Abstract
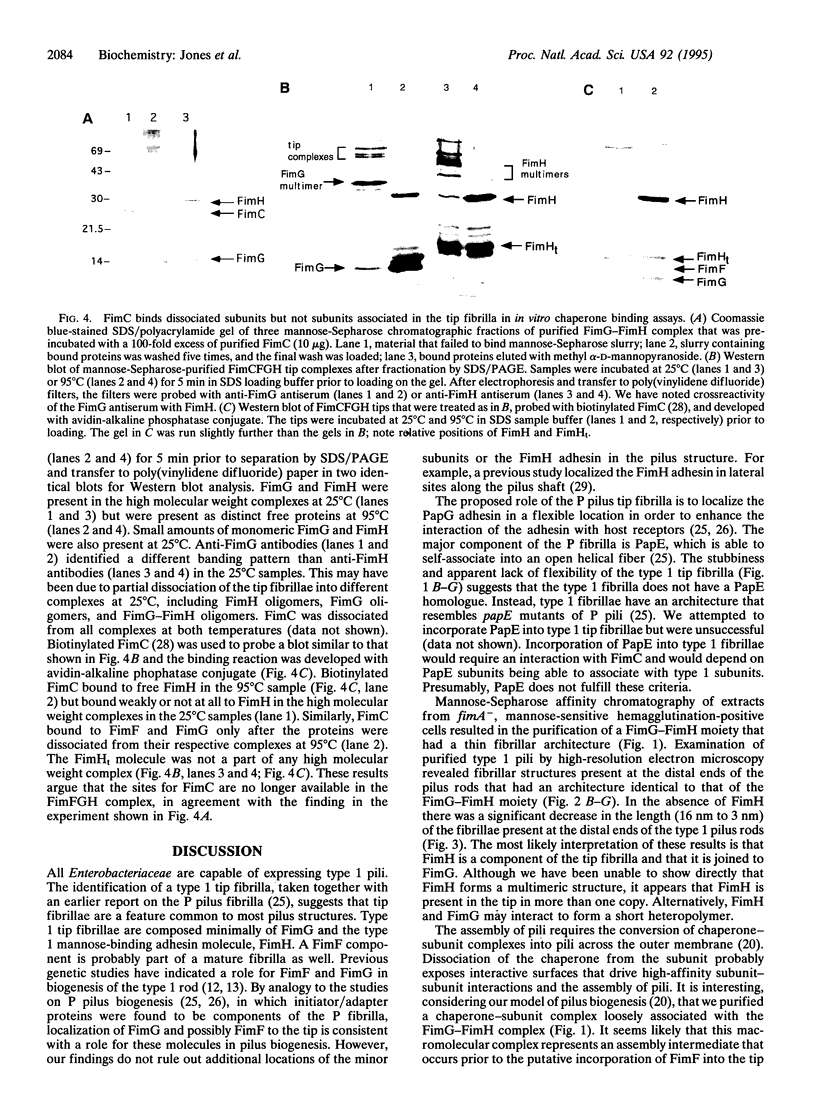
Type 1 pili are heteropolymeric mannosebinding fibers produced by all members of the Enterobacteriaceae family. The bulk of the fiber is composed of FimA. Two macromolecular complexes responsible for mediating an interaction with mannose-containing receptors were purified from fimA- Escherichia coli by mannose affinity chromatography and ion-exchange chromatography. One complex contained only the mannose-binding adhesin, FimH, associated with FimG, a minor component of the type 1 pilus. In the other complex the FimG-FimH moiety was loosely associated with a chaperone-minor subunit complex (FimC-FimF), possibly representing an intermediate in tip fibrilla assembly. The FimC chaperone has also been shown to form a preassembly complex with FimH that has been purified and characterized previously. Purified FimC did not bind to the FimG-FimH complex but did recognize FimH dissociated from the FimG-FimH complex. Quick-freeze deep-etch electron microscopy revealed that the FimG-FimH complex had a thin fibrillar architecture. High-resolution electron microscopy of type 1 pili revealed that a 16-nm fibrillar tip structure with an architecture identical to that of the FimG-FimH complex was joined end-to-end to the pilus rod. In a fimH- deletion mutant, the tip fibrillae joined to pilus rods were approximately 3 nm in length. The full-length tip fibrilla was restored by complementation with the fimH gene in trans. The bipartite nature of the type 1 pilus was also demonstrated on pili purified from clinical isolates of members of the Enterobacteriaceae family arguing that it is a conserved feature of the type 1 pilus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham S. N., Goguen J. D., Sun D., Klemm P., Beachey E. H. Identification of two ancillary subunits of Escherichia coli type 1 fimbriae by using antibodies against synthetic oligopeptides of fim gene products. J Bacteriol. 1987 Dec;169(12):5530–5536. doi: 10.1128/jb.169.12.5530-5536.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham S. N., Sun D., Dale J. B., Beachey E. H. Conservation of the D-mannose-adhesion protein among type 1 fimbriated members of the family Enterobacteriaceae. Nature. 1988 Dec 15;336(6200):682–684. doi: 10.1038/336682a0. [DOI] [PubMed] [Google Scholar]
- Bloch C. A., Stocker B. A., Orndorff P. E. A key role for type 1 pili in enterobacterial communicability. Mol Microbiol. 1992 Mar;6(6):697–701. doi: 10.1111/j.1365-2958.1992.tb01518.x. [DOI] [PubMed] [Google Scholar]
- Brinton C. C., Jr The structure, function, synthesis and genetic control of bacterial pili and a molecular model for DNA and RNA transport in gram negative bacteria. Trans N Y Acad Sci. 1965 Jun;27(8):1003–1054. doi: 10.1111/j.2164-0947.1965.tb02342.x. [DOI] [PubMed] [Google Scholar]
- Dodson K. W., Jacob-Dubuisson F., Striker R. T., Hultgren S. J. Outer-membrane PapC molecular usher discriminately recognizes periplasmic chaperone-pilus subunit complexes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3670–3674. doi: 10.1073/pnas.90.8.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbarah A., Gahmberg C. G., Ofek I., Jacobi U., Sharon N. Identification of the leukocyte adhesion molecules CD11 and CD18 as receptors for type 1-fimbriated (mannose-specific) Escherichia coli. Infect Immun. 1991 Dec;59(12):4524–4530. doi: 10.1128/iai.59.12.4524-4530.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach G. F., Clegg S., Allen B. L. Identification and characterization of the genes encoding the type 3 and type 1 fimbrial adhesins of Klebsiella pneumoniae. J Bacteriol. 1989 Mar;171(3):1262–1270. doi: 10.1128/jb.171.3.1262-1270.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampapa C. S., Abraham S. N., Chiang T. M., Beachey E. H. Isolation and characterization of a receptor for type 1 fimbriae of Escherichia coli from guinea pig erythrocytes. J Biol Chem. 1988 Apr 15;263(11):5362–5367. [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. Protocol for 3-D visualization of molecules on mica via the quick-freeze, deep-etch technique. J Electron Microsc Tech. 1989 Nov;13(3):244–263. doi: 10.1002/jemt.1060130310. [DOI] [PubMed] [Google Scholar]
- Hultgren S. J., Duncan J. L., Schaeffer A. J., Amundsen S. K. Mannose-sensitive haemagglutination in the absence of piliation in Escherichia coli. Mol Microbiol. 1990 Aug;4(8):1311–1318. doi: 10.1111/j.1365-2958.1990.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Jacob-Dubuisson F., Heuser J., Dodson K., Normark S., Hultgren S. Initiation of assembly and association of the structural elements of a bacterial pilus depend on two specialized tip proteins. EMBO J. 1993 Mar;12(3):837–847. doi: 10.1002/j.1460-2075.1993.tb05724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. H., Pinkner J. S., Nicholes A. V., Slonim L. N., Abraham S. N., Hultgren S. J. FimC is a periplasmic PapD-like chaperone that directs assembly of type 1 pili in bacteria. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8397–8401. doi: 10.1073/pnas.90.18.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. The fimD gene required for cell surface localization of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1990 Jan;220(2):334–338. doi: 10.1007/BF00260505. [DOI] [PubMed] [Google Scholar]
- Klemm P., Christiansen G. Three fim genes required for the regulation of length and mediation of adhesion of Escherichia coli type 1 fimbriae. Mol Gen Genet. 1987 Jul;208(3):439–445. doi: 10.1007/BF00328136. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Heuser J., Normark S., Hultgren S. J. P pili in uropathogenic E. coli are composite fibres with distinct fibrillar adhesive tips. Nature. 1992 Mar 19;356(6366):252–255. doi: 10.1038/356252a0. [DOI] [PubMed] [Google Scholar]
- Kuehn M. J., Ogg D. J., Kihlberg J., Slonim L. N., Flemmer K., Bergfors T., Hultgren S. J. Structural basis of pilus subunit recognition by the PapD chaperone. Science. 1993 Nov 19;262(5137):1234–1241. doi: 10.1126/science.7901913. [DOI] [PubMed] [Google Scholar]
- Kukkonen M., Raunio T., Virkola R., Lähteenmäki K., Mäkelä P. H., Klemm P., Clegg S., Korhonen T. K. Basement membrane carbohydrate as a target for bacterial adhesion: binding of type I fimbriae of Salmonella enterica and Escherichia coli to laminin. Mol Microbiol. 1993 Jan;7(2):229–237. doi: 10.1111/j.1365-2958.1993.tb01114.x. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Kaper J. B., Black R. E., Clements M. L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983 Dec;47(4):510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minion F. C., Abraham S. N., Beachey E. H., Goguen J. D. The genetic determinant of adhesive function in type 1 fimbriae of Escherichia coli is distinct from the gene encoding the fimbrial subunit. J Bacteriol. 1986 Mar;165(3):1033–1036. doi: 10.1128/jb.165.3.1033-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orndorff P. E., Falkow S. Organization and expression of genes responsible for type 1 piliation in Escherichia coli. J Bacteriol. 1984 Aug;159(2):736–744. doi: 10.1128/jb.159.2.736-744.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponniah S., Endres R. O., Hasty D. L., Abraham S. N. Fragmentation of Escherichia coli type 1 fimbriae exposes cryptic D-mannose-binding sites. J Bacteriol. 1991 Jul;173(13):4195–4202. doi: 10.1128/jb.173.13.4195-4202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell P. W., Orndorff P. E. Lesions in two Escherichia coli type 1 pilus genes alter pilus number and length without affecting receptor binding. J Bacteriol. 1992 Sep;174(18):5923–5935. doi: 10.1128/jb.174.18.5923-5935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slonim L. N., Pinkner J. S., Brändén C. I., Hultgren S. J. Interactive surface in the PapD chaperone cleft is conserved in pilus chaperone superfamily and essential in subunit recognition and assembly. EMBO J. 1992 Dec;11(13):4747–4756. doi: 10.1002/j.1460-2075.1992.tb05580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokurenko E. V., Courtney H. S., Ohman D. E., Klemm P., Hasty D. L. FimH family of type 1 fimbrial adhesins: functional heterogeneity due to minor sequence variations among fimH genes. J Bacteriol. 1994 Feb;176(3):748–755. doi: 10.1128/jb.176.3.748-755.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker R., Jacob-Dubuisson F., Freiden C., Hultgren S. J. Stable fiber-forming and nonfiber-forming chaperone-subunit complexes in pilus biogenesis. J Biol Chem. 1994 Apr 22;269(16):12233–12239. [PubMed] [Google Scholar]
- Tewari R., MacGregor J. I., Ikeda T., Little J. R., Hultgren S. J., Abraham S. N. Neutrophil activation by nascent FimH subunits of type 1 fimbriae purified from the periplasm of Escherichia coli. J Biol Chem. 1993 Feb 5;268(4):3009–3015. [PubMed] [Google Scholar]