Abstract
The ventral premammillary nucleus (PMV) expresses dense collections of sex steroid receptors and receptors for metabolic cues, including leptin, insulin and ghrelin. The PMV responds to opposite sex odor stimulation and projects to areas involved in reproductive control, including direct innervation of gonadotropin releasing hormone neurons. Thus, the PMV is well positioned to integrate metabolic and reproductive cues, and control downstream targets that mediate reproductive function. In fact, lesions of PMV neurons blunt female reproductive function and maternal aggression. However, although the projections of PMV neurons have been well documented, little is known about the neuronal inputs received by PMV neurons. To fill this gap, we performed a systematic evaluation of the brain sites innervating the PMV neurons of male and female rats using the retrograde tracer subunit B of the cholera toxin (CTb). In general, we observed that males and females show a similar pattern of afferents. We also noticed that the PMV is preferentially innervated by neurons located in the forebrain, with very few projections coming from brainstem nuclei. The majority of inputs originated from the medial nucleus of the amygdala, the bed nucleus of the stria terminalis and the medial preoptic nucleus. A moderate to high density of afferents was also observed in the ventral subiculum, the arcuate nucleus and the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus. Our findings strengthen the concept that the PMV is part of the vomeronasal system and integrates the brain circuitry controlling reproductive functions.
Keywords: Cholera toxin b, Sexual dimorphism, Hypothalamus, Reproduction
1. Introduction
The ventral premammillary nucleus (PMV) is a small, compact group of neurons in the medial zone of the hypothalamus, located immediately caudal to the ventromedial nucleus of the hypothalamus and ventral to the dorsal premammillary nucleus (Canteras et al., 1992b; Donato and Elias, 2011). Initial studies using extensive electrolytic lesions of the premammillary area of rats suggested the involvement of the PMV in male conspecific aggression (van den Berg et al., 1983). These findings were later reinforced by neuroanatomical studies using Fos immunoreactivity as a marker of neuronal activation (Hoffman and Lyo, 2002). The PMV of male rats and hamsters shows increased Fos expression after aggressive conspecific encounters, but notably also after sexual behavior (Coolen et al., 1996; Kollack-Walker and Newman, 1995; Veening et al., 2005). In fact, conspecific opposite sex odors alone induce Fos expression in PMV neurons of rodents. These neurons coexpress nitric oxide synthases and cocaine- and amphetamine-regulated transcript (CART) (Cavalcante et al., 2006a; Donato et al., 2010; Yokosuka et al., 1999). We further demonstrated that CART mRNA is increased in the PMV of males following exposure to female odors and that PMV CART neurons project to areas controlling gonadotropin releasing hormone (GnRH) secretion (Cavalcante et al., 2006a; Rondini et al., 2004).
The PMV expresses high to moderate density of sex steroids receptors and projects to the sexually dimorphic circuitry, to nuclei of the vomeronasal system and reproductive control sites (Canteras et al., 1992b; Gautron et al., 2013; Merchenthaler et al., 2004; Rondini et al., 2004; Simerly et al., 1990). Therefore, PMV neurons may integrate external (odor) and internal (sex steroids) cues to modulate the downstream targets in neuroendocrine and behavioral responses. In agreement with this model, electrolytic lesions of the PMV abolished the luteinizing hormone (LH) rise induced by male odor or electrical stimulation of the medial nucleus of the amygdala (Beltramino and Taleisnik, 1985). Moreover, using excitotoxic lesions, we and others have shown that the PMV plays a role in female neuroendocrine regulation of estrous cycles, sexual behavior and maternal aggression (Donato et al., 2009, 2013; Motta et al., 2013).
The PMV also expresses receptors for several metabolic cues including leptin, insulin and ghrelin, and may represent a key integrative site for metabolic regulation (Donato and Elias, 2011; Elias et al., 2000; Elmquist et al., 1998; Frazao et al., 2014; Leshan et al., 2009; Zigman et al., 2006). Nutrition is fundamental to reproductive function (Frisch and McArthur, 1974; Hill et al., 2008; Kennedy and Mitra, 1963). States of negative energy balance decreases reproductive capacity and delays puberty. On the other hand, excess energy also disrupts fertility (Bluher and Mantzoros, 2004; Chan and Mantzoros, 2001). Leptin signaling-deficient mice and humans are obese and infertile, and the genetic deletion of insulin receptors in the brain disrupts fertility in mice (Bruning et al., 2000; Chua et al., 1996; Clement et al., 1998; Coleman, 1978; Farooqi et al., 2002; Tartaglia et al., 1995; Zhang et al., 1994). However, the exact brain sites involved in the metabolic control of reproduction are not completely known. We have shown that endogenous re-expression of leptin receptors only in the PMV of mice otherwise null for leptin receptor induces puberty and improves fertility (Donato et al., 2011). These findings indicate that the PMV plays a fundamental role in the integration of metabolism and reproduction. However, although the projections of PMV neurons have been well documented (Canteras et al., 1992b; Gautron et al., 2013; Leshan et al., 2009; Rondini et al., 2004), little is known about the neuronal inputs received by PMV neurons. To fill this gap and contribute to the understanding of the hypothalamic circuitry controlling complex physiological systems, we performed a systematic evaluation of the brain sites innervating the PMV neurons of male and female rats. In the present study, we chose to use a standard neuroanatomical tracer to acquire knowledge on the complete network of neuronal populations with the potential to modulate PMV output.
2. Results
Neurons projecting to the PMV from the entire brain were mapped using the cholera toxin b subunit (CTb) as a retrograde tracer. CTb is an efficient, allowing restricted injections combined with robust and consistent retrograde labeling with virtually no uptake from fibers of passage (Luppi et al., 1990). We observed that the projections to the PMV of male and female rats were predominantly ipsilateral with a few retrogradely labeled neurons in contralateral nuclei. The pattern of distribution of CTb immunoreactive (CTb-ir) neurons was virtually identical between sexes, with few differences in the density of retrogradely labeled cells in specific areas with apparent higher number of CTb-ir neurons in the female brain. Because injection sites were variable, quantification was not possible. Figs. 1 and 2 illustrate the injection sites of cases with CTb centered inside the boundaries of the PMV (n=4 males and n=3 females) and used in our mapping study. Fig. 3 shows examples of cases with injection sites restricted to the PMV (W282 male and W422 female). Injections outside the PMV were used as controls (see examples in Fig. 4). A comparative analysis of retrogradely labeled neurons in males and females is presented in Table 1. Abbreviations follow the nomenclature proposed by Swanson (1992).
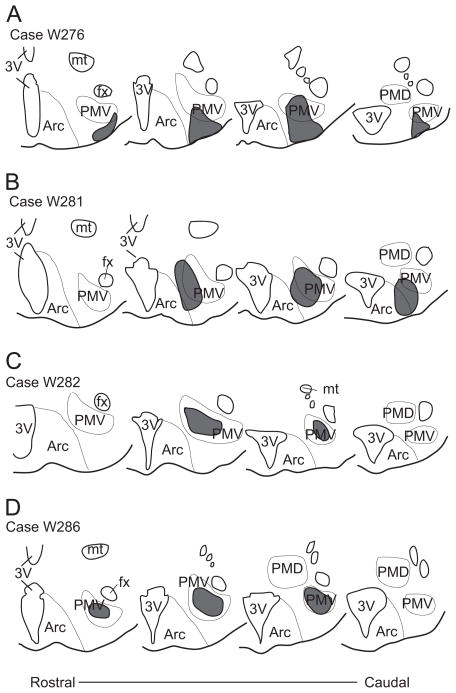
Fig. 1.
Schematic illustration of the rostro-to-caudal extent of the injection sites in male rats (W276, W281, W282 and W286) with the retrograde tracer cholera toxin b (CTb) centered in the ventral premammillary nucleus (PMV). Abbreviations: 3V, third ventricle; Arc, arcuate nucleus; fx, fornix; mt, mammillothalamic tract; PMD, dorsal premammillary nucleus.
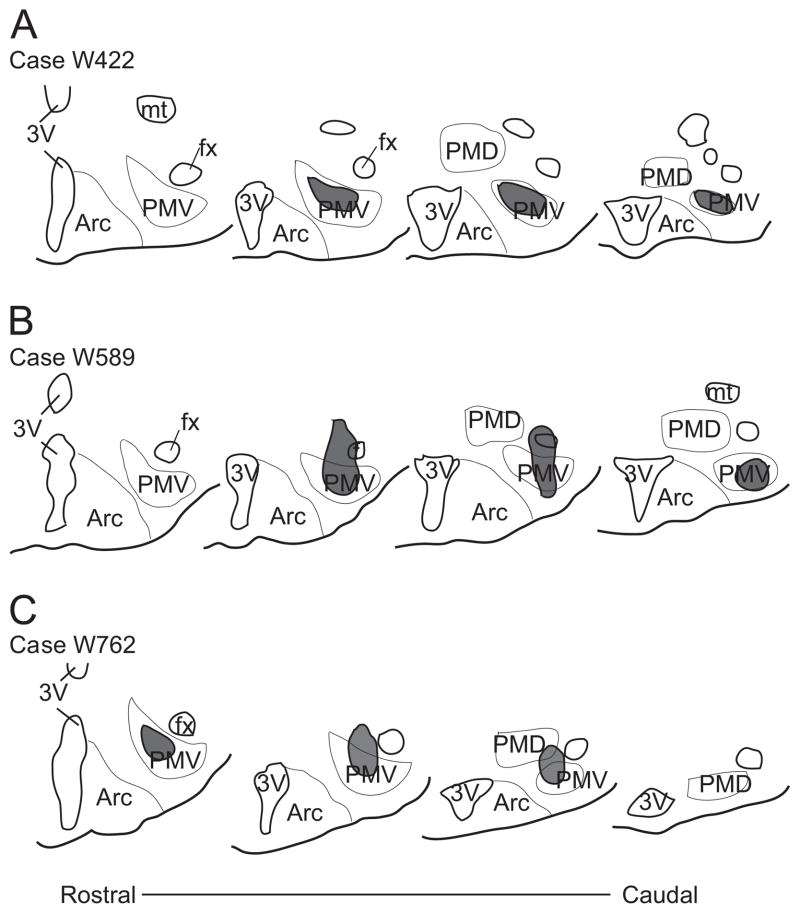
Fig. 2.
Schematic illustration of the rostro-to-caudal extent of the injection sites in female rats (W422, W589 and W762) with the retrograde tracer cholera toxin b (CTb) centered in the ventral premammillary nucleus (PMV). Abbreviations: 3V, third ventricle; Arc, arcuate nucleus; fx, fornix; mt, mammillothalamic tract; PMD, dorsal premammillary nucleus.
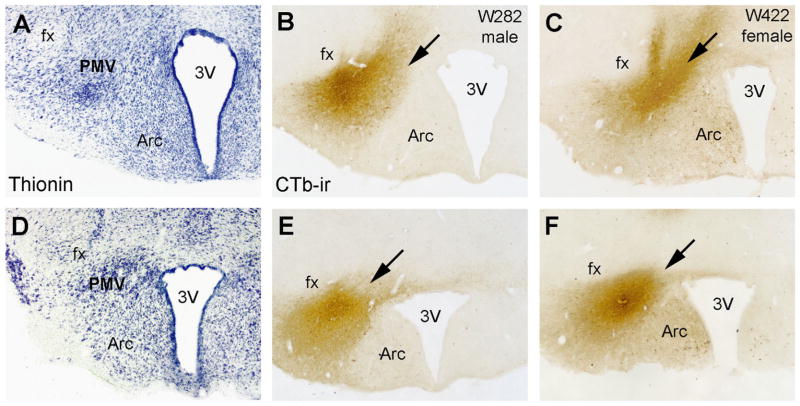
Fig. 3.
Injections of the retrograde tracer cholera toxin b (CTb) into the ventral premammillary nucleus (PMV) of male and female rats. A, D Brightfield images of a reference brain stained with thionin showing two rostro-to-caudal levels of the PMV (A, rostral and D, caudal). B, C, E, F Brightfield images showing hypothalamic sections of a male (B, E, W282) and a female (C, F, W422) rat with CTb injections centered in the PMV. Abbreviations: 3V, third ventricle; Arc, arcuate nucleus; fx, fornix. Scale bar: A–F=500 μm.
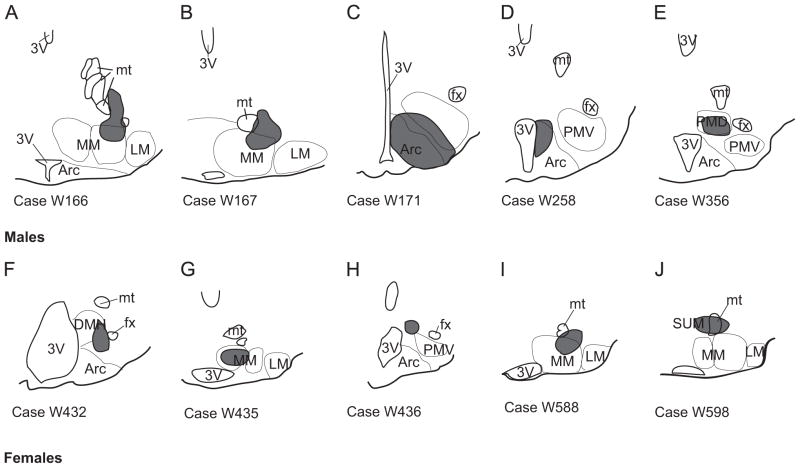
Fig. 4.
Schematic illustration of injection sites outside the ventral premammillary nucleus of males and females used as controls. A–E, control cases for males; F–J, control cases for females. Abbreviations: 3V, third ventricle; Arc, arcuate nucleus; DMH, dorsomedial nucleus of the hypothalamus; fx, fornix; LM, lateral mammillary nucleus; MM, medial mammillary nucleus; mt, mammillothalamic tract; PMD, dorsal premammillary nucleus.
Table 1.
Comparative distribution and density of retrogradely labeled neurons following CTb injection into the PMV of male and female rats.
| Brain areas | Male | Female |
|---|---|---|
| Forebrain | ||
| Cerebral cortex | ||
| Prelimbic cortex | ++ | + |
| Infralimbic cortex | ++ | + |
| Insular agranular cortex | +/− | +/− |
| Anterior ventral cingulate cortex | +/− | +/− |
| Olfactory cortex | ||
| Dorsal tenia tecta | ++ | ++ |
| Amigdalopiriform transition | +/− | + |
| Entorhinal lateral area | − | +/− |
| Septum | ||
| Lateral subdivision | +++ | +++ |
| Ventral subdivision | +++ | +++ |
| Intermediate subdivision | + | + |
| Bed nuclei of the stria terminalis | ||
| Anterodorsal subdivision | ++ | ++ |
| Dorsomedial subdivision | + | + |
| Principal subdivision | ++++ | ++++ |
| Interfascicular subdivision | ++ | ++ |
| Ventral subdivision | +/− | +/− |
| Bed nucleus of the accessory olfactory tract | ++ | ++ |
| Amygdala | ||
| Medal nucleus | ||
| Anterodorsal subdivision | ++ | ++ |
| Anteroventral subdivision | +/− | +/− |
| Posterodorsal subdivision | +++ | ++++ |
| Posteroventral subdivision | ++ | +++ |
| Cortical nucleus | ||
| Anterior subdivision | + | + |
| Posterolateral subdivision | + | + |
| Posteromedial subdivision | + | + |
| Basomedial nucleus | ||
| Anterior subdivision | + | + |
| Posterior subdivision | +/− | +/− |
| Posterior nucleus (amigdalohippocampal area) | +++ | ++++ |
| Amygdalopiriform transition | + | + |
| Hippocampus, ventral subiculum | +++ | ++++ |
| Thalamus | ||
| Paraventricular nucleus | − | +/− |
| Paratenial nucleus | +/− | +/− |
| Subparafascicular parvicellular nucleus | +/− | +/− |
| Hypothalamus | ||
| Anteroventral periventricular nucleus | +/− | + |
| Median preoptic nucleus | +/− | +/− |
| Paraventricular nucleus, periventricular subdivision | + | + |
| Paraventricular nucleus, anterior parvicellular subdivision | +/− | +/− |
| Paraventricular nucleus, medial parvicellular subdivision | +/− | +/− |
| Retrochiasmatic area | +/− | +/− |
| Arcuate nucleus | ++ | ++ |
| Anterodorsal preoptic nucleus | + | + |
| Anteroventral preoptic nucleus | + | + |
| Medial preoptic nucleus, lateral subdivision | ++ | ++ |
| Medial preoptic nucleus, central subdivision | +++ | ++++ |
| Medial preoptic nucleus, medial subdivision | +++ | +++ |
| Anterior nucleus | ++ | ++ |
| Ventromedial nucleus, dorsomedial subdivision | +/− | +/− |
| Ventromedial nucleus, central subdivision | + | + |
| Ventromedial nucleus, ventrolateral subdivision | ++ | +++ |
| Dorsomedial nucleus, anterior subdivision | + | + |
| Dorsomedial nucleus, ventral subdivision | + | ++ |
| Tuberal nucleus, intermediate part | + | + |
| Posterior nucleus | +/− | +/− |
| Supramammillar nucleus | +/− | +/− |
| Lateral preoptic area | +/− | +/− |
| Perifornical nucleus | + | + |
| Lateral hypothalamic area, anteroventral zone | +/− | +/− |
| Lateral hypothalamic area, justaventromedial region | +/− | +/− |
| Lateral hypothalamic area, ventromedial zone | +/− | +/− |
| Brainstem | ||
| Periaqueductal gray, ventrolateral column | +/− | +/− |
| Laterodorsal tegmental nucleus | +/− | +/− |
| Ventral tegmental area | +/− | +/− |
| Locus coeruleus | − | +/− |
| Dorsal nucleus of the raphe | +/− | +/− |
| Parabrachial lateral nucleus, central division | +/− | +/− |
| Parabrachial lateral nucleus, external division | +/− | +/− |
| Parabrachial lateral nucleus, superior division | +/− | +/− |
| Nucleus incertus | − | +/− |
Ratings reflect a qualitative evaluation of the density of retrogradely labeled cells. (−) lack of CTb-ir neurons, (+/−) very low, (+) low, (++) moderate, (+++) high, (++++) very high density of retrogradely labeled neurons.
2.1. Telencephalon
In the telencephalon, CTb-ir neurons were mainly concentrated in the medial and posterior amygdala, the bed nucleus of the stria terminalis and the lateral septal nucleus (Fig. 5, Fig. 7B–J). The medial prefrontal cortex, the bed nucleus of the olfactory accessory tract and the ventral subiculum also showed a consistent medium to small projections to the PMV (Figs. 7A, G and 8A–B).
Fig. 5.
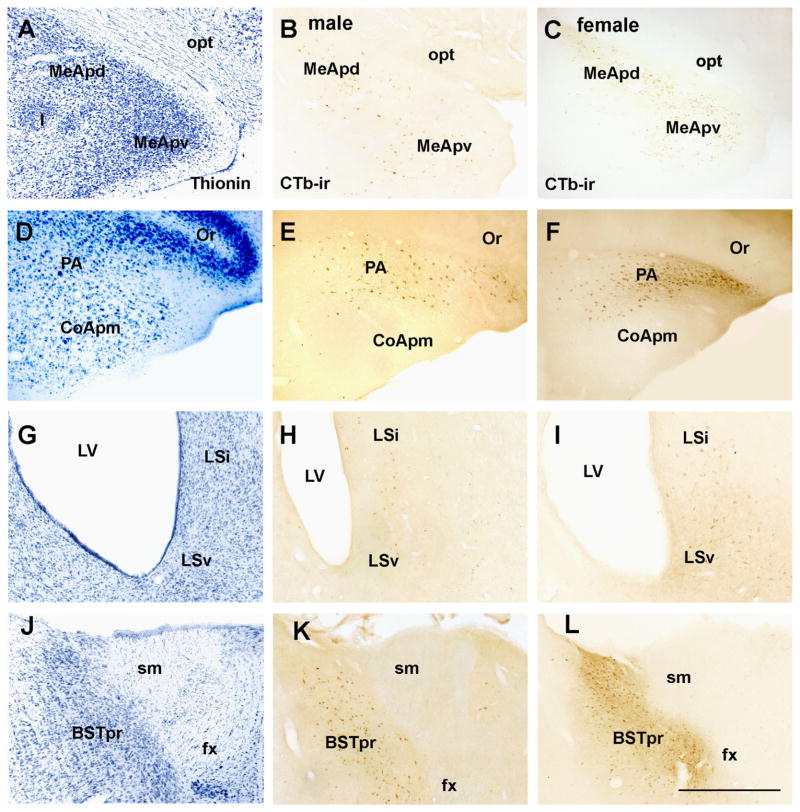
Distribution of retrogradely labeled neurons in the male and female forebrain. A, D, G, J Brightfield images of a reference brain stained with thionin showing the cytoarchitecture of a control (female) brain. B and C Brightfield images showing cholera toxin b immunoreactive neurons (CTb-ir) in the posterodorsal and posteroventral subdivisions of the medial nucleus of the amygdala (MeApd and MeApv, respectively) of male (B) and female (C) rats. E and F Brightfield images showing CTB-ir neurons in the posterior nucleus of the amygdala (PA) of male (E) and female (F) rats. H and I Brightfield images showing CTb-ir neurons in the lateral septum ventral (LSv) and lateral septum intermediate (LSi) of male (E) and female (F) rats. J and K Brightfield images showing CTb-ir neurons in the principal subdivision of the bed nucleus of the stria terminalis (BSTpr) of male (H) and female (I) rats. Abbreviations: CoApm, subdivision posteromedial of the cortical nucleus of the amygdala; fx, fornix; I, intercalate nucleus of the amygdala; LV, lateral ventricle; opt, optic tract; Or, stratus oriens; sm, stria medularis. Scale bar: 300 μm.
Fig. 7.
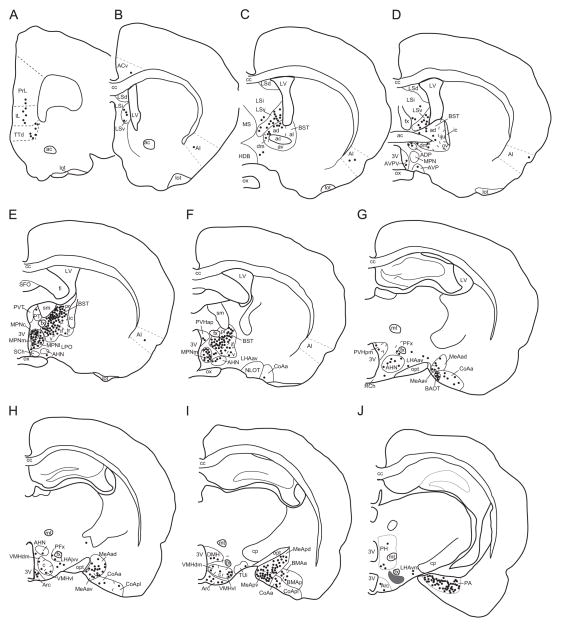
Schematic illustration of the distribution of retrogradely labeled forebrain neurons projecting to the ventral premammillary nucleus of a representative male case (W286). The drawing illustrates coronal rostro-to-caudal sections of the forebrain. Each dot represents 3–5 cells. Abbreviations: 3v, third ventricle; ac, anterior commissure; ACv, ventral anterior cingulate cortex; AHN, anterior hypothalamic nucleus; AI, agranular insular cortex; ADP, anterodorsal preoptic nucleus; Arc, arcuate nucleus; AVP, anteroventral preoptic nucleus; AVPV, anteroventral periventricular nucleus; BAOT, bed nucleus of the accessory olfactory tract; BMAa, anterior basomedial nucleus of the amygdala; BMAp, posterior basomedial nucleus of the amygdala; BST, bed nucleus of the stria terminalis (ad, anterodorsal; al, anterolateral; av, anteroventral; dm, dorsomedial; if, interfascicular; ju, justacapsular; ov, oval; pr, principal; tr, transversal; v, ventral); cc, corpus callosum; CoAa, anterior cortical nucleus of the amygdala; CoApl, posterolateral cortical nucleus of the amygdala; cp, cerebral peduncle; DMH, dorsomedial nucleus of the hypothalamus; fi, fimbria; fx, fornix; HDB, nucleus of the horizontal limb of the diagonal band; ic, internal capsule; IL, infralimbic cortex; LHAav, lateral hypothalamic area, anteroventral zone; LHAjvv, lateral hypothalamic area, justaventromedial region; LHAvm, lateral hypothalamic area, ventromedial zone; lot, lateral olfactory tract; LPO, lateral preoptic area; LSd, lateral septum dorsal; LSi, lateral septum intermediate; LSv, lateral septum ventral; LV, lateral ventricle; MeAad, medial nucleus of the amygdala, anterodorsal subdivision; MeAav, medial nucleus of the amygdala, anteroventral subdivision; MeApd, medial nucleus of the amygdala, posterodorsal subdivision; MeApv, medial nucleus of the amygdala, posteroventral subdivision; MPNc, medial preoptic nucleus, central subdivision; MPNl, medial preoptic nucleus, lateral subdivision; MPNm, medial preoptic nucleus, medial subdivision; MS, medial septum; mt, mamillothalamic tract; opt, optic tract; ox, optic chiasm; NLOT, nucleus of the lateral optic tract; PA, posterior nucleus of the amygdala; PFx, perifornicial area; PH, posterior hypothalamic nucleus; PrL, prelimbic cortex; PVHap, paraventricular nucleus of the hypothalamus, anterior parvocellular; PVHpm, paraventricular nucleus of the hypothalamus, parvocellular medial; PT, paratenial nucleus, PVT, paraventricular nucleus of the thalamus; RCh, retrochiasmatica area; SCh, suprachiasmatic nucleus; SFO, subfornical organ; sm, stria medullaris of the thalamus; VMHdm, ventromedial nucleus of the hypothalamus, dorsomedial subdivision; VMHvl, ventromedial nucleus of the hypothalamus, ventrolateral subdivision (c, central subdivision); TTd, tenia tecta dorsal; TUi, tuberal nucleus, intermediate part.
Fig. 8.
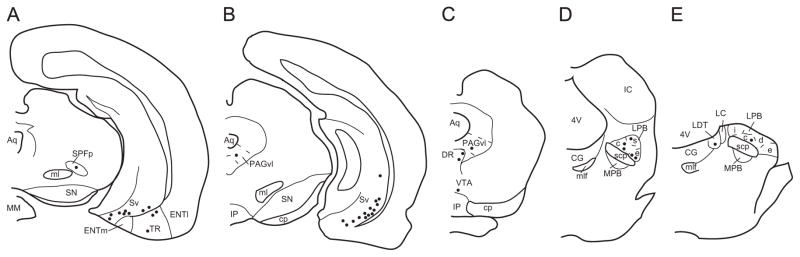
Schematic illustration of the distribution of retrogradely labeled hippocampus and brainstem neurons projecting to the ventral premammillary nucleus (PMV) of a representative male case (W286). The drawings illustrate coronal rostro-to-caudal sections of the hippocampus and brainstem. Each dot represents 3–5 cells. Abbreviations: 4V, fourth ventricle; Aq, aqueduct; CG, central gray; cp, cerebral peduncle; DR, dorsal raphe nucleus; ENTl, entorhinal lateral cortex; ENTm, entorhinal medial cortex; IC, inferior colliculus; IP, interpeduncular nucleus; LC, locus coeruleus; LDT, laterodorsal tegmental nucleus; LPB, lateral parabrachial nucleus (c, central; d, dorsal; e, external; i, internal; s, superior); ml, medial lemniscus; mlf, medial longitudinal fasciculus; MM, medial mamillary nucleus; MPB, medial parabrachial nucleus; PAGvl, ventrolateral column of the periaqueductal gray matter; scp, superior cerebellar peduncle; SN, substantia nigra; SPFp, parvocelullar division of the subparafascicular nucleus; Sv, subiculum ventral; TR, amygdalopiriform transition; VTA, ventral tegmental area.
The medial nucleus of the amygdala (MeA) comprises the major source of afferents to the PMV. CTb-ir neurons were spread through most of the MeA, with the highest concentration in the posterodorsal and posteroventral subdivisions (MeApd and MeApv, respectively, Figs. 5A–C, 7G–I). The posterior nucleus of the amygdala (PA) also showed a high density of CTb-ir neurons (Fig. 5D–F, Fig. 7J), while more caudally, a small number of retrogradely labeled neurons were observed inside the postpiriform transition area. Several other nuclei of the amygdala showed a low density of CTb-ir neurons, including the anterior and posterior parts of the cortical nucleus and the anterior and posterior parts of the basomedial nucleus (Fig. 7F–I, Table 1). Moderate to small number of retrogradely labeled neurons were also found in the bed nucleus of the accessory olfactory tract (Fig. 7G).
Retrogradely labeled neurons were concentrated in the ventral subdivisions of the lateral septal nucleus (LS) and a few scattered CTb-ir neurons were found in the LS intermediate subdivision (Figs. 5G–I, 7B–D). CTb-ir neurons were very dense in the principal subdivision of the bed nucleus of the stria terminalis (BSTpr, Fig. 5J–L; Fig. 7E, F). The interfascicular and anterodorsal subdivisions of the BST showed a moderate concentration of retrogradely labeled neurons, and the BST ventral and dorsomedial subdivisions showed only a few CTb-ir neurons (Fig. 7C–F).
In the cerebral cortex, small groups of retrograde labeled neurons were observed in the infralimbic, prelimbic and dorsal tenia tecta (Fig. 7A). A few CTb-ir neurons were found in the ventral anterior cingulate and agranular insular cortices (Fig. 7B–E). In the hippocampal formation, the ventral subiculum contained a moderate to high density of retrogradely labeled neurons (Figs. 6A–C, 8A, B).
Fig. 6.
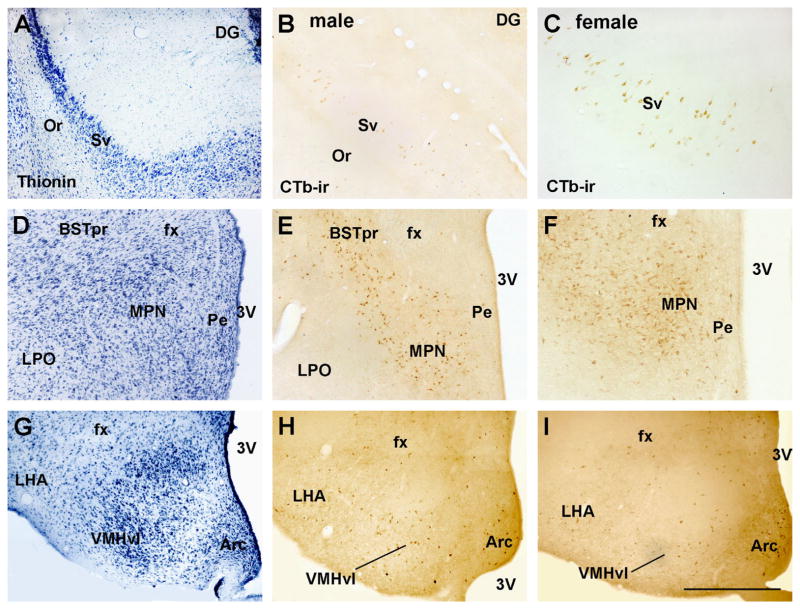
Distribution of retrogradely labeled neurons in the male and female hippocampus and hypothalamus. A, D, G Brightfield images of a reference brain stained with thionin showing the cytoarchitecture of a control (female) brain. B and C Brightfield images showing cholera toxin b immunoreactive neurons (CTb-ir) in the ventral subiculum (Sv) of male (B) and female (C) rats. E and F Brightfield images showing CTb-ir neurons in the medial preoptic nucleus (MPN) of male (E) and female (F) rats. H and G Brightfield images showing CTb-ir neurons in the ventrolateral subdivision of the ventromedial nucleus of the hypothalamus (VMHvl), lateral hypothalamic area (LHA) and arcuate nucleus (Arc) of male (H) and female (I) rats. Abbreviations: 3V, third ventricle; BSTpr, bed nucleus of the stria terminalis, principal subdivision; DG, dentate gyrus; fx, fornix; LPO, lateral preoptic area; Or, stratus oriens; Pe, periventricular nucleus. Scale bar: A–B, D–I=300 μm; C=150 μm.
2.2. Diencephalon
We observed that multiple hypothalamic nuclei innervate the PMV of male and female rats. In the periventricular zone of the hypothalamus, a moderate number of retrogradely labeled neurons were observed in the arcuate nucleus (Fig. 7G–I) whereas only a few CTb-ir neurons were scattered in the anteroventral periventricular nucleus, the median preoptic nucleus, the retrochiasmatic area, and the parvocelullar anterior, medial and periventricular subdivisions of the paraventricular nucleus of the hypothalamus (Fig. 7D–J).
In the medial zone, a few CTb-ir neurons were observed in the anterodorsal preoptic and ventrolateral preoptic nuclei (Fig. 7D). Retrogradely labeled neurons were also observed in the medial preoptic area, with most of them concentrated in the medial preoptic nucleus (MPN, Figs. 6D–F, 7E, F). Inside the MPN, a high density of CTb-ir neurons was observed in the medial (MPNm) and central subdivisions (MPNc) in both males and females. Retrogradely labeled neurons were scattered in the anterior region of the hypothalamus, with a higher concentration observed in the anterior hypothalamic nucleus (Fig. 7F–H). In the tuberal region, the ventromedial nucleus of the hypothalamus showed a moderate number of retrogradely labeled neurons, primarily in the ventrolateral subdivision (VMHvl, Fig. 6G–I, Fig. 7H, I). Only a few retrogradely labeled neurons were also observed in the dorsomedial nucleus of the hypothalamus (Fig. 7I). In the posterior region, scattered CTb-ir neurons were found in the posterior hypothalamic nucleus and the supramammillary nucleus (Fig. 7J).
A small number of retrogradely labeled neurons was observed in the lateral zone of the hypothalamus. According to the topographic organization of the lateral hypothalamic area proposed by Swanson et al. (2005), we observed retrogradely labeled neurons in the ventral and intermediate zones of the anterior region, the lateral zone of the ventral region, the juxtaventromedial and juxtadorsomedial regions, and the intermediate part of the medial tuberal nucleus (Fig. 7G, H). A few CTb-ir neurons were also found in the lateral preoptic area.
In the thalamus, only a few CTb-ir neurons were found in the parataenial (Fig. 7E), the subparaventricular, the paraventricular, and the parvicellular subdivision of the subparafascicular nucleus (Fig. 8A).
2.3. Brainstem
Projections from the brainstem to the PMV were scarce (Fig. 8A–E). Very few retrogradely labeled neurons were observed in the ventrolateral column of the periaqueductal gray matter, the dorsal nucleus of the raphe, the laterodorsal tegmental nucleus, the ventral tegmental nucleus, the lateral parabrachial nucleus and the locus coeruleus (Fig. 8, Table 1).
2.4. Control mice
Injections outside the PMV were used as controls (Fig. 4). Injections in cases W166 and W167 (males), and W435 and W588 (females) contaminated the medial mammillary nucleus (Fig. 4A–B, G, I) and showed high numbers of retrogradely labeled neurons in the medial septal nucleus, nucleus of the diagonal band of Broca, lateral hypothalamic area and zona incerta. Injections in cases W171 and W258 contaminated the arcuate nucleus and showed similar distribution of retrogradely labeled neurons compared to the PMV except that higher numbers of neurons were observed in the anteroventral periventricular nucleus, lateral parabrachial nucleus and periaqueductal gray matter. Injection in case W356 contaminated the dorsal premammillary nucleus (Fig. 4E) showing high number of retrogradely labeled neurons in the anterior hypothalamic area. Injection in the case W432 contaminated the dorsomedial nucleus of the hypothalamus (Fig. 4F) with high number of CTb-ir cells in the paraventricular nucleus of the hypothalamus and in the suprachiasmatic nucleus. Injection in case W436 was very small and centered in the dorsal tuberomammillary nucleus (Fig. 4H) showing few cells throughout the brain mostly in the periventricular hypothalamic area. The injection in case W598 was centered in the mammillothalamic tract and supramammillary nucleus (Fig. 4J) and showed high number of cells in the lateral and medial septal nuclei, dorsal subiculum and anterior thalamic nuclei.
3. Discussion
In the present study, we showed that the PMV of males and females receive inputs from many areas throughout the brain, with a predominance of projections originating in the forebrain. No clear difference was noticed in the innervation pattern comparing both sexes. The MeA, the PA, the BSTpr and the MPN are the major sources of inputs. Moderate innervation originates from the ventral subdivision of the LS and the ventral subiculum. In the hypothalamus, moderate input to the PMV originates in the anterior hypothalamic area, the arcuate nucleus and the VMHvl, whereas the brainstem has a very discrete set of afferents.
The major inputs to the PMV originate from nuclei pertaining to the vomeronasal system, also known as the accessory olfactory system. The vomeronasal system is comprised of a group of nuclei that receive and process olfactory information related to conspecific (socially relevant) and heterospecific (socially non-relevant) interactions (Halpern and Martinez-Marcos, 2003). The vomeronasal organ is located in the nasal cavity and provides odorant signals to the accessory olfactory bulb (AOB). From the AOB, olfactory signals reach multiple forebrain nuclei, including the BSTpr and subdivisions of the medial, cortical and posterior nuclei of the amygdala (Halpern and Martinez-Marcos, 2003; Scalia and Winans, 1975). No direct projection from the AOB to the PMV has been described, and our present study gives further support to this lack of a direct connection. However, most of the secondary relays of the vomeronasal system, including those mentioned above, innervate the PMV.
The PMV of rats and mice express Fos immunoreactivity in response to opposite sex odor exposure (Cavalcante et al., 2006a; Donato et al., 2010; Yokosuka et al., 1999). The expression of the gene c-fos or the Fos protein is a powerful tool for assessing the neuronal groups activated by a specific stimulus (Hoffman et al., 2002). Because the PMV is not directly innervated by the AOB, odor-induced neuronal activation (Fos expression) likely originates from the MeA, PA and/or BSTpr projections.
Odorant signals from the opposite sex are potent stimulators of luteinizing hormone (LH) and sex steroids secretion in male and female rodents (Beltramino and Taleisnik, 1983; Coquelin et al., 1984; Kamel et al., 1977; Macrides et al., 1975; Maruniak and Bronson, 1976; Purvis and Haynes, 1978). This neuroendocrine response was recapitulated by studies using electrical stimulation of vomeronasal relays, including the OAB, the MeA and the BSTpr (Beltramino and Taleisnik, 1978, 1979, 1980). However, interestingly, lesions of the PMV blunted these endocrine outputs (Beltramino and Taleisnik, 1985), indicating that the neuroendocrine reproductive response to odor stimulation requires an intact PMV. In agreement with these data, recent studies have demonstrated that bilateral lesions of the PMV of female rats significantly decreased female sexual behavior and maternal aggression towards a male intruder (Donato et al., 2013; Motta et al., 2013); both responses are highly dependent on the processing of olfactory cues (Bean and Wysocki, 1989; Ferreira and Hansen, 1986; Stowers et al., 2002). Because the MeA is a key site in olfactory discrimination (Donato et al., 2010; Meredith and Westberry, 2004; Petrulis, 2009), it is tempting to hypothesize that most of the olfactory cues targeting the PMV originate from MeA inputs. Choi and co-workers using molecular, tracer and neurochemical tools showed that the LIM homeodomain gene 6 (Lhx6) marks an amygdalo-hypothalamic GABAergic pathway for reproductive behavior in mice, including MeApd-PMV (Choi et al., 2005). Interestingly, we have previously demonstrated that a subset of MeApd neurons projecting to the PMV express urocortin 3 (Cavalcante et al., 2006b). Whether the MeApd-PMV GABAergic and/or urocortinergic innervations transmit olfactory signals needs further investigation.
Other potentially important sources of olfactory cues to the PMV are the PA and the BSTpr. Both nuclei express sex steroids receptors and display reciprocal connections with the PMV (Canteras et al., 1992a, 1992b; Merchenthaler et al., 2004; Simerly et al., 1990). The BSTpr directly respond to socially relevant odor stimulation (Kollack-Walker and Newman, 1995) whereas the PA seems to be modulated by dense projections from the MeApd presumably conducting odorant signals (Canteras et al., 1992a). In addition, the BSTpr and the PA receive dense projections from sexually dimorphic circuitry both being apt to integrate signals from the internal and external milieu and modulate the reproductive physiology via projections to the PMV(Canteras et al., 1992a).
In the hypothalamus, the MPN and the VMHvl also receive strong innervation from the MeA and BSTpr and densely project to the PMV (Canteras et al., 1994; Simerly and Swanson, 1988). Both nuclei express a dense collection of sex steroids receptors (Simerly et al., 1990), are sexually dimorphic (Madeira et al., 1999, 2001) and are involved with the modulation of male (MPN) and female (VMHvl) sexual behaviors (Hansen et al., 1982). These observations and the strong reciprocal connections of these sites with the PMV strengthen the concept that PMV neurons are well positioned to integrate sexually relevant signals (e.g., odor) and reproductive status to modulate behavioral (e.g., female sexual behavior and maternal aggression) and neuroendocrine (LH secretion) responses (Donato et al., 2009, 2013; Donato and Elias, 2011; Leshan et al., 2009; Motta et al., 2013). In line with this concept is the demonstration by different laboratories that the PMV directly innervates GnRH neurons, the final neural output in the neuroendocrine reproductive axis (Boehm et al., 2005; Leshan et al., 2009; Rondini et al., 2004; Yoon et al., 2005).
The main olfactory pathway runs parallel to the vomeronasal system and is classically known to process different sets of chemosensory stimuli (Boehm et al., 2005; Keller et al., 2009; Suárez et al., 2012; Yoon et al., 2005). However, both systems converge in the amygdala, and recent studies have shown that, in fact, both systems conduct vomeronasal and main odorant signals and interact in the control of mate recognition and sexual behavior (Achiraman et al., 2010; Boehm, 2006; Restrepo et al., 2004; Xu et al., 2005). In agreement with previous studies using anterograde tracers (Petrovich et al., 1996; Price et al., 1991), we observed that several nuclei of the main olfactory circuitry, including the basomedial, anterior and posterolateral nuclei of the amygdala, the dorsal tenia tecta and the postpiriform transition area displayed moderate to small projections to the PMV. The physiological significance of these projections is currently unknown, but we hypothesize that these sites also integrate the brain circuitry that modulates male and female sexual behaviors in response to olfactory stimuli.
A small number of CTb-ir neurons were observed in the prelimbic and the infralimbic cortices, in agreement with previous studies using anterograde tracers (Floyd et al., 2001; Hurley et al., 1991; Sesack et al., 1989; Takagishi and Chiba, 1991). These cortical areas are visceromotor sites of the medial prefrontal cortex that send major projections to the brainstem, mainly to the nucleus of the solitary tract, to regulate autonomic responses (Cechetto and Saper, 1987; Terreberry and Neafsey, 1983, 1987). Moreover, stimulation of the medial prefrontal cortex, including the prelimbic and infralimbic areas, blocked the LH surge and ovulation of proestrus rats (Caceres and Taleisnik, 1980a). The same approach blunted the LH release provoked by electrical stimulation of the medial preoptic area (Caceres and Taleisnik, 1980a, 1980b). Interestingly, interruption of PMV innervation eliminated the inhibitory effect of medial pre-frontal cortex stimulation on LH secretion (Caceres and Taleisnik, 1981). Our findings demonstrate that the PMV receives a small number of projections from these sites, but the physiological relevance of this direct cortical innervation of the PMV needs further evaluation. However, we found that the ventral subiculum contained a high density of retrogradely labeled neurons, in agreement with previous publications using anterograde tracers (Canteras and Swanson, 1992). The strong projection from the ventral subiculum to hypothalamic sites is thought to be involved in the modulation of anticipatory adjustments to endocrine and behavioral responses (Herman and Mueller, 2006; O’Mara, 2006). Studies have also demonstrated that the ventral subiculum expresses sex steroid receptors and shows sexually dimorphic organization of synaptic contacts and dendritic arborization (Andrade et al., 2000; Simerly et al., 1990).
The thalamus sends a very small number of inputs to the PMV; of those, the SPFp caught our attention. The SPFp expresses Fos after mating and receives projections from the lumbar spinal cord that conduct sensory inputs from the genital organs (Coolen et al., 1996; Truitt et al., 2003). Hence, the SPFp may provide somatic information from the genitals to the PMV, reinforcing the idea of an integrative role for the PMV in reproductive functions.
In summary, in the present study we demonstrated that the PMV of male and female rats is innervated by forebrain nuclei involved in odor processing and reproductive functions. Virtually no differences between males and females were noted in the innervation pattern. Our findings strengthen the concept that the PMV integrates external and internal cues to modulate neuroendocrine outputs and behaviors associated with reproductive function and species survival.
4. Experimental procedures
4.1. Animals
Adult male and female Wistar rats (280–320 g) were housed two per cage in the animal facility of the Department of Anatomy, Institute of Biomedical Sciences, University of São Paulo, SP – Brazil. Animals were maintained on a 12-h light/dark cycle (lights on at 7 am) in a temperature-controlled environment (21±2 °C) and were given free access to rat chow and water. All experiments were carried out in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996) and by the University of São Paulo Committee for Research and Animal Care. We attempted to minimize the number of rats used, and every effort was made to ensure that no rat suffered unnecessarily.
4.2. Retrograde tracer injection and histology
Rats were anesthetized with a subcutaneous injection of a solution containing ketamine (5 mg/100 g), xylazine (1 mg/100 g), and acepromazine (0.2 mg/100 g) and received a stereotaxic injection of CTb (1%; List Biological Laboratories, Campbell, CA, USA) into the PMV (n=32 males and n=30 females). The CTb was injected iontophoretically from a glass micropipette (10–20 μm of tip internal diameter) by applying a +5 μA current pulsed at 7-s intervals over 10–12 min. After 15 days, rats were perfused with 4% formaldehyde, the brains were dissected and cryoprotected overnight at 4 °C in 0.1 M phosphate-buffered saline (PBS), pH 7.4, containing 30% sucrose. Brains were cut in the frontal plane in 30-μm sections on a freezing microtome. Five series were collected in antifreeze solution and stored at −20 °C.
4.3. Immunohistochemistry
One series from each animal was submitted to a standard immunoperoxidase reaction. Sections were pretreated with hydrogen peroxide and blocked in 2% normal donkey serum (Jackson Laboratories, West Grove, PA, USA) and 0.3% Triton X-100 (Sigma). The sections were then incubated with antisera against CTb (raised in goat, 1:50,000; List Biological Laboratories, Campbell, CA, USA) overnight at room temperature. The CTb antisera have been validated before (Cavalcante et al., 2006b; Kusnoor et al., 2010). This was followed by incubation for 1 h in biotin-conjugated donkey anti-goat IgG (1:1000, Jackson Laboratories) and for 1 h in avidin–biotin complex (1:500, Vector Labs, Burlingame, CA, USA). The tissue was then submitted to an immunoperoxidase reaction using 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) as the chromogen and 0.03% hydrogen peroxide dissolved in 0.02 M potassium PBS (KPBS), pH 7.4, for 5–6 min. The reaction was terminated with rinses in KPBS. Sections were mounted onto gelatin-coated slides, dehydrated, delipidated and coverslipped with DPX mounting medium (BDH, Poole, England). One adjacent series was submitted to thionin staining and used as a reference.
4.4. Data analysis and production of photomicrographs
Brain sections were analyzed with a Zeiss M2 microscope. Photomicrographs were produced by capturing images with a Zeiss Axiocam HRc digital camera and AxioVision software. Only the sharpness, contrast, and brightness were adjusted. Adobe Creative Suite (CS7) was used to integrate photomicrographs into plates. Drawings were produced using Adobe Illustrator (CS7) and Canvas 12.
Acknowledgments
We would like to thank Joelcimar Martins da Silva for expert technical assistance. Financial support was provided in the form of Grants and fellowships from the FAPESP (00/11569-4, 03/06022-4, 04/00585-0, and 05/50951-5).
Footnotes
Support: This study was supported by grants and fellowships from the FAPESP (to J.C.C. fellowship 2001/11103-8, J.C.B. Grant 2004/13849-5 and C.F.E. Grant 05/59286-4) and the NIH (HD61539 and HD69702 to C.F.E.).
References
- Achiraman S, et al. Detection of estrus by male mice: synergistic role of olfactory-vomeronasal system. Neurosci Lett. 2010;477:144–148. doi: 10.1016/j.neulet.2010.04.051. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Madeira MD, Paula-Barbosa MM. Sexual dimorphism in the subiculum of the rat hippocampal formation. Brain Res. 2000;875:125–137. doi: 10.1016/s0006-8993(00)02605-6. [DOI] [PubMed] [Google Scholar]
- Bean NJ, Wysocki CJ. Vomeronasal organ removal and female mouse aggression: the role of experience. Physiol Behav. 1989;45:875–882. doi: 10.1016/0031-9384(89)90209-6. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Facilitatory and inhibitory effects of electrochemical stimulation of the amygdala on the release of luteinizing hormone. Brain Res. 1978;144:95–107. doi: 10.1016/0006-8993(78)90437-7. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Effect of electrochemical stimulation in the olfactory bulbs on the release of gonadotropin hormones in rats. Neuroendocrinology. 1979;28:320–328. doi: 10.1159/000122879. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Dual action of electrochemical stimulation of the bed nucleus of the stria terminalis on the release of LH. Neuroendocrinology. 1980;30:238–242. doi: 10.1159/000123007. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Release of LH in the female rat by olfactory stimuli. Effect of the removal of the vomeronasal organs or lesioning of the accessory olfactory bulbs. Neuroendocrinology. 1983;36:53–58. doi: 10.1159/000123436. [DOI] [PubMed] [Google Scholar]
- Beltramino C, Taleisnik S. Ventral premammillary nuclei mediate pheromonal-induced LH release stimuli in the rat. Neuroendocrinology. 1985;41:119–124. doi: 10.1159/000124164. [DOI] [PubMed] [Google Scholar]
- Bluher S, Mantzoros CS. The role of leptin in regulating neuroendocrine function in humans. J Nutr. 2004;134:2469S–2474S. doi: 10.1093/jn/134.9.2469S. [DOI] [PubMed] [Google Scholar]
- Boehm U, Zou Z, Buck LB. Feedback loops link odor and pheromone signaling with reproduction. Cell. 2005;123:683–695. doi: 10.1016/j.cell.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Boehm U. The vomeronasal system in mice: from the nose to the hypothalamus- and back! Semin Cell Dev Biol. 2006;17:471–479. doi: 10.1016/j.semcdb.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Bruning JC, et al. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Caceres A, Taleisnik S. Blockade of ovulation and release of LH in the rat by electrochemical stimulation of the frontal lobe cortex. Brain Res. 1980a;188:411–423. doi: 10.1016/0006-8993(80)90041-4. [DOI] [PubMed] [Google Scholar]
- Caceres A, Taleisnik S. Inhibition of secretion of luteinizing hormone induced by electrochemical stimulation of the anterior cingulate cortex mediated by a beta-adrenergic mechanism. J Endocrinol. 1980b;87:419–429. doi: 10.1677/joe.0.0870419. [DOI] [PubMed] [Google Scholar]
- Caceres A, Taleisnik S. Pathways by which stimuli originating in the cingulate cortex inhibiting LH secretion reach the hypothalamus. Neuroendocrinology. 1981;32:317–324. doi: 10.1159/000123180. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Connections of the posterior nucleus of the amygdala. J Comp Neurol. 1992a;324:143–179. doi: 10.1002/cne.903240203. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Projections of the ventral premammillary nucleus. J Comp Neurol. 1992b;324:195–212. doi: 10.1002/cne.903240205. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324:180–194. doi: 10.1002/cne.903240204. [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW. Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol. 1994;348:41–79. doi: 10.1002/cne.903480103. [DOI] [PubMed] [Google Scholar]
- Cavalcante JC, Bittencourt JC, Elias CF. Female odors stimulate CART neurons in the ventral premammillary nucleus of male rats. Physiol Behav. 2006a;88:160–166. doi: 10.1016/j.physbeh.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Cavalcante JC, et al. Distribution of urocortin 3 neurons innervating the ventral premammillary nucleus in the rat brain. Brain Res. 2006b;1089:116–125. doi: 10.1016/j.brainres.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Cechetto DF, Saper CB. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- Chan JL, Mantzoros CS. Leptin and the hypothalamic-pituitary regulation of the gonadotropin-gonadal axis. Pituitary. 2001;4:87–92. doi: 10.1023/a:1012947113197. [DOI] [PubMed] [Google Scholar]
- Choi GB, et al. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Chua SC, Jr, et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor [see comments] Science. 1996;271:994–996. doi: 10.1126/science.271.5251.994. [DOI] [PubMed] [Google Scholar]
- Clement K, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction [see comments] Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Fos immunoreactivity in the rat brain following consummatory elements of sexual behavior: a sex comparison. Brain Res. 1996;738:67–82. doi: 10.1016/0006-8993(96)00763-9. [DOI] [PubMed] [Google Scholar]
- Coquelin A, et al. Pheromonally induced release of luteinizing hormone in male mice: involvement of the vomeronasal system. J Neurosci. 1984;4:2230–2236. doi: 10.1523/JNEUROSCI.04-09-02230.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, et al. The ventral premammillary nucleus links fasting-induced changes in leptin levels and coordinated luteinizing hormone secretion. J Neurosci. 2009;29:5240–5250. doi: 10.1523/JNEUROSCI.0405-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, et al. Male and female odors induce Fos expression in chemically defined neuronal population. Physiol Behav. 2010;99:67–77. doi: 10.1016/j.physbeh.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Donato J, Jr, et al. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Investig. 2011;121:355–368. doi: 10.1172/JCI45106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, Elias CF. The ventral premammillary nucleus links metabolic cues and reproduction. Front Endocrinol (Lausanne) 2011;2:57. doi: 10.3389/fendo.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato J, Jr, et al. Lesions of the ventral premammillary nucleus disrupt the dynamic changes in Kiss1 and GnRH expression characteristic of the proestrus-estrus transition. Neuroscience. 2013;241:67–79. doi: 10.1016/j.neuroscience.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias CF, et al. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- Elmquist JK, et al. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Farooqi IS, et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J Clin Investig. 2002;110:1093–1103. doi: 10.1172/JCI15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira A, Hansen S. Sensory control of maternal aggression in Rattus norvegicus. J Comp Psychol. 1986;100:173–177. [PubMed] [Google Scholar]
- Floyd NS, et al. Orbitomedial prefrontal cortical projections to hypothalamus in the rat. J Comp Neurol. 2001;432:307–328. doi: 10.1002/cne.1105. [DOI] [PubMed] [Google Scholar]
- Frazao R, et al. Estradiol modulates Kiss1 neuronal response to ghrelin. Am J Physiol Endocrinol Metab. 2014;306:E606–E614. doi: 10.1152/ajpendo.00211.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science. 1974;185:949–951. doi: 10.1126/science.185.4155.949. [DOI] [PubMed] [Google Scholar]
- Gautron L, et al. Discrete melanocortin-sensitive neuroanatomical pathway linking the ventral premmamillary nucleus to the paraventricular hypothalamus. Neuroscience. 2013;240:70–82. doi: 10.1016/j.neuroscience.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern M, Martinez-Marcos A. Structure and function of the vomeronasal system: an update. Prog Neurobiol. 2003;70:245–318. doi: 10.1016/s0301-0082(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Hansen S, et al. Effects of ibotenic acid-induced neuronal degeneration in the medial preoptic area and the lateral hypothalamic area on sexual behavior in the male rat. Brain Res. 1982;239:213–232. doi: 10.1016/0006-8993(82)90843-5. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elmquist JK, Elias CF. Hypothalamic pathways linking energy balance and reproduction. Am J Physiol Endocrinol Metab. 2008;294:E827–E832. doi: 10.1152/ajpendo.00670.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman CS, et al. GABAergic drugs alter hypothalamic serotonin release and lordosis in estrogen-primed rats. Brain Res. 2002;946:96–103. doi: 10.1016/s0006-8993(02)02867-6. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? J Neuroendocrinol. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Hurley KM, et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Kamel F, et al. The influence of mating and related stimuli on plasma levels of luteinizing hormone, follicle stimulating hormone, prolactin, and testosterone in the male rat. Endocrinology. 1977;101:421–429. doi: 10.1210/endo-101-2-421. [DOI] [PubMed] [Google Scholar]
- Keller M, et al. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behav Brain Res. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kennedy GC, Mitra J. Body weight and food intake as initiating factors for puberty in the rat. J Physiol. 1963;166:408–418. doi: 10.1113/jphysiol.1963.sp007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66:721–736. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kusnoor SV, et al. Extracerebellar role for Cerebellin1: modulation of dendritic spine density and synapses in striatal medium spiny neurons. J Comp Neurol. 2010;518:2525–2537. doi: 10.1002/cne.22350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshan RL, et al. Direct innervation of GnRH neurons by metabolic- and sexual odorant-sensing leptin receptor neurons in the hypothalamic ventral premammillary nucleus. J Neurosci. 2009;29:3138–3147. doi: 10.1523/JNEUROSCI.0155-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppi PH, Fort P, Jouvet M. Iontophoretic application of unconjugated cholera toxin B subunit (CTb) combined with immunohistochemistry of neurochemical substances: a method for transmitter identification of retrogradely labeled neurons. Brain Res. 1990;534:209–224. doi: 10.1016/0006-8993(90)90131-t. [DOI] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Leal S, Paula-Barbosa MM. Stereological evaluation and Golgi study of the sexual dimorphisms in the volume, cell numbers, and cell size in the medial preoptic nucleus of the rat. J Neurocytol. 1999;28:131–148. doi: 10.1023/a:1007076206828. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Ferreira-Silva L, Paula-Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432:329–345. doi: 10.1002/cne.1106. [DOI] [PubMed] [Google Scholar]
- Maruniak JA, Bronson FH. Gonadotropic responses of male mice to female urine. Endocrinology. 1976;99:963–969. doi: 10.1210/endo-99-4-963. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, et al. Distribution of estrogen receptor alpha and beta in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473:270–291. doi: 10.1002/cne.20128. [DOI] [PubMed] [Google Scholar]
- Meredith M, Westberry JM. Distinctive responses in the medial amygdala to same-species and different-species pheromones. J Neurosci. 2004;24:5719–5725. doi: 10.1523/JNEUROSCI.1139-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta SC, et al. Ventral premammillary nucleus as a critical sensory relay to the maternal aggression network. Proc Natl Acad Sci USA. 2013;110:14438–14443. doi: 10.1073/pnas.1305581110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara S. Controlling hippocampal output: the central role of subiculum in hippocampal information processing. Behav Brain Res. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. J Comp Neurol. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Petrulis A. Neural mechanisms of individual and sexual recognition in Syrian hamsters (Mesocricetus auratus) Behav Brain Res. 2009;200:260–267. doi: 10.1016/j.bbr.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Slotnick BM, Revial MF. Olfactory projections to the hypothalamus. J Comp Neurol. 1991;306:447–461. doi: 10.1002/cne.903060309. [DOI] [PubMed] [Google Scholar]
- Purvis K, Haynes NB. Effect of the odour of female rat urine on plasma testosterone concentrations in male rats. J Reprod Fertil. 1978;53:63–65. doi: 10.1530/jrf.0.0530063. [DOI] [PubMed] [Google Scholar]
- Restrepo D, et al. Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice. Horm Behav. 2004;46:247–256. doi: 10.1016/j.yhbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Rondini TA, et al. Hypothalamic cocaine- and amphetamine-regulated transcript neurons project to areas expressing gonadotropin releasing hormone immunoreactivity and to the anteroventral periventricular nucleus in male and female rats. Neuroscience. 2004;125:735–748. doi: 10.1016/j.neuroscience.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Sesack SR, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270:209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Simerly RB, et al. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Stowers L, et al. Loss of sex discrimination and male–male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Suárez R, García-González D, De Castro F. Mutual influences between the main olfactory and vomeronasal systems in development and evolution. Front Neuroanat. 2012:6. doi: 10.3389/fnana.2012.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. Brain Maps: Structure of the Rat Brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- Swanson LW, Sanchez-Watts G, Watts AG. Comparison of melanin-concentrating hormone and hypocretin/orexin mRNA expression pattern in the new parceling scheme of the lateral hypothalamic zone. Neurosci Lett. 2005;387:80–84. doi: 10.1016/j.neulet.2005.06.066. [DOI] [PubMed] [Google Scholar]
- Takagishi M, Chiba T. Efferent projections of the infralimbic (area 25) region of the medial prefrontal cortex in the rat: an anterograde tracer PHA-L study. Brain Res. 1991;566:26–39. doi: 10.1016/0006-8993(91)91677-s. [DOI] [PubMed] [Google Scholar]
- Tartaglia LA, et al. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995;83:1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. Rat medial frontal cortex: a visceral motor region with a direct projection to the solitary nucleus. Brain Res. 1983;278:245–249. doi: 10.1016/0006-8993(83)90246-9. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Truitt WA, et al. Activation of a subset of lumbar spinothalamic neurons after copulatory behavior in male but not female rats. J Neurosci. 2003;23:325–331. doi: 10.1523/JNEUROSCI.23-01-00325.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg M, ter Horst G, Koolhaas J. The nucleus premammillaris ventralis (PMV) and aggressive behavior in the rat. Aggress Behav. 1983;9:41–47. [Google Scholar]
- Veening JG, et al. Do similar neural systems subserve aggressive and sexual behaviour in male rats? Insights from c-Fos and pharmacological studies. Eur J Pharmacol. 2005;526:226–239. doi: 10.1016/j.ejphar.2005.09.041. [DOI] [PubMed] [Google Scholar]
- Xu F, et al. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489:491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yokosuka M, et al. Female-soiled bedding induced fos immunoreactivity in the ventral part of the premammillary nucleus (PMv) of the male mouse. Physiol Behav. 1999;68:257–261. doi: 10.1016/s0031-9384(99)00160-2. [DOI] [PubMed] [Google Scholar]
- Yoon H, Enquist LW, Dulac C. Olfactory inputs to hypothalamic neurons controlling reproduction and fertility. Cell. 2005;123:669–682. doi: 10.1016/j.cell.2005.08.039. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zigman JM, et al. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]