-limonene and its metabolites. J Biol Chem 266:17679–17685. [PubMed] [Google Scholar]
- 43. Manuele MG, Arcos ML, Davicino R, Ferraro G, Cremaschi G, et al. (2009) Mechanism of the antiproliferative action of limonene on a lymphoma cell line: participation of nitric oxide. antiproliferative action of limonene on a lymphoma cell line. Phytother Res 23:1011–1017. [DOI] [PubMed] [Google Scholar]
- 44. Manuele MG, Barreiro Arcos ML, Davicino R, Ferraro G, Cremaschi G, et al. (2010) Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: relationship with oxidative stress. Cancer Invest 28:135–145. [DOI] [PubMed] [Google Scholar]
- 45. Miller JA, Lang JE, Ley M, Nagle R, Hsu CH, et al. (2013) Human breast tissue disposition and bioactivity of limonene in women with early-stage breast cancer. Cancer Prev Res (Phila) 6:577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chaudhary SC, Siddiqui MS, Athar M, Alam MS (2012) D-Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum Exp Toxicol 31:798–811. [DOI] [PubMed] [Google Scholar]
- 47. Elson CE, Maltzman TH, Boston JL, Tanner MA, Gould MN (1988) Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis 9:331–332. [DOI] [PubMed] [Google Scholar]
- 48. Maltzman TH, Hurt LM, Elson CE, Tanner MA, Gould MN (1989) The prevention of nitrosomethylurea-induced mammary tumors by d-limonene and orange oil. Carcinogenesis 10:781–783. [DOI] [PubMed] [Google Scholar]
- 49. White E (2012) Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer 12:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.