Abstract
Background
Bicuspid aortic valve (BAV), the most common form of congenital heart disease, is a leading cause of aortic stenosis (AS) and aortic insufficiency (AI). AS is typically due to calcific valve disease. Recently, microRNAs (miRNAs) have been shown to modulate gene expression. This study examined miRNAs that were altered in aortic valve leaflets of patients with AS compared to patients with AI. Additionally, in vitro experiments were performed to examine if these miRNAs modulate calcification-related genes.
Materials and Methods
Aortic valve samples (fused or unfused leaflets) were collected from 9 patients (mean age 44.9±13.8 years) undergoing aortic valve replacement. PIQOR™ miRXplore Microarrays containing 1421 miRNAs were used and hybridized to fused leaflet samples labeled with Cy5; unfused samples were used as control and labeled with Cy3. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed to validate the miRNA array results. Cultured human aortic valve cells (AVICs) were treated with miRNA mimics and qRT-PCR was performed to determine changes in mRNAs.
Results
Seven miRNAs were statistically different between the AS and AI patients by microarray. MiR-26a and miR-195 levels were reduced by 65% and 59% respectively with p<0.05 in the stenotic samples by qRT-PCR. MiR-30b was reduced by 62% (p<0.06) in the stenotic samples by qRT-PCR. Human AVICs treated with miR-26a or miR-30b mimics had decreased mRNA levels of calcification-related genes. MiR-26a repressed BMP2 by 36%, ALKALINE PHOSPHATASE (ALPL) by 38%, and SMAD1 by 26%. MiR-30b reduced expression of SMAD1 by 18% and SMAD3 by 12%. Whereas miR-195 treated AVICs had increased mRNA levels of calcification-related genes such as BMP2 by 68% and RUNX2 by 11%.
Conclusions
MiR-26a, miR-30b, and miR-195 were decreased in the aortic valves of patients requiring valve replacement due to AS compared to those being replaced due to AI. These miRNAs appear to modulate calcification related genes in vitro.
Keywords: Aortic valve calcification, miRNAs, Gene regulation, Bicuspid Aortic Valve
Introduction
Aortic valve calcification/stenosis has a significant clinical impact since it is the third leading cause of adult heart disease (1) and the most common form of acquired valvular disease in developed countries (2). The risk factor most closely linked to calcific aortic stenosis is bicuspid aortic valve (BAV), present in 1–2% of the population (2). Examination of human calcified aortic valve tissue and in vitro experiments using Aortic Valve Interstitial Cells (AVICs) have demonstrated that there are several gene pathways involved in calcification including BMP2(3, 4), NOTCH1(5–8), and the SMADs(3, 4, 9). miRNA are a class of highly conserved small noncoding RNAs that have key roles in modulation of gene expression(10). miRNAs are primarily involved in repressing mRNA transcripts by binding specific sequences in 3’ untranslated region (3’UTR) of the mRNA. There is a rapidly growing body of literature examining the role of miRNAs in cardiovascular development and disease (11–16). While miRNA profiling has been performed in hepatocellular(17) and neck carcinoma(18, 19), sepsis(20), and heart failure(21), we are not aware of any reports profiling miRNAs associated with aortic valve disease and their possible role in calcification. Since BAV predisposes to both stenosis and insufficiency (regurgitation) in our cohort, we sought to evaluate the differentially expressed miRNAs in BAV with AS as compared to AI. Additionally, we examined if these miRNAs could alter expression of calcification related genes.
Materials and Methods
Aortic Valve Collection
The study protocol was approved by the institutional ethics committee and written informed consent was obtained from all patients. Preoperatively, the functional state of the aortic valve was determined by echocardiography and/or angiocardiography according to guidelines of the American Heart Association and the American College of Cardiology(22). All of the patients examined for altered miRNA expression had type 1 bicuspid aortic valve leaflets(23), with fusion of the right and left coronary leaflets, that was confirmed at the time of aortic valve replacement. The fused and unfused leaflets were inspected at the time of surgery for morphology. The leaflets were collected separately frozen in liquid nitrogen. Patients’ demographics are shown in table 1.
Table 1.
Patient demographics and clinical characteristics.
| Bicuspid aortic valve (BAV) | N = 9 |
|---|---|
| Demographic | |
| Age (years) | 44.9 ± 13.8 |
| Gender (male/female) | 9 m |
| Body mass index | 26.4±3.0 |
| Reason of aortic valve replacement | |
| Aortic valve insufficiency | 5 |
| Aortic valve stenosis | 4 |
| Systemic disease | |
| Hypertension | 0 |
| Chronic obstructive pulmonary disease | 0 |
| Aneurysm of ascending aorta (Ø mm) | 41.3±7.3 |
RNA Isolation and miRNA Array
RNA was isolated from the leaflets using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The RNA from the fused leaflet was labeled with Cy5 while the unfused leaflet RNA, which served as a control, was labeled with Cy3 using the PIQOR MiRXplore protocol (Miltenyi Biotech, Bergisch Gladbach, Germany). The samples were hybridized to PIQOR miRXplore Microarrays (Miltenyi Biotech) according to the manufacturer’s recommendations.
Statistical Analysis of miRNA Array Data
Normalization
Imagene files were processed using GeneSpring 7.3.1 from Agilent. The files were normalized by dividing the Cy5 channel (fused leaflet) with its matched Cy3 channel (unfused leaflet) creating a ratio. The next step in the normalization was to scale the ratios from each matched set to the 50th intensity from the matched set. This calculation was done for each matched set, this scales each matched set to the most representative intensity. In total there are 9 matched sets so this procedure was done 9 times. This step was performed to ensure that systematic error was removed from the data.
Assessment of group homogeneity
Principal Component Analysis and hierarchical clustering (Spearman correlation, average linkage) were performed using the normalized ratio data. As a common result, three samples (microarrays 6170041, 3320029, 6620035) were reported as outliers from the respective sample group. (Table 2). These samples were excluded from further analysis.
Table 2.
Sample quality control. Principle Component and Hierarchical Clustering analyses excluded three microarrays from the statistical analysis since these arrays were outliers.
| Microarray | Reason of Aortic valve replacement | Sample QC |
|---|---|---|
| 6170041 | Aortic valve insufficiency | Bad |
| 6620037 | Aortic valve insufficiency | Good |
| 6620029 | Aortic valve insufficiency | Bad |
| 6620030 | Aortic valve insufficiency | Good |
| 6620031 | Aortic valve insufficiency | Good |
| 6620032 | Aortic valve stenosis | Good |
| 6620033 | Aortic valve stenosis | Good |
| 6620034 | Aortic valve stenosis | Good |
| 6620035 | Aortic valve stenosis | Bad |
Probe Quality Control
The next step was to filter the data based on the signal error within the data, this removed miRNAs that have a large amount of signal noise from statistical analysis. This filter was applied to all the data and miRNA only passed if they achieved the specified signal/noise ratio in all samples. This ensured that the data used in the proceeding statistical analysis is sound. The total size of the miRNA array was 1421, which is a combination of human, murine, viral and other species miRNAs. 66 miRNA remained after applying this filter.
Statistical Analysis
The remaining 6 samples equated to 3 replicates in the stenosis and insufficient groups. The data was analyzed for significantly differentially expressed miRNA between the groups. The miRNA used in this analysis were the 66 miRNA remaining after the signal/noise ratio filter. The test applied to this data was a Student t-test with a p-value cutoff of 0.05. No multiple testing was applied.
QRT-PCR
miRNA qRT-PCR was performed on the fused leaflet valve samples used in the miRNA array experiment (AI group n=5 and AS group n=4) to validate the miRNA microarray results. The TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) with miRNA specific primers was used according to manufacture’s protocol. Δ ΔΔCT values were calculated; U6 was used as an endogenous control.
Transfection of human Aortic Valve Interstitial Cells (AVICs)
Human aortic valve leaflets were harvested from recipient hearts at the time of cardiac transplant with IRB approval. Human AVICs were cultured according to standard protocols(24, 25). Fully confluent AVICs were transfected with either 100pM miRNA mimic (Dharmacon, Lafayette, CO) or Block-it using Lipofectamine 2000 (Invitrogen) according to manufacture’s protocols(8). As a transfection control and to determine transfection efficiency, the cells were transfected with non-functioning siRNA (Block-It, Invitrogen)(26). After 96 hours, RNA was isolated using Trizol. cDNA was prepared using Superscript III (Invitrogen). qRT-PCR was performed using TaqMan primers (Applied Biosystems) for a panel of calcification-related genes. ΔΔCT values were calculated; GADPH served as the control.
Statistical Analysis
The statistical significance of differences between groups was determined with the unpaired t test. P≤0.05 was considered significant.
Results
miR-26a, miR-30b, and miR-195 were reduced in AS samples
miRNA Microarray Results
To identify possible miRNAs whose expression levels were altered in bicuspid aortic valve (BAV), miRNA array analysis was performed. Statistical analysis of the array data demonstrated that seven miRNAs (miR-16, miR-26a, miR-27a, miR-30b, miR-130, miR-195, and miR-497) were altered in BAV. (Table 3). All seven miRNAs were downregulated in AS group as compared to the AI group, of which three miRNAs from the same family (miR-16, miR-195, and miR-497) are found to be significant for their difference between AS and AI phenotypes.
Table 3.
miRNA microarray results. Seven miRNAs were found to have a statistically significant decrease in AS as compared to AI valve samples.
| Name | Fold change | Regulated | p-value |
|---|---|---|---|
| hsa-miR-130A | −1.48148 | down in AVS | 0.00259 |
| hsa-miR-30B | −1.83486 | down in AVS | 0.00748 |
| hsa-miR-497 | −1.58983 | down in AVS | 0.0119 |
| hsa-miR-195 | −1.9802 | down in AVS | 0.0158 |
| hsa-miR-26A | −1.62075 | down in AVS | 0.0265 |
| hsa-miR-16 | −1.9685 | down in AVS | 0.0353 |
| hsa-miR-27A | −1.88679 | down in AVS | 0.0406 |
miRNA qRT-PCR
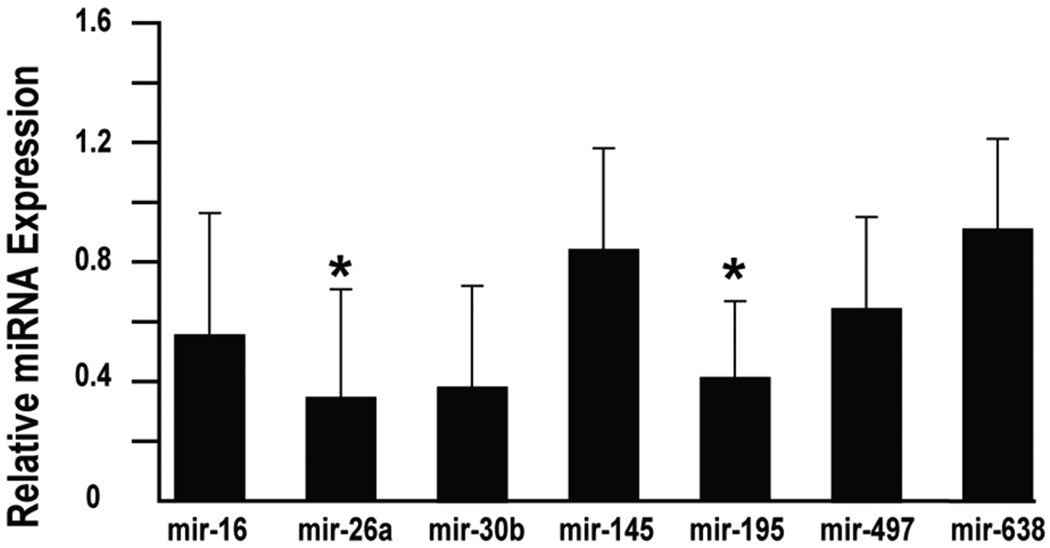
To validate the miRNA arrays results, miRNA qRT-PCR was performed on the fused leaflets from both groups. Since the fused leaflet tends to be the predominate foci of disease (data not shown), the unfused leaflet was used as an internal control. miR-26a and miR-195 levels were reduced by 65% and 59% respectively with p<0.05 in the stenotic leaflets as compared to the insufficient leaflets by qRT-PCR. miR-30b was reduced by 62% in the stenotic samples by qRT-PCR with a p-value that approached significance at p<0.06. (Figure 1) The remaining miRNAs were not altered in a statistically significant manner.
Figure 1.
miR-26a and miR-195 were expressed at lower levels in AS valves. QRT-PCR was performed on the AS (n=4) and AI (n=5) valve samples to validate the miRNA microarray results. miR-26a was reduced by 65% in the AS valves while miR-195 was decreased by 59% (*p<0.05 for both). Of note, miR-30b was reduced by 62% with p<0.06.
miR-26a, miR-30b, and miR-195 modulate calcification related genes
To examine if miR-26a, miR-30b, and miR-195 have roles in modulating calcification-related genes in AVICs, mimics for these miRNAs were transfected into human AVICs and RNA was harvested for qRT-PCR after four days. A panel of calcification and valve related genes were examined via qRT-PCR.
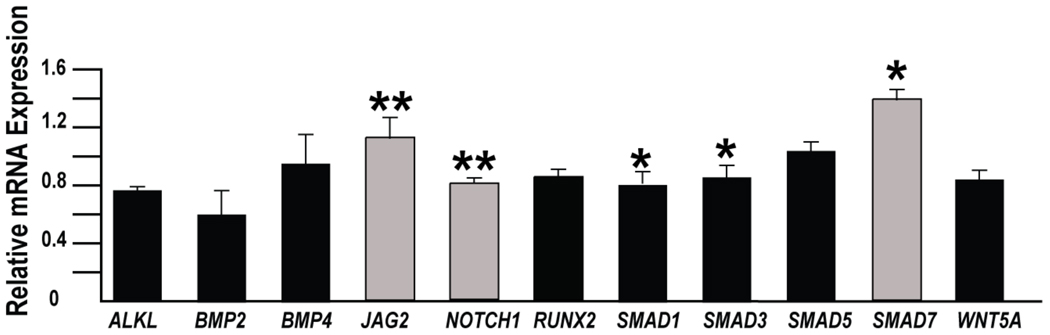
mir-26a repressed several of the calcification-related genes in vitro (Fig 2). ALKL mRNA levels were reduced by 38% (p<0.01), BMP2 mRNA was reduced by 36% (p<0.04), SMAD1 by 26% (p<0.01). MiR-26a increased the mRNA level of genes that may have roles in inhibiting calcification including JAG2 (31% p<0.01) and SMAD7 (15% p<0.01). Of note, there was an increase in two genes that are considered pro-calcific, RUNX2 was increased by 16% p<0.01 and SMAD5 increased by 21% p<0.01.
Figure 2.
MiR-26a predominately repressed pro-calcification genes (black bars) while increasing expression of anti-calcification genes (grey bars) in human AVICs. Specifically, miR-26a treatment resulted in decreases expression of ALPL (38%), BMP2 (36%), and SMAD1 (26%). At the same time, JAG2 and SMAD7 were increased by 31% and 15% respectively in cells treated with miR-26a. However, the increases in the pro-calcification genes RUNX2 (16%) and SMAD5 (21%) goes against the trend. (n=3; *p<0.05; **p<0.01).
miR-30b also appeared to down regulate calcification-related gene pathways (fig 3). miR-30b repressed SMAD1 by 18% p<0.02 and SMAD3 by 12% p<0.05. BMP2 was repressed by 39% with a p-value that approached significance at p<0.07. JAG2 was increased by 14% p<0.01 and SMAD7 by 40% p<0.02. In contrast, NOTCH1 expression was decreased by 19% p<0.01.
Figure 3.
MiR-30b predominately repressed pro-calcification genes (black bars) while increasing expression of anti-calcification genes (grey bars) in human AVICs. AVICs treated with miR-30b mimic had decreased SMAD1 (18%) and SMAD3 (12%) mRNA levels. JAG2 and SMAD7, two anti-calcification genes, had 14% and 40% increase in mRNA expression respectively. The 19% decreased in NOTCH1 expression goes against the trend. (n=3; *p<0.05; **p<0.01). Of note, BMP2 was reduced by 68% with a p<0.07.
miR-195 activated calcification related genes by BMP2 68% p<0.01, RUNX2 11% p<0.01, SMAD1 9% p<0.04, SMAD3 4% p<0.02, SMAD5 17% p<0.01 (fig 4). There was an increase in JAG2 and SMAD7 expression by 13% p<0.01 and 26% respectively (p<0.01) which are two genes that may repress calcification.
Figure 4.
MiR-195 predominately activates pro-calcification gene expression (black bars) while repressing anti-calcification genes (grey bars). BMP2 (68%), RUNX2 (11%), SMAD1 (9%), SMAD3 (4%), and SMAD5 (17%) were increased in miR-195 treated AVICs. The increases in JAG2 (13%) and SMAD7 (26%) go against the trend. (n=3; *p<0.05; **p<0.01).
Discussion
To our knowledge, this is the first report of altered miRNA levels in aortic valve disease and their possible role in aortic valve calcification. Given that BAV, present in 1–2% of the population, is the most the most common risk factor for aortic valve calcification, we focused our efforts on comparing miRNA levels in BAV with AS or AI. We compared the miRNA levels in the fused leaflets using miRNA arrays and qPCR. The miRNA arrays identified seven miRNAs with differentially expression in the fused leaflets of BAV compared to the unfused leaflet. It is interesting that three miRNAs (miR-16, miR-195, and miR-497) from the same family are found to be significant for their difference between AS and AI phenotypes. miRNA qPCR demonstrated that miR-26a and miR-195 were decreased in AS patients as compared to AI patients in a statistically significant manner. miR-30b was downregulated with a p-value of 0.06, which approached statistically significance. We acknowledge the likelihood that additional miRNAs may be altered but were not detected by the microarray since calcification is not a uniform process throughout the leaflet and our experimental design examined the expression patterns in the entire leaflet. Furthermore, miRNAs may have a different role in the early pathogenesis while the valve samples were obtained in the late stages of the disease process.
In order to examine the role of miR-26a, miR-195, and miR-30b on calcification related genes, we transfected cultured human AVICs with miRNA mimics and performed qPCR for calcification related genes. Interestingly, we found that miR-26a repressed pro-calcification genes such as BMP2, RUNX2, SMAD1, and SMAD5, at the same time there was an increasing of anti-calcification genes such as SMAD7, and JAG2. JAG2, a ligand for the Notch signaling pathway, may repress aortic valve calcification via NOTCH1, which has been shown to repress aortic valve calcification(5, 8). SMAD7 has inhibitory roles to BMP signaling and inhibits TGF-beta signaling (27, 28). miR-30b has a similar pattern of repression calcification related genes and activating SMAD7 and JAG2. In contrast, MiR-195 appears to activate several pro-calcification related genes. Our findings indicate that these miRNAs (miR-26a, miR-195, and miR-30b) in vitro modulated the mRNA levels of calcification related genes. It appears that miRNAs may have a key role in the evolving understanding of the genetic pathways involved in aortic valve calcification and merit study as a possible treatment modality.
In our future directions, we have embarked upon the examination of these miRNAs in a large cohort of BAVs with different clinical phenotypes. One aspect of these studies will be to characterize the role of the miRNAs in different cell types within the aortic valve.
Study limitations
A limitation of the study is the significant age difference of the patients in the AI group as compared to the AS group. This difference is typical for the patient populations because AS typically requires surgery later in life. The age difference raises the possibility that age is a contributing factor to the alterations of the miRNA. The decreases in mir-26a and -30b could contribute to the calcification of the aortic valve since they modulate genes related to calcification. Further study will be required to understand how miRNAs are being regulated in the aortic valve leaflets including role of aging on miRNA expression.
Another limitation is that it was not possible to identify specific calcification-related genes that are being targeted by the miRNA studied in this report. We did attempt to perform in silico analysis using publically available target prediction programs. However, none of the genes that had altered expression in response to treatment with miRNA mimics had predicted binding sites for the relevant miRNA. As the understanding of relationships between miRNAs and their target genes improves, it likely will be easier to identify the specific calcification-related genes targeted by the miRNAs.
We found that miR-26a, miR-30b, and miR-195 were reduced in the aortic valve leaflets of patients undergoing valve replacement of the aortic valve due to calcific stenosis as compared to patients undergoing valve replacement due to insufficiency. In vitro, increased levels of miR-26a and miR-30b repress the calcification pathways by modulating calcification-related mRNAs. Conversely, miR-195 tended to activate the calcification pathways.
Acknowledgments
Acknowledgements and Scientific Contribution
The authors would like to take members of their respective laboratories for helpful discussions. Dr. Nigam designed and performed the qPCR and in vitro experiments. Dr. Nigam was funded by NIH K08 HL086775-01. Drs. Sievers and Sier assisted with the patient valve collection. Drs. Jensen and Simpson provided access to the Cardiac Tissue Bank at UCSF from which the aortic valve leaflets used for AVIC culture were obtained. Dr. Srivastava contributed critical scientific guidance and review of the manuscript. Dr. Mohamed was responsible for designing the project, collection of the patient valves, and microarray data. Drs. Nigam and Mohamed prepared the manuscript.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005;111:3316–3326. doi: 10.1161/CIRCULATIONAHA.104.486738. [DOI] [PubMed] [Google Scholar]
- 3.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 4.Kaden JJ, Bickelhaupt S, Grobholz R, Vahl CF, Hagl S, Brueckmann M, et al. Expression of bone sialoprotein and bone morphogenetic protein-2 in calcific aortic stenosis. J Heart Valve Dis. 2004;13:560–566. [PubMed] [Google Scholar]
- 5.Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, et al. Mutations in NOTCH1 cause aortic valve disease. Nature. 2005;437:270–274. doi: 10.1038/nature03940. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed SA, Aherrahrou Z, Liptau H, Erasmi AW, Hagemann C, Wrobel S, et al. Novel missense mutations (p.T596M and p.P1797H) in NOTCH1 in patients with bicuspid aortic valve. Biochem Biophys Res Commun. 2006;345:1460–1465. doi: 10.1016/j.bbrc.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 7.McKellar SH, Tester DJ, Yagubyan M, Majumdar R, Ackerman MJ, Sundt TM., 3rd Novel NOTCH1 mutations in patients with bicuspid aortic valve disease and thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2007;134:290–296. doi: 10.1016/j.jtcvs.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Nigam V, Srivastava D. Notch1 represses osteogenic pathways in aortic valve cells. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cushing MC, Mariner PD, Liao JT, Sims EA, Anseth KS. Fibroblast growth factor represses Smad-mediated myofibroblast activation in aortic valvular interstitial cells. FASEB J. 2008;22:1769–1777. doi: 10.1096/fj.07-087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Samal E, Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature. 2005;436:214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 13.da Costa MartinsPA, Bourajjaj M, Gladka M, Kortland M, van Oort RJ, Pinto YM, et al. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation. 2008;118:1567–1576. doi: 10.1161/CIRCULATIONAHA.108.769984. [DOI] [PubMed] [Google Scholar]
- 14.Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, et al. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B, et al. The muscle-specific microRNA miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1 and KCNJ2. Nat Med. 2007;13:486–491. doi: 10.1038/nm1569. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 17.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SS, Jiang WW, Smith I, Poeta LM, Begum S, Glazer C, et al. MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008;123:2791–2797. doi: 10.1002/ijc.23831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramdas L, Giri U, Ashorn CL, Coombes KR, El-Naggar A, Ang KK, et al. miRNA expression profiles in head and neck squamous cell carcinoma and adjacent normal tissue. Head Neck. 2009;31:642–654. doi: 10.1002/hed.21017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vasilescu C, Rossi S, Shimizu M, Tudor S, Veronese A, Ferracin M, et al. MicroRNA Fingerprints Identify miR-150 as a Plasma Prognostic Marker in Patients with Sepsis. PLoS One. 2009;4:e7405. doi: 10.1371/journal.pone.0007405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naga PrasadSV, Duan ZH, Gupta MK, Surampudi VS, Volinia S, Calin GA, et al. Unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. J Biol Chem. 2009;284:27487–27499. doi: 10.1074/jbc.M109.036541. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006;48:e1–e148. doi: 10.1016/j.jacc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 23.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, et al. The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart. 2008;94:1634–1638. doi: 10.1136/hrt.2007.132092. [DOI] [PubMed] [Google Scholar]
- 24.Jian B, Narula N, Li QY, Mohler ER, 3rd, Levy RJ. Progression of aortic valve stenosis: TGF-beta1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann Thorac Surg. 2003;75:457–465. doi: 10.1016/s0003-4975(02)04312-6. discussion 465-456. [DOI] [PubMed] [Google Scholar]
- 25.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114:I547–I552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 26.Peng Y, Laser J, Shi G, Mittal K, Melamed J, Lee P, et al. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res. 2008;6:663–673. doi: 10.1158/1541-7786.MCR-07-0370. [DOI] [PubMed] [Google Scholar]
- 27.Casellas R, Brivanlou AH. Xenopus Smad7 inhibits both the activin and BMP pathways and acts as a neural inducer. Dev Biol. 1998;198:1–12. doi: 10.1006/dbio.1998.8893. [DOI] [PubMed] [Google Scholar]
- 28.Souchelnytskyi S, Nakayama T, Nakao A, Moren A, Heldin CH, Christian JL, et al. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-beta receptors. J Biol Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]