Abstract
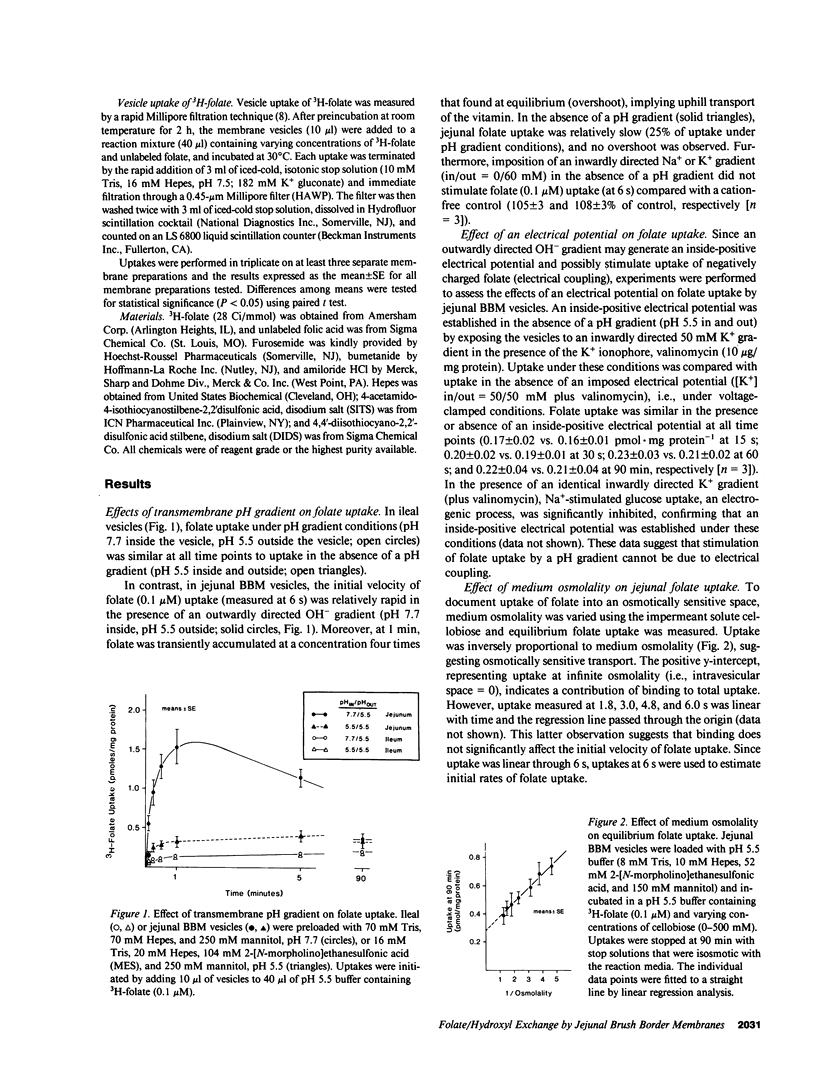
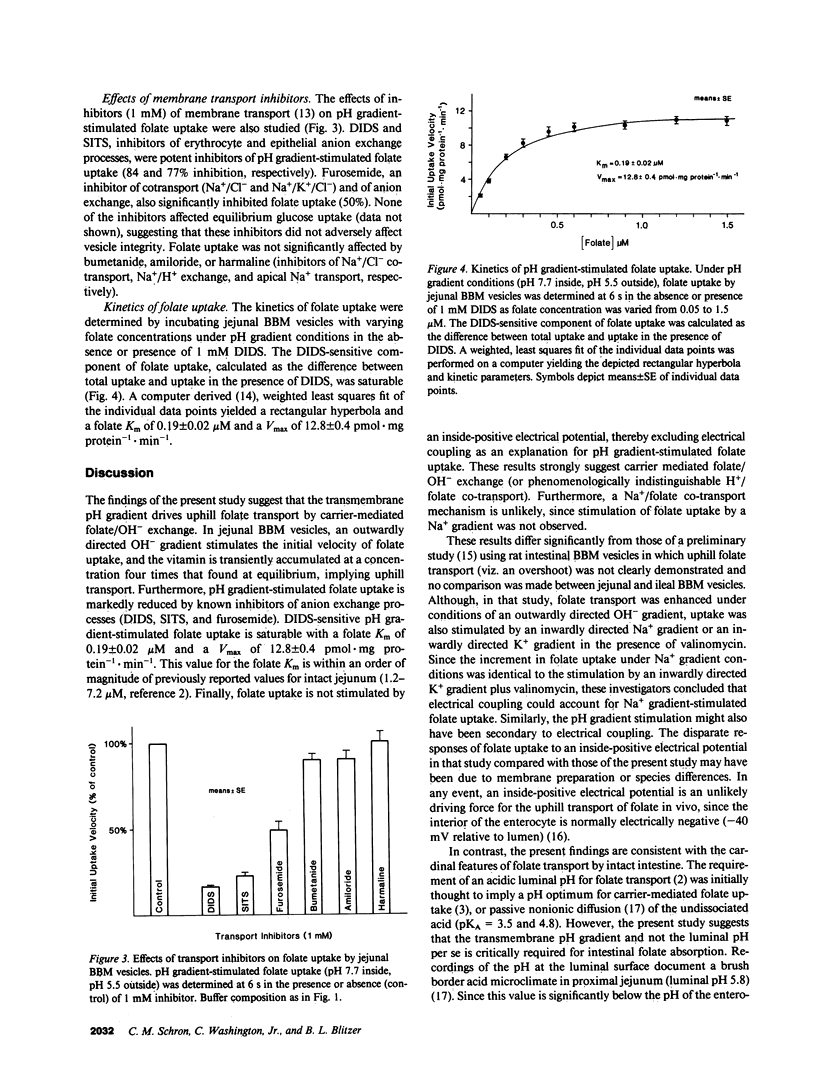
In rabbit jejunal, but not ileal brush border membrane vesicles, an outwardly directed OH- gradient (pH 7.7 inside, pH 5.5 outside) markedly stimulated the initial velocity of folate (0.1 microM) uptake compared with uptake in the absence of a pH gradient. Under pH gradient conditions, folate was transiently accumulated at a concentration four times that found at equilibrium (over-shoot), implying uphill transport of the vitamin. Equilibrium folate uptake was inversely proportional to medium osmolality, suggesting uptake into an osmotically sensitive space. pH gradient-stimulated folate uptake was markedly reduced by inhibitors of anion exchange (4,4'-diisothiocyano-2,2'-disulfonic acid stilbene; 4-acetamido-4-isothiocyanostilbene-2,2'-disulfonic acid; furosemide), and was saturable (folate Km = 0.19 +/- 0.02 microM; Vmax = 12.8 +/- 0.4 pmol X mg protein-1 X min-1). Imposition of an inside-positive electrical potential did not stimulate folate uptake, suggesting that stimulation by a pH gradient was not due to an induced electrical potential. In contrast, an inwardly directed Na+ or K+ gradient did not stimulate folate uptake. These findings provide evidence for a carrier on the jejunal brush border membrane that mediates folate/OH- exchange (or H+/folate co-transport), and are consonant with the known presence of an outwardly directed OH- gradient in vivo (brush border acid microclimate), an acidic pH optimum for intestinal folate uptake, and the primary role of the jejunum in folate absorption.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleland W. W. Statistical analysis of enzyme kinetic data. Methods Enzymol. 1979;63:103–138. doi: 10.1016/0076-6879(79)63008-2. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- Eilam Y., Ariel M., Jablonska M., Grossowicz N. On the mechanism of folate transport in isolated intestinal epithelial cells. Am J Physiol. 1981 Feb;240(2):G170–G175. doi: 10.1152/ajpgi.1981.240.2.G170. [DOI] [PubMed] [Google Scholar]
- Knickelbein R., Aronson P. S., Atherton W., Dobbins J. W. Sodium and chloride transport across rabbit ileal brush border. I. Evidence for Na-H exchange. Am J Physiol. 1983 Oct;245(4):G504–G510. doi: 10.1152/ajpgi.1983.245.4.G504. [DOI] [PubMed] [Google Scholar]
- Kurtin P., Charney A. N. Intestinal ion transport and intracellular pH during acute respiratory alkalosis and acidosis. Am J Physiol. 1984 Jul;247(1 Pt 1):G24–G31. doi: 10.1152/ajpgi.1984.247.1.G24. [DOI] [PubMed] [Google Scholar]
- Liedtke C. M., Hopfer U. Mechanism of Cl- translocation across small intestinal brush-border membrane. II. Demonstration of Cl--OH- exchange and Cl- conductance. Am J Physiol. 1982 Mar;242(3):G272–G280. doi: 10.1152/ajpgi.1982.242.3.G272. [DOI] [PubMed] [Google Scholar]
- Lucas M. L., Blair J. A. The magnitude and distribution of the acid microclimate in proximal jejunum and its relation to luminal acidification. Proc R Soc Lond B Biol Sci. 1978 Jan 24;200(1138):27–41. doi: 10.1098/rspb.1978.0003. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Keeffe E. B., Blankenship N. M., Ockner R. K. Validation of a recording spectrophotometric method for measurement of membrane-associated Mg- and NaK-ATPase activity. J Lab Clin Med. 1979 May;93(5):790–799. [PubMed] [Google Scholar]
- Selhub J., Rosenberg I. H. Folate transport in isolated brush border membrane vesicles from rat intestine. J Biol Chem. 1981 May 10;256(9):4489–4493. [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. E. Mechanisms of folate homeostasis. Am J Physiol. 1984 Apr;246(4 Pt 1):G319–G324. doi: 10.1152/ajpgi.1984.246.4.G319. [DOI] [PubMed] [Google Scholar]
- Yang C. H., Sirotnak F. M., Dembo M. Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. J Membr Biol. 1984;79(3):285–292. doi: 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]



